Abstract
Background
Penile cancer (PC) is a rare cancer in western countries, but is more common in parts of the developing world. Due to its rarity and the consequent lack of randomized trials, current therapy is based on retrospective studies and small prospective trials.
Design
Studies of PC therapy were searched in PubMed and abstracts at major conferences.
Results
PC is generally an aggressive malignancy characterized by early locoregional lymph node (LN) spread and later metastases in distant sites. Given the strong predictive value of LN involvement for overall survival, evaluating regional LNs is critical. Advanced LN involvement is increasingly being treated with multimodality therapy incorporating chemotherapy and/or radiation. A single superior cisplatin-based regimen has not been defined. Further advances may occur with a better collaboration on an international scale and comprehensive understanding of tumor biology. To this end, the preventive role of circumcision and understanding of the oncogenic roles of Human Papilloma Virus-16, and smoking may yield advances. Preliminary data suggest a role for agents targeting epidermal growth factor receptor and angiogenesis.
Conclusion
Advances in therapy for PC will require efficient trial designs, synergistic collaboration, incentives to industry and the efforts of patient advocacy groups and venture philanthropists.
Keywords: biologic agents, chemotherapy, combined modality therapy, molecular targets, penile cancer, radiotherapy
introduction
Penile cancer (PC) is relatively rare in the developed countries, but higher incidences have been observed in the less developed countries. In 2012, 1570 new cases and 310 deaths from PC are predicted to occur in the USA, although the incidence declined from 1973 to 2002 (Table 1) [1, 2]. Conversely, the incidence climbs to 8.3 per 100 000 in parts of Asia, Africa and South America [3, 4].
Table 1.
Staging, incidence and outcomes in penile cancer
| Stagea | 2012 incidence in US | 2012 deaths in US | ∼5-year survival (%) |
|---|---|---|---|
| 0 (Tis or Ta, N0M0) | NA | NA | 100 |
| 1 (T1aN0M0) | NA | NA | 90 |
| 2 (T1b–T3N0M0) | NA | NA | 50 |
| 3 (T1–T3N1–N2M0) | NA | NA | 30 |
| 4 (any T4, any N3 or M1) | NA | NA | 5 |
| All stages | 1570 [1] | 310 | NA |
aAdapted from American Joint Committee on Cancer (AJCC) Staging Manual, seventh edition (2010) published by Springer, New York, Inc.
Tis: carcinoma in situ; Ta: noninvasive verrucous carcinoma; T1a: tumor invades subepithelial connective tissue without lymphovascular invasion (LVI) and is not high grade 3–4; T1b: tumor invades subepithelial connective tissue with LVI or is grade 3–4; T2: tumor invades corpus spongiosum or cavernosum; T3: tumor invades urethra; T4: tumor invades other adjacent structures; N1: mobile unilateral inguinal lymph node; N2: mobile multiple or bilateral inguinal lymph nodes; N3: palpable fixed inguinal nodal mass or pelvic lymphadenopathy; M1: distant metastasis; NA: not available.
In a study of registries including 6539 men with PC in the US during 1995–2003, Hispanic men had the highest incidence rates (6.58 per million) followed by black men (4.02 per million), white nonHispanic men (3.90 per million), native American men (2.81 per million) and Asian-Pacific Islanders (2.40 per million) [5]. The median age of diagnosis was 60–62 years, although the incidence increased among older subgroups. The majority (61%) of cases were diagnosed at a localized stage, although Hispanic and black men tended to be diagnosed with more advanced stages. The highest incidence occurred in the south (4.42 per million) and lowest in the west (3.28 per million). Other studies suggest an association with lower socioeconomic status [2, 6].
PC is a highly aggressive malignancy characterized by early locoregional spread with subsequent potential for distant dissemination. Studies of PC therapy were identified in PubMed and abstracts at major conferences to highlight recent advances in our knowledge regarding the management and molecular biology of the disease, the importance of multidisciplinary management, and suggest strategies to engender advances in therapy.
etiology, risk factors and prevention
Neonatal circumcision has been recognized to reduce the incidence of PC, possibly by inhibiting chronic inflammation [7, 8]. In fact, chronic inflammatory conditions such as balanopostitis and lichen sclerosus et atrophicus are among the strongest risk factors for PC [odds ratio (OR) >10], with 4%–8% of men with lichen sclerosus et atrophicus developing PC [9, 10]. PC virtually does not occur in the Jewish and Muslim populations, in which early circumcision is common. In a series of 458 cases in Ugandan Africans, the incidence was extremely low where circumcision was practiced [11]. Intriguingly, differences in incidence in the uncircumcised were observed over small distances, suggesting that unknown factors varying with geographical location may be operative. Moreover, early circumcision during infancy may be critical [12]. In a study including 110 men with PC and 355 matched controls, relative to men circumcised at birth, the risk for PC was 3.2 times greater among men who were never circumcised and 3.0 times greater among men who were circumcised after the neonatal period [12]. Those with a history of genital warts had 5.9 times the risk. Of 67 tumors tested for Human Papilloma Virus (HPV) DNA, 49% were positive, the majority of them being HPV-16 (70%). Among men uncircumcised at birth, the presence of smegma and difficulty in retracting the foreskin conferred a relative risk of 2.1 and 3.5, respectively. However, the role of smegma resulting from phimosis remains controversial [13, 14].
HPV, particularly HPV-16 and -18, appears to participate in pathogenesis, although HPV may only play a minor role in nonndemic areas [15–22]. Indeed, circumcision may exert its protective effect against the development of PC partly by preventing HPV infection [23, 24]. In a case–control study, penile HPV-DNA was detected in 166 of 847 uncircumcised men (19.6%) and in 16 of 292 circumcised men (5.5%) [23]. Monogamous women whose male partners had ≥6 sexual partners and were circumcised had a lower risk of cervical cancer than women whose partners had similar sexual history and were uncircumcised (OR 0.42). Studies of vaccination in men to prevent HPV-associated morbidities are ongoing [16, 25]. The increased risk of PC in patients with human immunodeficiency virus (HIV) infection may be mediated by coinfection with HPV, although the role of HIV in directly causing the malignancy is unclear [26].
Additional risk factors may include tobacco exposure as well as psoralens and ultraviolet A (PUVA) photochemotherapy; familial predisposition has not yet been identified. Current smoking conferred a higher risk compared with never smokers, albeit a causal link is unclear [27, 28]. In a prospective study of 892 men with psoriasis who had received PUVA, the standard morbidity ratio, which compares morbidity in the sample population with that expected on the basis of population incidence data, was 58.8 for invasive and in situ penile tumors [29]. Moreover, the incidence was dose dependent.
diagnosis and staging
The glans penis is the most common site of origin followed by the prepuce, coronal sulcus and shaft [2, 30]. Most patients present with localized disease as a mass, ulcer or inflammatory lesion (Table 1) [31]. Inguinal lymphadenopathy by physical examination exhibits low positive and negative predictive values. In one report of 100 men with PC treated according to the European Association of Urology (EAU) guidelines in a single institution, 72% of men with palpable lymph nodes (LNs) and 18% with impalpable LNs had pathological LN involvement [32]. Hence, an inguinal fine needle aspiration (FNA) biopsy has been recommended by the National Comprehensive Cancer Network (NCCN, v. 1.2012) to guide therapy in patients with palpable inguinal nodes. Subsequent excisional biopsy has been recommended if the FNA is negative (to avoid sampling error), and proceeding with full inguinal LN dissection is recommended if the FNA is positive for tumor. In those with impalpable LNs, surveillance for low-risk patients (≤T1G1) and sentinel LN biopsy in high-risk patients has been recommended.
A fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan may be useful in detecting LN metastasis, but more data are needed [33–37]. Magnetic resonance imaging appeared highly accurate in locoregional staging according to one study (n = 55) [38]. For now, staging with computerized tomography imaging of the pelvis should be standard for all men presenting with T1 or greater disease, with abdomen and chest imaging added for poorly differentiated tumors or >N2 stage. The most common sites of distant metastases are lung, liver and bone.
pathology
The vast majority of malignancies of the penis are squamous cell cancers (SCCs), but other histologic types are observed in ∼5% of cases, such as melanomas, basal cell carcinomas and sarcomas [39]. The World Health Organization (WHO) classifies penile SCC, or PC, as usual, basaloid, verrucous, warty (condylomatous), papillary, sarcomatoid, adenosquamous and mixed [40]. In a surgical series of 333 patients receiving homogeneous surgery, basaloid, sarcomatoid and adenosquamous carcinomas displayed the highest histological grade and deep tissue infiltration, while verrucous, papillary and condylomatous (warty) carcinomas were associated with low grade and superficial invasion. This relationship translated into distinct clinical behavior, with a higher 10-year survival rate for verrucous, adenosquamous, mixed, papillary and warty carcinoma (100%, 100%, 97%, 92% and 90%, respectively), while patients with the usual and basaloid types had 78% and 76% 10-year survival, respectively. Of note, 75% of patients with sarcomatoid carcinoma died, usually within a year of diagnosis [41]. Interestingly, verrucous carcinomas appear to exhibit low p16 and HPV expression [42]. Grading has an established prognostic role for PC with crucial clinical implications [43, 44]. Higher grade and basaloid and warty tumors are more consistently associated with HPV, suggesting that distinct pathogenic pathways may drive tumors [20, 45, 46].
molecular biology
Epidermal growth factor receptor (EGFR) overexpression appears to be almost universal and correlated with the grade, but not the stage [47–49]. In an American series, KRAS (Kirsten rat sarcoma) mutations and ERCC1 (excision repair cross-complementing group 1) amplification appeared rare or absent, which may portend responsiveness to EGFR inhibitors and platinum chemotherapy. EGFR had the highest relative expression followed by thymidylate synthetase. However, in a Spanish series (n = 28), 22% of evaluable tumors had mis-sense mutations in KRAS, suggesting that there may be regional differences in biology [50]. In another study, somatic mis-sense mutations in PIK3CA, HRAS and KRAS were found in 11 of 28 (39%) PC samples [51]. PIK3CA mutations were found in all grades and stages, whereas HRAS and KRAS mutations were found in more advanced tumors. The mutations were mutually exclusive, suggesting that dysregulation of either pathway is sufficient for tumor growth. A preliminary examination of the COSMIC dataset (n = 28) revealed p53 or PIK3CA mutations in 8 of the 28 (29%) tumors (http://www.sanger.ac.uk/cosmic, 18 June 2012, date last accessed) [52]. EGFR, HER3 and HER4 protein overexpression was found in one study of 148 cases, although no EGFR gene amplification was detected [53]. In this study, HPV-negative tumors expressed significantly more phosphorylated EGFR than HPV-positive cancers, which correlated with the phosphorylation and activation of Akt signaling. Conversely, HER3 expression was significantly more common in HPV-positive cases, which correlated with cytoplasmic localization of Akt1. PTEN protein expression was reduced in 62% of tumors, but PTEN gene loss occurred only in 4%.
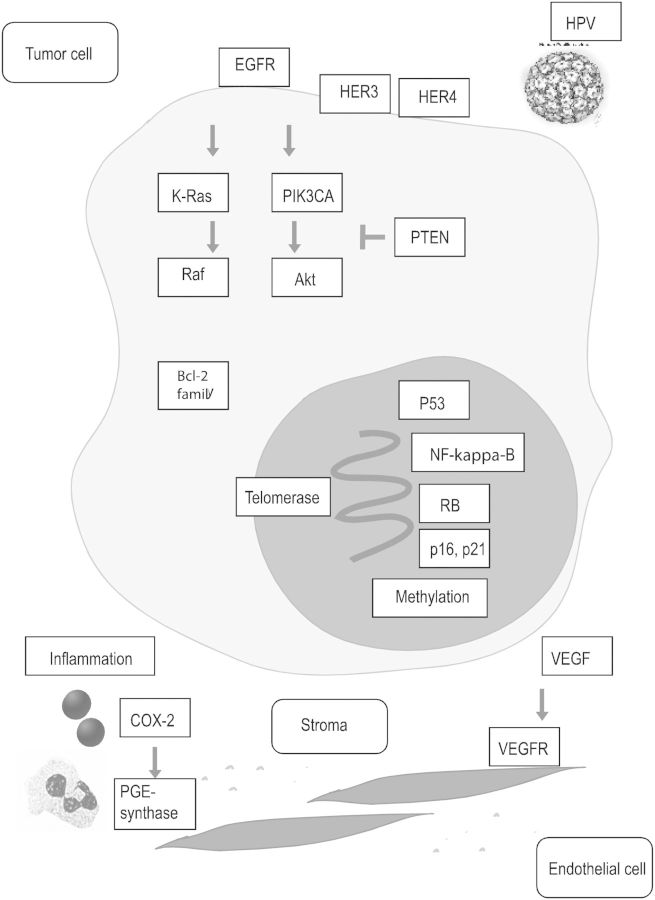
The epigenetic inactivation of thrombospondin-1 and RAS (rat sarcoma) association domain family-1A genes by hypermethylation seemed to confer prognostic significance in one study (n = 24) [54]. LN metastasis was significantly associated with negative p16 and combined LOH (loss of heterozygosity) and promoter hypermethylation, but not with p53 alterations [55]. Similarly, another study of 148 PCs demonstrated that HPV infection may engender p16 and p21 expression and RB suppression, but no association with p53 expression was detected [56]. Nevertheless, p53 protein expression has been related to LN metastasis and poor survival in other studies [57–59]. Moreover, studies indicate the potential importance of cell-cycle regulators and pro-survival proteins, e.g. p16, p21, telomerase and the Bcl-2 family [49, 60–62] (Figure 1).
Figure 1.
Potential molecular pathways driving growth and resistance of penile cancer. Human Papilloma Virus (HPV) may play an initiating role, but no dominant molecular driver has emerged. The EGFR and Her3/Her4 family, signaling via Ras-Raf and PI3K-Akt, transcription factors (NF-kappa-B), tumor suppressor gene alterations (RB and p53), epigenetic factors (methylation), cell-cycling regulators (p16 and p21), pro-survival molecules (Bcl-2 family and telomerase), pro-inflammatory (COX-2) and pro-angiogenic molecules (VEGF axis) appear to play a role in subsets.
Another study of 26 cases reported DNA sequence copy number alterations (CNAs) similar to oral and esophageal SCCs [63]. The most frequent copy number gains occurred in 8q24, 16p11-12, 20q11-13, 22q, 19q13 and 5p15, while the most common deletions occurred in 13q21-22, 4q21-32 and the X chromosome. The number of CNAs exhibited a possible correlation with clinical outcome, but the biological mechanisms remain undefined. Increased cyclo-oxygenase (COX)-2 and microsomal prostaglandin E synthase-1 were detected in penile intraepithelial neoplasia and carcinoma in one study, suggesting a pathogenic role for inflammation and a therapeutic role for COX-2 inhibitors [64]. The potential role of angiogenesis was suggested by a case series reporting the activity of sorafenib and sunitinib [65].
prognostic factors
Pathologic TNM staging provides prognostic stratification after surgery (Table 1) [66]. Furthermore, extranodal extension in inguinal LNs and pelvic LN involvement appear to be independently associated with decreased 5-year cancer-specific survival (42% and 22%, respectively) [67]. Nomograms have been reported for patients following penectomy to better predict cancer-specific survival and LN metastasis [68–70]. These nomograms incorporate multiple variables in addition to stage to enhance prognostication including grade, venous or lymphatic embolization and the type of surgery. Other studies have reported LN density, lack of koilocytosis and clear cell subtype to be prognostic [67, 70–84].
Additionally, molecular prognostic markers are suggested by some studies, e.g. p53, Ki-67, E-cadherin, MMP-9 (matrix metalloproteinase-9), annexins I and IV and decreased KAI1/CD82, a metastasis suppressor gene [57, 59, 85–88]. Although HPV has been associated with high-grade tumors, the impact on outcomes is unclear with one study even demonstrating a favorable impact of HPV and another study showing a positive association with survival of p16, which is related to HPV [17, 46, 89, 90].
surgery
Noninvasive tumors are amenable to local measures, e.g. topical 5-fluorouracil (5-FU) or imiquimod, laser or local excision. A partial and glans-sparing penectomy provides psychosocial benefits, preserves sexual function and is generally feasible for a T1 tumor [91]. A 2-cm margin has been advocated historically, although some recent data suggest a 5- to 10-mm margin may be adequate [92]. Total penectomy is preferred for ≥T2 tumors, although some T2 tumors are amenable to partial penectomy based on location. Penile-sparing surgical modalities including Mohs' micrographic surgery and laser ablation are considered for small tumors, particularly if located on the glans and margins ≥3 mm can be attained.
Controversies surround the role and extent of immediate inguinal lymphadenectomy with or without sentinel LN dissection in those without clinical lymphadenopathy, as well as the role of pelvic LN dissection [93–105]. In a large surgical series of 688 patients, immediate lymphadenectomy (n = 251) was associated with a better 10-year disease-specific survival than delayed (n = 81) lymphadenectomy (71% vs. 30%, P = 0.002) [99]. The authors reported divergent 10-year disease-specific survivals for low-risk (T1G1-2), intermediate (T2-3G1-2) and high-risk (T1-3G3 and T4G1-3) patients ranging from ∼75% to ∼40%. The 10-year disease-free survival rates for patients with negative and positive pathological nodal involvement in the immediate lymphadenectomy group were 96% and 35%, respectively. Despite the caveats of a retrospective analysis, these data suggest the powerful favorable impact of inguinal LN dissection. Video-endoscopic inguinal lymphadenectomy appeared feasible without compromising tumor control in those without palpable adenopathy in small retrospective series [106]. However, a larger experience and longer follow-up are necessary before routine adoption. Similarly, sentinel LN dissection has been carried out to guide additional dissection, although the false negative rate (15%–20%) suggests that further refinement of the methodology is necessary [101].
