Abstract
Background. Intravenous zanamivir is a neuraminidase inhibitor suitable for treatment of hospitalized patients with severe influenza.
Methods. Patients were treated with intravenous zanamivir 600 mg twice daily, adjusted for renal impairment, for up to 10 days. Primary outcomes included adverse events (AEs), and clinical/laboratory parameters. Pharmacokinetics, viral load, and disease course were also assessed.
Results. One hundred thirty patients received intravenous zanamivir (median, 5 days; range, 1–11) a median of 4.5 days (range, 1–7) after onset of influenza; 83% required intensive care. The most common influenza type/subtype was A/H1N1pdm09 (71%). AEs and serious AEs were reported in 85% and 34% of patients, respectively; serious AEs included bacterial pulmonary infections (8%), respiratory failure (7%), sepsis or septic shock (5%), and cardiogenic shock (5%). No drug-related trends in safety parameters were identified. Protocol-defined liver events were observed in 13% of patients. The 14- and 28-day all-cause mortality rates were 13% and 17%. No fatalities were considered zanamivir related. Pharmacokinetic data showed dose adjustments for renal impairment yielded similar zanamivir exposures. Ninety-three patients, positive at baseline for influenza by quantitative polymerase chain reaction, showed a median decrease in viral load of 1.42 log10 copies/mL after 2 days of treatment.
Conclusions. Safety, pharmacokinetic and clinical outcome data support further investigation of intravenous zanamivir.
Clinical Trials Registration NCT01014988.
Keywords: intravenous zanamivir, Influenza, hospitalized, safety, zanamivir, pandemic influenza, A/H1N1pdm09
Pandemic influenza and seasonal influenza epidemics are significant public health threats [1] that cause substantial worldwide morbidity and mortality [2]. The neuraminidase inhibitors oseltamivir and zanamivir are the only widely used antivirals active against currently circulating influenza A and B viruses. Peramivir, laninamivir, and other nonneuraminidase inhibitors are available in some countries. Neuraminidase inhibitors act by inhibiting viral cleavage of sialic acid residues from surface glycoconjugates of infected cells, preventing virus release and spread in the respiratory tract [3]. Oseltamivir (capsule and suspension) and zanamivir (oral inhalation powder) are approved for treatment and prophylaxis of acute uncomplicated influenza [4, 5]. However, no antiviral treatments have been proved to be effective for complicated or severe influenza in controlled trials, and no parenteral influenza antiviral formulations are licensed outside China, Japan, and Korea.
Enzyme kinetics and crystallography studies show that zanamivir binding to the neuraminidase active site results in a low propensity for resistance [6]. The H275Y neuraminidase mutation is the most commonly reported influenza virus mutation, conferring high-level resistance to oseltamivir among N1-containing viruses, but retaining full susceptibility to zanamivir [7]. In 2008, this mutation emerged in the seasonal H1N1 strain and spread in less than a year. Subsequently, oseltamivir resistance was estimated at 1% during the 2009 A/H1N1 pandemic, 2.1% in the United States during the 2011–2012 season [8], and 15% in a community outbreak in Australia [9].
Treatment options for influenza remain limited, and further development of resistance continues to be a serious public health threat. The 2009 pandemic disproportionately affected children, younger adults, and pregnant women, and resulted in high rates of acute respiratory distress syndrome, multiorgan failure, and death among hospitalized populations. A medical need exists for clinically proven safe and efficacious treatments with diverse resistance profiles for severe influenza. Reliable systemic intravenous administration may best suit hospitalized patients [10, 11].
Here we report findings from the adult cohort of a study evaluating the safety and tolerability of an investigational intravenous formulation of zanamivir in hospitalized patients with influenza. Assessments of pharmacokinetics, viral load, and course of illness were also performed. The study is ongoing in adolescent and pediatric patients.