Both the EAU and NCCN guidelines, which share a number of similarities, suggest adapting the extent of LN dissection to clinical stage [44, 94]. Generally, for low-risk compliant patients (pTis, pTa and pT1G1) without palpable LNs, surveillance was recommended. For all other patients without palpable LNs, a modified bilateral lymphadenectomy or sentinel LN dissection was recommended. Radical inguinal lymphadenectomy was recommended for patients with histologically proven LN metastasis. In addition, pelvic LN dissection was recommended in patients with multiple inguinal LNs, extranodal extension or node of Cloquet involvement.
patterns of recurrence after surgery
In a large retrospective study of 700 patients, the rate of recurrences was compared between patients undergoing penile-preserving treatments and partial/total amputation [107]. Regional recurrences were compared between patients surgically staged as pN0 or pN+ and clinically node-negative (cN0) patients who chose a watchful waiting approach. Of these 700 patients, 205 (29.3%) displayed a recurrence, including 18.6% local, 9.3% regional and 1.4% distant recurrences. The vast majority of recurrences (86%) occurred within 2 years. Local recurrences occurred in a higher proportion (27.7%) after penile-preserving therapy compared with following amputation (5.3%), although this did not appear to compromise survival due to the efficacy of surgical salvage. The regional recurrence rate was 2.3% in patients with pN0, 19.1% with pN+ and 9.1% undergoing a watchful waiting approach. The 5-year disease-specific survival rate was 92% after a local recurrence and 32.7% after regional recurrence, while all patients with a distant recurrence died within 22 months.
radiotherapy
External beam radiotherapy (XRT) has been employed for localized T1–T2 disease as organ-sparing therapy or as adjuvant therapy following surgery [108–113]. In retrospective reports of <100 patients each, local and regional recurrence rates (∼20% and ∼5%, respectively) appear higher than observed with surgery, but salvage resection is generally effective. However, there are no trials comparing XRT and surgery. Similarly, brachytherapy may be an excellent penile-sparing modality for T1 and T2 tumors <4 cm in size located on the glans [114–116]. The 10-year local recurrence rate in the largest retrospective study of brachytherapy for cancer of the glans penis (n = 144) was ∼20% and appeared to be dependent on tumor size. Surgical salvage rescued most recurrences, yielding 10-year cancer-specific survival in 92% [115]. Delayed complications included stenosis, necrosis, fibrosis and ulceration.
Anecdotal reports of the success of concurrent cisplatin or 5-FU-based chemotherapy and radiation for locally advanced unresectable disease suggest that further investigation of this modality is warranted [117, 118]. Prospective studies of concurrent chemoradiation are unavailable at this time, although extrapolation from similar perineal SCCs, e.g. vulvar and anal cancer, suggest the potential efficacy of chemoradiation followed by salvage surgery for persistent or recurrent disease [119, 120].
Adjuvant XRT may be considered in high-risk node positive patients following surgery, given the high risk of locoregional recurrence [109]. In a retrospective study, regional failure rates after inguinal LN dissection for pathological inguinal LN metastasis were 11% (1 of 9) and 60% (3 of 5) in patients with and without adjuvant XRT.
perioperative chemotherapy
In patients with multiple, fixed or bulky inguinal LNs (≥4 cm), or involved pelvic LNs, multimodality therapy including primary chemotherapy followed by surgery and node resection is preferred. Small retrospective studies including 5–20 patients have examined bleomycin–vincristine–methotrexate (BVM) and bleomycin–methotrexate–cisplatin (BMP; Table 1) [121–124]. A recent prospective trial investigated four cycles of neoadjuvant ifosfamide, paclitaxel, cisplatin (ITP) and demonstrated the feasibility and activity of this regimen (Table 2) [122, 124, 125]. Thirty men received ITP in this trial of whom 15 (50.0%) had an objective response and 22 (73.3%) underwent surgery. Three (10%) patients exhibited a pathologic complete response (pCR), which was a marginally substantial predictor of improved survival. Serious adverse events related to chemotherapy were infrequent, with grade 3 infection being the most common severe toxicity occurring in ∼16% of patients. Nine (30.0%) patients remained alive and free of recurrence after a median follow-up of 34 months. The estimated median time-to-progression (TTP) was 8.1 months, and median OS was 17.1 months.
Table 2.
Reported studies of ≥10 patients receiving preoperative therapy
| Author | Regimen | Design | N | Surgery N (%) | Clinical stage | Clinical response N (%) | pCR N (%) | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|---|---|
| Pagliaro et al. [125] | ITP | Phase II trial | 30 | 22 (73.3) | Any T, N2–N3 | 15 (50) | 3 (10) | 8.1 months | 17.1 months |
| Leijte et al. [122] | BMP, BVM, CF, CI | Retrospective | 20 | 9 (45) | Any N3 or T4 | 12 (60) | 2 (10) | NR | 5 years: 32% |
| Bermejo et al. [124] | BMP, PCa, TIP | Retrospective | 10 | 10 (100) | Variable, N1–N3 or M1 | 5 (50) | 3 (30) | NR | 26 months |
Surgery consisted of bilateral inguinal lymphadenectomy with unilateral or bilateral pelvic lymphadenectomy.
ITP: ifosfamide, paclitaxel, cisplatin; BVM: bleomycin–vincristine–methotrexate; BMP: bleomycin–methotrexate–cisplatin; CF: cisplatin–5-FU; CI: cisplatin–irinotecan; PCa: paclitaxel–carboplatin; NR: not reported.
Improved long-term outcomes were substantially associated with response to chemotherapy and the absence of bilateral residual tumor/extranodal extension/skin involvement. Trends toward shorter survival were noted with poor performance status, immobile groin mass, skin ulceration and leukocytosis. Preliminarily, the FDG-PET scan has appeared useful in monitoring response to neoadjuvant chemotherapy in a small study [126].
There are no prospective studies of adjuvant chemotherapy, although small retrospective reports have been presented [44, 121, 127]. Long-term disease-free survival occurred in 84% of 25 consecutive node positive patients treated with adjuvant BVM during 1979–1990 versus 39% of 38 consecutive patients undergoing radical LN dissection (with or without XRT) from 1960 to 1978 [121]. Given the high risk of locoregional recurrence, a potential role may exist for adjuvant combination chemotherapy and XRT. In the absence of randomized trials, clinical judgment and appropriate patient selection are necessary before embarking on adjuvant therapy. The EAU recommends adjuvant chemotherapy only for ≥pN2 disease, whereas the NCCN recommends it for LN size ≥4 cm.
chemotherapy for advanced disease
A substantial variability of employed first-line regimens exists in practice [128]. Cisplatin alone displayed modest activity with four partial responses in 26 (15.4%) patients and a median survival of only 4.7 months (Table 3) [129]. Historical data with combination BMP demonstrated a median survival of only 28 weeks [130–132]. In the largest prospective study of this regimen, there were five complete and eight partial responses in 40 assessable patients for a 32.5% response rate [132]. Unfortunately, in this study, five treatment-related deaths occurred and six other patients had 1 or more life threatening toxic episodes. Hence, the toxic effects of bleomycin–containing regimens have been recognized and considered to be prohibitive.
Table 3.
Reported studies of ≥10 patients receiving chemotherapy for advanced penile cancer
| Author | Line of therapy | Regimen | Design | N | Clinical response N (%) | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|
| Gagliano et al. [129] | First | Cisplatin | Phase II trial | 26 | 4 (15.4) | NR | 4.7 months |
| Haas et al. [132] | First | BMP | Phase II trial | 40 | 13 (32.5) | NR | 28 weeks |
| Dexeus et al. [131] | First | BMP | Retrospectivea | 14 | 10 (72) | NR | NR |
| Corral et al. [130] | First | BMP | Phase II trialb | 30 | 16 (55) | NR | 11.5 months |
| Di Lorenzo et al. [138] | First | CF | Retrospective | 25 | 8 (32) | 20 weeks | 8 months |
| Theodore et al. [140] | First | CI | Phase II trial | 28 | 8 (30.8) | NR | NR |
| Di Lorenzo et al. [142] | Second | Paclitaxelc | Phase II trial | 25 | 5 (20) | 11 weeks | 23 weeks |
aTwelve of the 14 patients had penile primary site.
bTrial enrolled patients with squamous cell carcinoma of the penis, scrotum, bladder, renal pelvis, ureter or urethra.
cPaclitaxel every 3 weeks.
BMP: bleomycin–methotrexate–cisplatin; CF: cisplatin–5-FU; CI: cisplatin–irinotecan; NR: not reported.
Thereafter, other cisplatin-based regimens were employed that omitted bleomycin. Small retrospective reports of regimens containing cisplatin–5-FU with or without taxane have demonstrated modest activity [132–139]. In the largest retrospective study employing cisplatin–5-FU, 25 patients exhibited a response rate of 32%, and median PFS (progression-free-survival) and OS of 20 weeks and 8 months, respectively [138]. In contrast to BMP, cisplatin–5-FU displayed excellent tolerance, with a 20% incidence of grade 3–4 neutropenia and an 8% incidence of grade 3–4 anemia. One trial investigated the combination of cisplatin and irinotecan in locoregionally advanced or metastatic disease [140]. Patients were treated in the neoadjuvant setting for T3 or N1–N2 disease either with up to four cycles before surgery or up to eight cycles for T4 or N3 or M1 disease. There were eight clinical responses in 26 assessable patients (30.8%) including two complete clinical responses, and three pCRs at LN dissection were noted. Anecdotal benefit has been observed when employing cisplatin–gemcitabine [141]. ITP may also be a rational regimen in metastatic disease, based on the activity in the neoadjuvant setting [125].
Second-line therapy is also not established, and taxanes have been used with marginal activity [142]. In a prospective, multicenter phase II trial, 25 patients were enrolled and treated with paclitaxel 175 mg/m2 every 3 weeks [142]. Partial responses were observed in 20%. The median PFS was only 11 weeks, and the median OS was 23 weeks. The anticipated, but manageable, toxic effects of paclitaxel were observed.
novel systemic regimens and biological agents
A potential role may exist for EGFR inhibiting monoclonal antibodies (panitumumab and cetuximab) [143–145]. In one retrospective study, all 13 patients with advanced PC expressed EGFR with 77% exhibiting 3+ levels of expression and received EGFR-targeted therapies, including erlotinib (n = 1), cetuximab (n = 3) or cetuximab, combined with platinum-based regimens (n = 9) [143]. The patients showed a median TTP of 3.2 months and a median OS of 9.8 months. Four (31%) patients survived between 13 and 48+ months, comparing favorably with historical survival when utilizing conventional chemotherapy. Anecdotal responses have been reported with panitumumab or combination docetaxel–cetuximab after cisplatin-based chemotherapy [144, 145]. EGFR monoclonal antibodies appear to warrant further study in combination with chemotherapy and radiation, given these promising signals.
Angiogenesis is also a promising target; in a retrospective report of six chemorefractory patients following at least two prior regimens treated with sunitinib or sorafenib, one patient achieved a partial response and four had stable disease [65]. Reduction in microvessel density and Ki-67 labeling index was observed in paired specimens. Serious adverse events were fatal infection and rupture of the femoral vessel.
future perspectives
Better understanding of the basic biology of the malignancy should guide the design and conduct of future clinical trials. Currently, a dominant molecular driver of the disease remains unknown. A large international consortium may overcome the barrier of slow accrual and has been demonstrated to be successful in other uncommon or rare malignancies [146–148]. In this context, the International Rare Cancer Initiative (IRCI) has been launched, which is composed of the UK National Cancer Research Network, Cancer Research UK, US National Cancer Institute (NCI) and the European Organization for the Research and Treatment of Cancer (EORTC). Given the rarity of the disease, referral to centers with demonstrated excellence in the management of PC should be considered, particularly with reported improvement in outcomes with this approach [149]. Simultaneously, the cooperation and partnership of regulatory bodies is essential in the early stages of drug development. There is a need to incentivize industry and a role for disease advocacy and venture philanthropy.
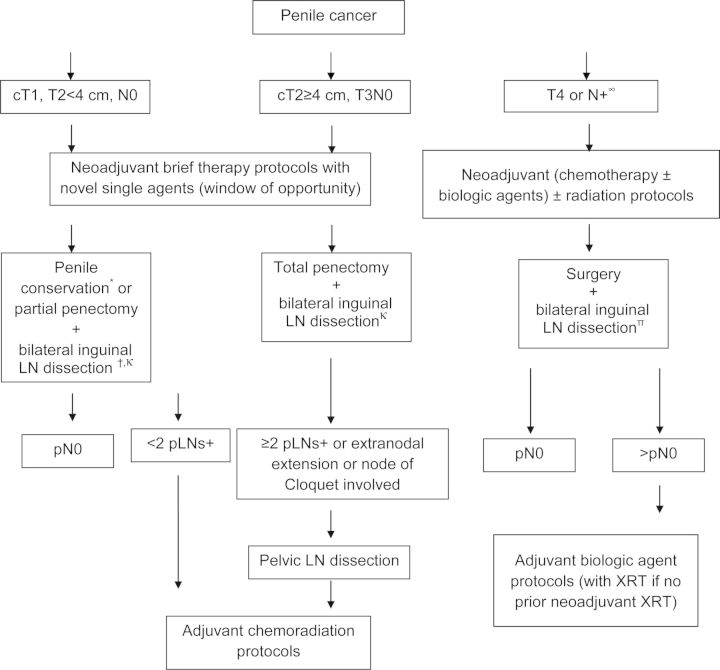
In addition, the classic paradigm of randomized trials may be difficult to execute in this rare malignancy. Therefore, a need for new paradigms and establishment of intermediate surrogates for long-term outcomes appear necessary, e.g. pathologic or radiographic response, or PFS. A Bayesian trial design may be well suited to studying new agents in rare cancers. The neoadjuvant paradigm may be particularly useful and could potentially be employed even in earlier stages of node-negative disease in ‘window-of-opportunity’ trials. Modifications of published recommendations are depicted in Figure 2 as a strategy to manage and expedite the development of therapy. We recommend an early aggressive perioperative approach with combined modality neoadjuvant therapy for T4 or node positive disease, since recurrence is associated with poor survival. These recommendations also underscore our belief that trials employing a neoadjuvant therapy approach and capitalizing on both ‘window-of-opportunity’ designs and combined modality regimens incorporating biologic agents (with chemotherapy and/or radiation) may be complementary. Opportunities also exist in the developing adjuvant regimens in those patients undergoing initial surgery. Moreover, phase I trials utilizing tumor molecular profiling to guide the enrollment of patients on protocols investigating specific agents may be exploited as an avenue to identify signals of activity [150].
Figure 2.
Proposed strategy to manage local and locoregional invasive penile cancer. ∞Consider LN biopsy to exclude false positive lymphadenopathy, *brachytherapy/external bean radiation/Mohs micrographic surgery/laser, †except for T1G1 where a role for surveillance exists, κpotential role for sentinel LN dissection followed by LN dissection if positive, πconsider pelvic LN dissection based on risk.
conclusion
A logical and effective therapeutic approach to PC is possible despite the lack of randomized trials (Figure 2). For localized disease, there are sophisticated approaches beyond mere amputation, such as glans-sparing partial penectomy, brachytherapy and reconstructive surgery. For metastatic disease in LNs, a curative neoadjuvant multidisciplinary paradigm is feasible instead of a palliative approach. Nevertheless, despite excellent outcomes in localized disease, locoregional and metastatic disease portend poor outcomes. Important research questions remain, such as the role of chemoradiation, and opportunities for targeted therapy. Unfortunately, in view of the rarity of the disease and little interest among pharmaceutical companies, few clinical trials have been conducted. Prevention and early detection appear critical. In particular, neonatal circumcision, smoking cessation and HPV vaccination may substantially reduce the incidence of PC. Indeed, HPV vaccination is already approved in the USA for males aged 9–26 years for preventing genital warts and anal cancer. Global collaboration is urgently necessary to make advances.
disclosure
GS: Research support and/or advisory board of Pfizer, BMS, Novartis, Celgene, and Sanofi-Aventis. LCP: Consultant for Janssen and Research support from Pfizer and Celgene. CB and RJL: these authors have declared no conflicts of interest. TBD: speaker for Pfizer and Bayer. GDL: research support from Pfizer and advisory board of Sanofi, Janssen, Cilag and Novartis.
references
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361–367. doi: 10.1016/j.urolonc.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Ornellas AA. Management of penile cancer. J Surg Oncol. 2008;97:199–200. doi: 10.1002/jso.20893. [DOI] [PubMed] [Google Scholar]
- 4.Misra S, Chaturvedi A, Misra NC. Penile carcinoma: a challenge for the developing world. Lancet Oncol. 2004;5:240–247. doi: 10.1016/S1470-2045(04)01427-5. [DOI] [PubMed] [Google Scholar]
- 5.Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995–2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833–1839. doi: 10.1158/1055-9965.EPI-07-0221. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer. 2008;113:2883–2891. doi: 10.1002/cncr.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoen EJ. The relationship between circumcision and cancer of the penis. CA Cancer J Clin. 1991;41:306–309. doi: 10.3322/canjclin.41.5.306. [DOI] [PubMed] [Google Scholar]
- 8.Kochen M, McCurdy S. Circumcision and the risk of cancer of the penis. A life-table analysis. Am J Dis Child. 1980;134:484–486. doi: 10.1001/archpedi.1980.02130170034012. [DOI] [PubMed] [Google Scholar]
- 9.Dillner J, von Krogh G, Horenblas S, et al. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol Suppl. 2000:189–193. doi: 10.1080/00365590050509913. [DOI] [PubMed] [Google Scholar]
- 10.Clouston D, Hall A, Lawrentschuk N. Penile lichen sclerosus (balanitis xerotica obliterans) BJU Int. 108(Suppl 2):14–19. doi: 10.1111/j.1464-410X.2011.10699.x. [DOI] [PubMed] [Google Scholar]
- 11.Schmauz R, Jain DK. Geographical variation of carcinoma of the penis in Uganda. Br J Cancer. 1971;25:25–32. doi: 10.1038/bjc.1971.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19–24. doi: 10.1093/jnci/85.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Van Howe RS, Hodges FM. The carcinogenicity of smegma: debunking a myth. J Eur Acad Dermatol Venereol. 2006;20:1046–1054. doi: 10.1111/j.1468-3083.2006.01653.x. [DOI] [PubMed] [Google Scholar]
- 14.Waskett JH, Morris BJ. Re: ‘RS Van Howe, FM Hodges. The carcinogenicity of smegma: debunking a myth.’ An example of myth and mythchief making? J Eur Acad Dermatol Venereol. 2008;22:131. doi: 10.1111/j.1468-3083.2007.02439.x. ; author reply 131–132. [DOI] [PubMed] [Google Scholar]
- 15.Heideman DA, Waterboer T, Pawlita M, et al. Human papillomavirus-16 is the predominant type etiologically involved in penile squamous cell carcinoma. J Clin Oncol. 2007;25:4550–4556. doi: 10.1200/JCO.2007.12.3182. [DOI] [PubMed] [Google Scholar]
- 16.Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141–150. doi: 10.1007/s00345-008-0302-z. [DOI] [PubMed] [Google Scholar]
- 17.Bezerra AL, Lopes A, Santiago GH, et al. Human papillomavirus as a prognostic factor in carcinoma of the penis: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. Cancer. 2001;91:2315–2321. [PubMed] [Google Scholar]
- 18.Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449–457. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- 19.Gregoire L, Cubilla AL, Reuter VE, et al. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst. 1995;87:1705–1709. doi: 10.1093/jnci/87.22.1705. [DOI] [PubMed] [Google Scholar]
- 20.Miralles-Guri C, Bruni L, Cubilla AL, et al. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62:870–878. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- 21.Rubin MA, Kleter B, Zhou M, et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159:1211–1218. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannweiler S, Sygulla S, Beham-Schmid C, et al. Penile carcinogenesis in a low-incidence area: a clinicopathologic and molecular analysis of 115 invasive carcinomas with special emphasis on chronic inflammatory skin diseases. Am J Surg Pathol. 35:998–1006. doi: 10.1097/PAS.0b013e3182147e59. [DOI] [PubMed] [Google Scholar]
- 23.Castellsague X, Bosch FX, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–1112. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 24.Backes DM, Bleeker MC, Meijer CJ, et al. Male circumcision is associated with a lower prevalence of human papillomavirus-associated penile lesions among Kenyan men. Int J Cancer. 130:1888–1897. doi: 10.1002/ijc.26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barroso LF, II, Wilkin T. Human papillomavirus vaccination in males: the state of the science. Curr Infect Dis Rep. 13:175–181. doi: 10.1007/s11908-010-0163-7. [DOI] [PubMed] [Google Scholar]
- 26.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 27.Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606–616. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 28.Hellberg D, Valentin J, Eklund T, et al. Penile cancer: is there an epidemiological role for smoking and sexual behaviour? Br Med J (Clin Res Ed) 1987;295:1306–1308. doi: 10.1136/bmj.295.6609.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern RS. Genital tumors among men with psoriasis exposed to psoralens and ultraviolet A radiation (PUVA) and ultraviolet B radiation. The Photochemotherapy Follow-up Study. N Engl J Med. 1990;322:1093–1097. doi: 10.1056/NEJM199004193221601. [DOI] [PubMed] [Google Scholar]
- 30.Heyns CF, Mendoza-Valdes A, Pompeo AC. Diagnosis and staging of penile cancer. Urology. 76:S15–23. doi: 10.1016/j.urology.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Burgers JK, Badalament RA, Drago JR. Penile cancer. Clinical presentation, diagnosis, and staging. Urol Clin North Am. 1992;19:247–256. [PubMed] [Google Scholar]
- 32.Hegarty PK, Kayes O, Freeman A, et al. A prospective study of 100 cases of penile cancer managed according to European Association of Urology guidelines. BJU Int. 2006;98:526–531. doi: 10.1111/j.1464-410X.2006.06296.x. [DOI] [PubMed] [Google Scholar]
- 33.Schlenker B, Scher B, Tiling R, et al. Detection of inguinal lymph node involvement in penile squamous cell carcinoma by 18F-fluorodeoxyglucose PET/CT: a prospective single-center study. Urol Oncol. 30:55–59. doi: 10.1016/j.urolonc.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Graafland NM, Leijte JA, Valdes Olmos RA, et al. Scanning with 18F-FDG-PET/CT for detection of pelvic nodal involvement in inguinal node-positive penile carcinoma. Eur Urol. 2009;56:339–345. doi: 10.1016/j.eururo.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Souillac I, Rigaud J, Ansquer C, et al. Prospective evaluation of (18)F-fluorodeoxyglucose positron emission tomography-computerized tomography to assess inguinal lymph node status in invasive squamous cell carcinoma of the penis. J Urol. 187:493–497. doi: 10.1016/j.juro.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Rosevear HM, Williams H, Collins M, et al. Utility of (18)F-FDG PET/CT in identifying penile squamous cell carcinoma metastatic lymph nodes. Urol Oncol. 2012;30:723–6. doi: 10.1016/j.urolonc.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Leijte JA, Graafland NM, Valdes Olmos RA, et al. Prospective evaluation of hybrid 18F-fluorodeoxyglucose positron emission tomography/computed tomography in staging clinically node-negative patients with penile carcinoma. BJU Int. 2009;104:640–644. doi: 10.1111/j.1464-410X.2009.08450.x. [DOI] [PubMed] [Google Scholar]
- 38.Kayes O, Minhas S, Allen C, et al. The role of magnetic resonance imaging in the local staging of penile cancer. Eur Urol. 2007;51:1313–1318. doi: 10.1016/j.eururo.2006.11.014. discussion 1318–1319. [DOI] [PubMed] [Google Scholar]
- 39.Cubilla AL, Reuter V, Velazquez E, et al. Histologic classification of penile carcinoma and its relation to outcome in 61 patients with primary resection. Int J Surg Pathol. 2001;9:111–120. doi: 10.1177/106689690100900204. [DOI] [PubMed] [Google Scholar]
- 40.Eble J, Sauter G, Epstein J, et al. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs, Chapter 5. Lyon: IARC Press; 2004. pp. 281–290. [Google Scholar]
- 41.Ornellas AA, Seixas AL, Marota A, et al. Surgical treatment of invasive squamous cell carcinoma of the penis: retrospective analysis of 350 cases. J Urol. 1994;151:1244–1249. doi: 10.1016/s0022-5347(17)35222-9. [DOI] [PubMed] [Google Scholar]
- 42.Stankiewicz E, Kudahetti SC, Prowse DM, et al. HPV infection and immunochemical detection of cell-cycle markers in verrucous carcinoma of the penis. Mod Pathol. 2009;22:1160–1168. doi: 10.1038/modpathol.2009.77. [DOI] [PubMed] [Google Scholar]
- 43.Hainsworth JD, Meluch AA, Litchy S, et al. Paclitaxel, carboplatin, and gemcitabine in the treatment of patients with advanced transitional cell carcinoma of the urothelium. Cancer. 2005;103:2298–2303. doi: 10.1002/cncr.21078. [DOI] [PubMed] [Google Scholar]
- 44.Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol. 57:1002–1012. doi: 10.1016/j.eururo.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 45.Cubilla AL, Lloveras B, Alejo M, et al. The basaloid cell is the best tissue marker for human papillomavirus in invasive penile squamous cell carcinoma: a study of 202 cases from Paraguay. Am J Surg Pathol. 34:104–114. doi: 10.1097/PAS.0b013e3181c76a49. [DOI] [PubMed] [Google Scholar]
- 46.Cubilla AL, Lloveras B, Alejo M, et al. Value of p16(INK)(4)(a) in the pathology of invasive penile squamous cell carcinomas: a report of 202 cases. Am J Surg Pathol. 35:253–261. doi: 10.1097/PAS.0b013e318203cdba. [DOI] [PubMed] [Google Scholar]
- 47.Lavens N, Gupta R, Wood LA. EGFR overexpression in squamous cell carcinoma of the penis. Curr Oncol. 17:4–6. doi: 10.3747/co.v17i1.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorff TB, Schwartz R, Ma Y, et al. EGFR, TS, and ERCC1 expression in penile squamous cancer. J Clin Oncol. 2011;29(Suppl 7) abstr 219. [Google Scholar]
- 49.Kayes O, Ahmed HU, Arya M, et al. Molecular and genetic pathways in penile cancer. Lancet Oncol. 2007;8:420–429. doi: 10.1016/S1470-2045(07)70137-7. [DOI] [PubMed] [Google Scholar]
- 50.Valverde CM, Hernandez-Losa J, Ferrandiz-Pulido C, et al. BRAF and KRAS mutations in penile cancer and their correlation with clinical features. J Clin Oncol. 2011;29(Suppl 7) abstr 221. [Google Scholar]
- 51.Andersson P, Kolaric A, Windahl T, et al. PIK3CA, HRAS and KRAS gene mutations in human penile cancer. J Urol. 2008;179:2030–2034. doi: 10.1016/j.juro.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 52.Bamford S, Dawson E, Forbes S, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stankiewicz E, Prowse DM, Ng M, et al. Alternative HER/PTEN/Akt pathway activation in HPV positive and negative penile carcinomas. PLoS One. 6:e17517. doi: 10.1371/journal.pone.0017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guerrero D, Guarch R, Ojer A, et al. Hypermethylation of the thrombospondin-1 gene is associated with poor prognosis in penile squamous cell carcinoma. BJU Int. 2008;102:747–755. doi: 10.1111/j.1464-410X.2008.07603.x. [DOI] [PubMed] [Google Scholar]
- 55.Poetsch M, Hemmerich M, Kakies C, et al. Alterations in the tumor suppressor gene p16(INK4A) are associated with aggressive behavior of penile carcinomas. Virchows Arch. 458:221–229. doi: 10.1007/s00428-010-1007-4. [DOI] [PubMed] [Google Scholar]
- 56.Stankiewicz E, Prowse DM, Ktori E, et al. The retinoblastoma protein/p16 INK4A pathway but not p53 is disrupted by human papillomavirus in penile squamous cell carcinoma. Histopathology. 58:433–439. doi: 10.1111/j.1365-2559.2011.03762.x. [DOI] [PubMed] [Google Scholar]
- 57.Lopes A, Bezerra AL, Pinto CA, et al. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol. 2002;168:81–86. [PubMed] [Google Scholar]
- 58.Gunia S, Kakies C, Erbersdobler A, et al. Expression of p53, p21 and cyclin D1 in penile cancer: p53 predicts poor prognosis. J Clin Pathol. 65:232–236. doi: 10.1136/jclinpath-2011-200429. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Y, Zhou XY, Yao XD, et al. The prognostic significance of p53, Ki-67, epithelial cadherin and matrix metalloproteinase-9 in penile squamous cell carcinoma treated with surgery. BJU Int. 2007;100:204–208. doi: 10.1111/j.1464-410X.2007.06908.x. [DOI] [PubMed] [Google Scholar]
- 60.Kayes OJ, Loddo M, Patel N, et al. DNA replication licensing factors and aneuploidy are linked to tumor cell cycle state and clinical outcome in penile carcinoma. Clin Cancer Res. 2009;15:7335–7344. doi: 10.1158/1078-0432.CCR-09-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alves G, Fiedler W, Guenther E, et al. Determination of telomerase activity in squamous cell carcinoma of the penis. Int J Oncol. 2001;18:67–70. doi: 10.3892/ijo.18.1.67. [DOI] [PubMed] [Google Scholar]
- 62.Nascimento Pde S, Ornellas AA, Campos MM, et al. Bax and bcl-2 imbalance and HPB infection in penile tumors and adjacent tissues. Prog Urol. 2004;14:353–359. [PubMed] [Google Scholar]
- 63.Alves G, Heller A, Fiedler W, et al. Genetic imbalances in 26 cases of penile squamous cell carcinoma. Genes Chromosomes Cancer. 2001;31:48–53. doi: 10.1002/gcc.1117. [DOI] [PubMed] [Google Scholar]
- 64.Golijanin D, Tan JY, Kazior A, et al. Cyclooxygenase-2 and microsomal prostaglandin E synthase-1 are overexpressed in squamous cell carcinoma of the penis. Clin Cancer Res. 2004;10:1024–1031. doi: 10.1158/1078-0432.ccr-1032-3. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Y, Li H, Yao XD, et al. Feasibility and activity of sorafenib and sunitinib in advanced penile cancer: a preliminary report. Urol Int. 2010;85:334–340. doi: 10.1159/000315432. [DOI] [PubMed] [Google Scholar]
- 66.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. UICC International Union Against Cancer. 7th edition. Oxford, UK: Wiley-Blackwell; 2009. pp. 239–242. [Google Scholar]
- 67.Graafland NM, van Boven HH, van Werkhoven E, et al. Prognostic significance of extranodal extension in patients with pathological node positive penile carcinoma. J Urol. 2010;184:1347–1353. doi: 10.1016/j.juro.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Ficarra V, Zattoni F, Artibani W, et al. Nomogram predictive of pathological inguinal lymph node involvement in patients with squamous cell carcinoma of the penis. J Urol. 2006;175:1700–1704. doi: 10.1016/S0022-5347(05)01003-7. discussion 1704–1705. [DOI] [PubMed] [Google Scholar]
- 69.Kattan MW, Ficarra V, Artibani W, et al. Nomogram predictive of cancer specific survival in patients undergoing partial or total amputation for squamous cell carcinoma of the penis. J Urol. 2006;175:2103–2108. doi: 10.1016/S0022-5347(06)00313-2. discussion 2108. [DOI] [PubMed] [Google Scholar]
- 70.Zini L, Cloutier V, Isbarn H, et al. A simple and accurate model for prediction of cancer-specific mortality in patients treated with surgery for primary penile squamous cell carcinoma. Clin Cancer Res. 2009;15:1013–1018. doi: 10.1158/1078-0432.CCR-08-1888. [DOI] [PubMed] [Google Scholar]
- 71.de Kernion JB, Tynberg P, Persky L, et al. Proceedings: carcinoma of the penis. Cancer. 1973;32:1256–1262. doi: 10.1002/1097-0142(197311)32:5<1256::aid-cncr2820320534>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 72.Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354–1359. doi: 10.1016/j.juro.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 73.Sarin R, Norman AR, Steel GG, et al. Treatment results and prognostic factors in 101 men treated for squamous carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1997;38:713–722. doi: 10.1016/s0360-3016(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 74.Ficarra V, Akduman B, Bouchot O, et al. Prognostic factors in penile cancer. Urology. 2010;76:S66–S73. doi: 10.1016/j.urology.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Graafland NM, Lam W, Leijte JA, et al. Prognostic factors for occult inguinal lymph node involvement in penile carcinoma and assessment of the high-risk EAU subgroup: a two-institution analysis of 342 clinically node-negative patients. Eur Urol. 2010;58:742–747. doi: 10.1016/j.eururo.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 76.Svatek RS, Munsell M, Kincaid JM, et al. Association between lymph node density and disease specific survival in patients with penile cancer. J Urol. 2009;182:2721–2727. doi: 10.1016/j.juro.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 77.Lopes A, Hidalgo GS, Kowalski LP, et al. Prognostic factors in carcinoma of the penis: multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J Urol. 1996;156:1637–1642. doi: 10.1016/s0022-5347(01)65471-5. [DOI] [PubMed] [Google Scholar]
- 78.Guimaraes GC, Lopes A, Campos RS, et al. Front pattern of invasion in squamous cell carcinoma of the penis: new prognostic factor for predicting risk of lymph node metastases. Urology. 2006;68:148–153. doi: 10.1016/j.urology.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 79.Slaton JW, Morgenstern N, Levy DA, et al. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer: independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J Urol. 2001;165:1138–1142. [PubMed] [Google Scholar]
- 80.Thuret R, Sun M, Abdollah F, et al. Conditional survival predictions after surgery for patients with penile carcinoma. Cancer. 2011;117:3723–3730. doi: 10.1002/cncr.25974. [DOI] [PubMed] [Google Scholar]
- 81.Thuret R, Sun M, Abdollah F, et al. Tumor grade improves the prognostic ability of American Joint Committee on Cancer stage in patients with penile carcinoma. J Urol. 2011;185:501–507. doi: 10.1016/j.juro.2010.09.111. [DOI] [PubMed] [Google Scholar]
- 82.Ficarra V, Zattoni F, Cunico SC, et al. Lymphatic and vascular embolizations are independent predictive variables of inguinal lymph node involvement in patients with squamous cell carcinoma of the penis: Gruppo Uro-Oncologico del Nord Est (Northeast Uro-Oncological Group) Penile Cancer data base data. Cancer. 2005;103:2507–2516. doi: 10.1002/cncr.21076. [DOI] [PubMed] [Google Scholar]
- 83.Soria JC, Fizazi K, Piron D, et al. Squamous cell carcinoma of the penis: multivariate analysis of prognostic factors and natural history in monocentric study with a conservative policy. Ann Oncol. 1997;8:1089–1098. doi: 10.1023/a:1008248319036. [DOI] [PubMed] [Google Scholar]
- 84.Mannweiler S, Sygulla S, Tsybrovskyy O, et al. Clear-cell differentiation and lymphatic invasion, but not the revised TNM classification, predict lymph node metastases in pT1 penile cancer: a clinicopathologic study of 76 patients from a low incidence area. Urol Oncol. 2012 doi: 10.1016/j.urolonc.2012.01.017. Mar 13 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 85.Campos RS, Lopes A, Guimaraes GC, et al. E-cadherin, MMP-2, and MMP-9 as prognostic markers in penile cancer: analysis of 125 patients. Urology. 2006;67:797–802. doi: 10.1016/j.urology.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 86.Protzel C, Kakies C, Kleist B, et al. Down-regulation of the metastasis suppressor protein KAI1/CD82 correlates with occurrence of metastasis, prognosis and presence of HPV DNA in human penile squamous cell carcinoma. Virchows Arch. 2008;452:369–375. doi: 10.1007/s00428-008-0590-0. [DOI] [PubMed] [Google Scholar]
- 87.Protzel C, Knoedel J, Zimmermann U, et al. Expression of proliferation marker Ki67 correlates to occurrence of metastasis and prognosis, histological subtypes and HPV DNA detection in penile carcinomas. Histol Histopathol. 2007;22:1197–1204. doi: 10.14670/HH-22.1197. [DOI] [PubMed] [Google Scholar]
- 88.Protzel C, Richter M, Poetsch M, et al. The role of annexins I, II and IV in tumor development, progression and metastasis of human penile squamous cell carcinomas. World J Urol. 2011;29:393–398. doi: 10.1007/s00345-010-0575-x. [DOI] [PubMed] [Google Scholar]
- 89.Lont AP, Kroon BK, Horenblas S, et al. Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int J Cancer. 2006;119:1078–1081. doi: 10.1002/ijc.21961. [DOI] [PubMed] [Google Scholar]
- 90.Gunia S, Erbersdobler A, Hakenberg OW, et al. p16(INK4a) is a marker of good prognosis for primary invasive penile squamous cell carcinoma: a multi-institutional study. J Urol. 2012;187:899–907. doi: 10.1016/j.juro.2011.10.149. [DOI] [PubMed] [Google Scholar]
- 91.Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303–1307. doi: 10.1016/j.juro.2011.05.084. [DOI] [PubMed] [Google Scholar]
- 92.Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040–1043. doi: 10.1111/j.1464-410X.2005.05769.x. [DOI] [PubMed] [Google Scholar]
- 93.Marconnet L, Rigaud J, Bouchot O. Long-term followup of penile carcinoma with high risk for lymph node invasion treated with inguinal lymphadenectomy. J Urol. 2010;183:2227–2232. doi: 10.1016/j.juro.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 94.Protzel C, Alcaraz A, Horenblas S, et al. Lymphadenectomy in the surgical management of penile cancer. Eur Urol. 2009;55:1075–1088. doi: 10.1016/j.eururo.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 95.Heyns CF, Fleshner N, Sangar V, et al. Management of the lymph nodes in penile cancer. Urology. 2010;76:S43–57. doi: 10.1016/j.urology.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 96.Pompeo AC. Extended lymphadenectomy in penile cancer. Can J Urol. 2005;12(Suppl 1):30–36. discussion 97–98. [PubMed] [Google Scholar]
- 97.Kroon BK, Horenblas S, Lont AP, et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. 2005;173:816–819. doi: 10.1097/01.ju.0000154565.37397.4d. [DOI] [PubMed] [Google Scholar]
- 98.Lont AP, Kroon BK, Gallee MP, et al. Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J Urol. 2007;177:947–952. doi: 10.1016/j.juro.2006.10.060. ; discussion 952. [DOI] [PubMed] [Google Scholar]
- 99.Ornellas AA, Kinchin EW, Nobrega BL, et al. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. J Surg Oncol. 2008;97:487–495. doi: 10.1002/jso.20980. [DOI] [PubMed] [Google Scholar]
- 100.Spiess PE, Izawa JI, Bassett R, et al. Preoperative lymphoscintigraphy and dynamic sentinel node biopsy for staging penile cancer: results with pathological correlation. J Urol. 2007;177:2157–2161. doi: 10.1016/j.juro.2007.01.125. [DOI] [PubMed] [Google Scholar]
- 101.Izawa J, Kedar D, Wong F, et al. Sentinel lymph node biopsy in penile cancer: evolution and insights. Can J Urol. 2005;12(Suppl 1):24–29. [PubMed] [Google Scholar]
- 102.Hungerhuber E, Schlenker B, Frimberger D, et al. Lymphoscintigraphy in penile cancer: limited value of sentinel node biopsy in patients with clinically suspicious lymph nodes. World J Urol. 2006;24:319–324. doi: 10.1007/s00345-006-0073-3. [DOI] [PubMed] [Google Scholar]
- 103.Neto AS, Tobias-Machado M, Ficarra V, et al. Dynamic sentinel node biopsy for inguinal lymph node staging in patients with penile cancer: a systematic review and cumulative analysis of the literature. Ann Surg Oncol. 2011;18:2026–2034. doi: 10.1245/s10434-010-1546-6. [DOI] [PubMed] [Google Scholar]
- 104.Graafland NM, Valdes Olmos RA, Meinhardt W, et al. Nodal staging in penile carcinoma by dynamic sentinel node biopsy after previous therapeutic primary tumour resection. Eur Urol. 2010;58:748–751. doi: 10.1016/j.eururo.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 105.Pettaway CA, Pisters LL, Dinney CP, et al. Sentinel lymph node dissection for penile carcinoma: the M. D. Anderson Cancer Center experience. J Urol. 1995;154:1999–2003. [PubMed] [Google Scholar]
- 106.Tobias-Machado M, Tavares A, Ornellas AA, et al. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol. 2007;177:953–957. doi: 10.1016/j.juro.2006.10.075. discussion 958. [DOI] [PubMed] [Google Scholar]
- 107.Leijte JA, Kirrander P, Antonini N, et al. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. Eur Urol. 2008;54:161–168. doi: 10.1016/j.eururo.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 108.Franks KN, Kancherla K, Sethugavalar B, et al. Radiotherapy for node positive penile cancer: experience of the Leeds teaching hospitals. J Urol. 2011;186:524–529. doi: 10.1016/j.juro.2011.03.117. [DOI] [PubMed] [Google Scholar]
- 109.Chen MF, Chen WC, Wu CT, et al. Contemporary management of penile cancer including surgery and adjuvant radiotherapy: an experience in Taiwan. World J Urol. 2004;22:60–66. doi: 10.1007/s00345-003-0383-7. [DOI] [PubMed] [Google Scholar]
- 110.Mistry T, Jones RW, Dannatt E, et al. A 10-year retrospective audit of penile cancer management in the UK. BJU Int. 2007;100:1277–1281. doi: 10.1111/j.1464-410X.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 111.Gotsadze D, Matveev B, Zak B, et al. Is conservative organ-sparing treatment of penile carcinoma justified? Eur Urol. 2000;38:306–312. doi: 10.1159/000020298. [DOI] [PubMed] [Google Scholar]
- 112.Azrif M, Logue JP, Swindell R, et al. External-beam radiotherapy in T1-2 N0 penile carcinoma. Clin Oncol (R Coll Radiol) 2006;18:320–325. doi: 10.1016/j.clon.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 113.Zouhair A, Coucke PA, Jeanneret W, et al. Radiation therapy alone or combined surgery and radiation therapy in squamous-cell carcinoma of the penis? Eur J Cancer. 2001;37:198–203. doi: 10.1016/s0959-8049(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 114.Rozan R, Albuisson E, Giraud B, et al. Interstitial brachytherapy for penile carcinoma: a multicentric survey (259 patients) Radiother Oncol. 1995;36:83–93. doi: 10.1016/0167-8140(95)01574-z. [DOI] [PubMed] [Google Scholar]
- 115.de Crevoisier R, Slimane K, Sanfilippo N, et al. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N- or NX) Int J Radiat Oncol Biol Phys. 2009;74:1150–1156. doi: 10.1016/j.ijrobp.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 116.Crook J, Grimard L, Tsihlias J, et al. Interstitial brachytherapy for penile cancer: an alternative to amputation. J Urol. 2002;167:506–511. doi: 10.1016/S0022-5347(01)69074-8. [DOI] [PubMed] [Google Scholar]
- 117.Pedrick TJ, Wheeler W, Riemenschneider H. Combined modality therapy for locally advanced penile squamous cell carcinoma. Am J Clin Oncol. 1993;16:501–505. doi: 10.1097/00000421-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 118.Eliason M, Bowen G, Bowen A, et al. Primary treatment of verrucous carcinoma of the penis with fluorouracil, cis-diamino-dichloro-platinum, and radiation therapy. Arch Dermatol. 2009;145:950–952. doi: 10.1001/archdermatol.2009.160. [DOI] [PubMed] [Google Scholar]
- 119.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 120.van Doorn HC, Ansink A, Verhaar-Langereis M, et al. Neoadjuvant chemoradiation for advanced primary vulvar cancer. Cochrane Database Syst Rev. 2006;19:CD003752. doi: 10.1002/14651858.CD003752.pub2. [DOI] [PubMed] [Google Scholar]
- 121.Pizzocaro G, Piva L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 1988;27:823–824. doi: 10.3109/02841868809094366. [DOI] [PubMed] [Google Scholar]
- 122.Leijte JA, Kerst JM, Bais E, et al. Neoadjuvant chemotherapy in advanced penile carcinoma. Eur Urol. 2007;52:488–494. doi: 10.1016/j.eururo.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 123.Hakenberg OW, Nippgen JB, Froehner M, et al. Cisplatin, methotrexate and bleomycin for treating advanced penile carcinoma. BJU Int. 2006;98:1225–1227. doi: 10.1111/j.1464-410X.2006.06496.x. [DOI] [PubMed] [Google Scholar]
- 124.Bermejo C, Busby JE, Spiess PE, et al. Neoadjuvant chemotherapy followed by aggressive surgical consolidation for metastatic penile squamous cell carcinoma. J Urol. 2007;177:1335–1338. doi: 10.1016/j.juro.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 125.Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol. 2010;28:3851–3857. doi: 10.1200/JCO.2010.29.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Graafland NM, Valdes Olmos RA, Teertstra HJ, et al. 18F-FDG PET/CT for monitoring induction chemotherapy in patients with primary inoperable penile carcinoma: first clinical results. Eur J Nucl Med Mol Imaging. 2010;37:1474–1480. doi: 10.1007/s00259-010-1434-0. [DOI] [PubMed] [Google Scholar]
- 127.Maiche AG. Adjuvant treatment using bleomycin in squamous cell carcinoma of penis: study of 19 cases. Br J Urol. 1983;55:542–544. doi: 10.1111/j.1464-410x.1983.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 128.Protzel C, Ruppin S, Milerski S, et al. The current state of the art of chemotherapy of penile cancer: results of a nationwide survey of German clinics. Urologe A. 2009;48:1495–1498. doi: 10.1007/s00120-009-2108-z. [DOI] [PubMed] [Google Scholar]
- 129.Gagliano RG, Blumenstein BA, Crawford ED, et al. cis-Diamminedichloroplatinum in the treatment of advanced epidermoid carcinoma of the penis: a Southwest Oncology Group Study. J Urol. 1989;141:66–67. doi: 10.1016/s0022-5347(17)40590-8. [DOI] [PubMed] [Google Scholar]
- 130.Corral DA, Sella A, Pettaway CA, et al. Combination chemotherapy for metastatic or locally advanced genitourinary squamous cell carcinoma: a phase II study of methotrexate, cisplatin and bleomycin. J Urol. 1998;160:1770–1774. [PubMed] [Google Scholar]
- 131.Dexeus FH, Logothetis CJ, Sella A, et al. Combination chemotherapy with methotrexate, bleomycin and cisplatin for advanced squamous cell carcinoma of the male genital tract. J Urol. 1991;146:1284–1287. doi: 10.1016/s0022-5347(17)38069-2. [DOI] [PubMed] [Google Scholar]
- 132.Haas GP, Blumenstein BA, Gagliano RG, et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: a Southwest Oncology Group study. J Urol. 1999;161:1823–1825. [PubMed] [Google Scholar]
- 133.Pettaway CA, Pagliaro L, Theodore C, et al. Treatment of visceral, unresectable, or bulky/unresectable regional metastases of penile cancer. Urology. 2010;76:S58–S65. doi: 10.1016/j.urology.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 134.Pizzocaro G, Nicolai N, Milani A. Taxanes in combination with cisplatin and fluorouracil for advanced penile cancer: preliminary results. Eur Urol. 2009;55:546–551. doi: 10.1016/j.eururo.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 135.Trabulsi EJ, Hoffman-Censits J. Chemotherapy for penile and urethral carcinoma. Urol Clin North Am. 2010;37:467–474. doi: 10.1016/j.ucl.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 136.Hussein AM, Benedetto P, Sridhar KS. Chemotherapy with cisplatin and 5-fluorouracil for penile and urethral squamous cell carcinomas. Cancer. 1990;65:433–438. doi: 10.1002/1097-0142(19900201)65:3<433::aid-cncr2820650310>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 137.Shammas FV, Ous S, Fossa SD. Cisplatin and 5-fluorouracil in advanced cancer of the penis. J Urol. 1992;147:630–632. doi: 10.1016/s0022-5347(17)37327-5. [DOI] [PubMed] [Google Scholar]
- 138.Di Lorenzo G, Buonerba C, Federico P, et al. Cisplatin and 5-fluorouracil in inoperable, stage IV squamous cell carcinoma of the penis. BJU Int. 2012. September 10 [epub ahead of print] [DOI] [PubMed]
- 139.Necchi A, Nicolai N, Piva L, et al. A combination of cisplatin and 5-fluorouracil plus a taxane for advanced squamous-cell carcinoma (SCC) of the penis: a single institution series. Ann Oncol. 2010;21(Suppl 8):viii271–viii303. abstr 939P. [Google Scholar]
- 140.Theodore C, Skoneczna I, Bodrogi I, et al. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC PROTOCOL 30992) Ann Oncol. 2008;19:1304–1307. doi: 10.1093/annonc/mdn149. [DOI] [PubMed] [Google Scholar]
- 141.Power DG, Galvin DJ, Cuffe S, et al. Cisplatin and gemcitabine in the management of metastatic penile cancer. Urol Oncol. 2009;27:187–190. doi: 10.1016/j.urolonc.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 142.Di Lorenzo G, Federico P, Buonerba C, et al. Paclitaxel in pretreated metastatic penile cancer: final results of a phase 2 study. Eur Urol. 2011;60:1280–1284. doi: 10.1016/j.eururo.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 143.Carthon BC, Pettaway C, Pagliaro LC. Epidermal growth factor receptor (EGFR) targeted therapy in advanced metastatic squamous cell carcinoma (AMSCC) of the penis: updates and molecular analyses. J Clin Oncol. 2010;28(Suppl) abstr e15022. [Google Scholar]
- 144.Necchi A, Nicolai N, Colecchia M, et al. Proof of activity of anti-epidermal growth factor receptor-targeted therapy for relapsed squamous cell carcinoma of the penis. J Clin Oncol. 2011;29:e650–e652. doi: 10.1200/JCO.2011.34.8367. [DOI] [PubMed] [Google Scholar]
- 145.Rescigno P, Matano E, Raimondo L, et al. Combination of docetaxel and cetuximab for penile cancer: a case report and literature review. Anticancer Drugs. 2012;23:573–577. doi: 10.1097/CAD.0b013e328350ead7. [DOI] [PubMed] [Google Scholar]
- 146.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30:1107–1113. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 148.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 149.Lucky MA, Rogers B, Parr NJ. Referrals into a dedicated British penile cancer centre and sources of possible delay. Sex Transm Infect. 2009;85:527–530. doi: 10.1136/sti.2009.036061. [DOI] [PubMed] [Google Scholar]
- 150.Ivy SP, Siu LL, Garrett-Mayer E, et al. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin Cancer Res. 2010;16:1726–1736. doi: 10.1158/1078-0432.CCR-09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]