METHODS
Study Design
This open-label, multicenter, single-arm, phase II study (Clinical Trials registration NCT01014988; GSK NAI113678) was conducted in 8 countries (Australia, Canada, France, Russia, Spain, Thailand, United Kingdom, and United States). The study was performed in accordance with ICH GCP and the principles of the Declaration of Helsinki, and approved by local ethics committees. Informed consent was obtained from the patient or legal guardian before the study. Enrollment criteria included age ≥18 years, hospitalization with severe or progressive laboratory-confirmed influenza [12, 13] while receiving approved influenza antiviral medications or not being suitable for treatment with approved antivirals (eg, unable to receive oral or inhaled medication), and ability to receive the first dose of intravenous zanamivir within 7 days after onset of influenza symptoms. Patients were excluded if they required concurrent therapy with another influenza antiviral medication or had elevated alanine aminotransferase (ALT) ≥3 times the upper limit of normal (ULN) and total bilirubin ≥2 × ULN, ALT >5 × ULN, or unstable cardiac disease or arrhythmia at baseline (detailed criteria available at www.clinicaltrials.gov).
Treatment
Intravenous zanamivir (600 mg) was administered over a 30-minute period twice daily for 5 days, with the option to extend treatment for up to 5 more days at the investigator's discretion. Intravenous zanamivir dosing was adjusted for patients with renal impairment based on daily calculated creatinine clearance (CLcr) or on estimated clearance while receiving continuous renal replacement therapy (CLCRRT) [14, 15] after an initial 600-mg loading dose. The maintenance dose was 600 mg for subjects with a CLcr/CLCRRT ≥80 mL/min, reduced as follows for those with lower CLcr/CLCRRT values: 400 mg for 50 to <80 mL/min, 250 mg for 30 to <50 mL/min, 150 mg for 15 to <30 mL/min, and 60 mg for <15 mL/min. The interval between the initial dose and the start of maintenance dosing was 24 hours for patients with a CLcr/CLCRRT of 15 to <30 mL/min and 48 hours for those with a CLcr/CLCRRT of <15 mL/min. For all other patients, maintenance doses were administered every 12 hours (Supplementary Table 1). The dose rationale was based on the prophylactic efficacy of intravenous zanamivir [16] concentrations in lung epithelial lining fluid (range, 216–1163 ng/mL) were many times greater than the median inhibitory concentration for a range of influenza A and B neuraminidases after intravenous administration of zanamivir (600 mg); these concentrations were 55%–79% of the corresponding serum zanamivir concentrations [17].
Outcome Measures
Primary safety outcomes included adverse events (AEs) classified according to the DAIDS toxicity scale [18], serious AEs (SAEs), incidents of hepatic injury (liver AEs defined as ALT ≥5 × ULN; liver SAEs defined as ALT ≥3 × ULN and total bilirubin ≥2 × ULN; laboratory criteria of Hy's law [19]), clinical laboratory measurements, electrocardiographic data, and vital signs.
Secondary outcome measures included serum pharmacokinetic parameters and change in influenza viral load over time (by quantitative real-time polymerase chain reaction [qRT-PCR] and quantitative virus culture [qVC]; Quest Diagnostics). Clinical end points included mortality rate, length of hospitalization (as measured from study day 1), intensive care unit (ICU) stay (total length of stay), and time until return to normal vital signs, according to defined criteria. Exploratory outcomes included influenza viral load quantification in samples obtained from endotracheal aspirates and correlation analyses between pharmacokinetic parameters, viral load, and clinical outcomes.
Study Procedures
Safety and clinical outcomes were assessed daily during treatment, then after treatment on days 2, 5, 9, 16, and 23 after the last dose of intravenous zanamivir. Poststudy deaths attributable to AEs that began during the study (or occurring during the period of hospitalization) were also recorded, even if death occurred after the end-of-study assessment.
Serum pharmacokinetic sampling was optional. Pharmacokinetic samples for the initial dose were scheduled for collection before and at 25–30 minutes (end of infusion) and 1–2, 4–6, and 11–12 hours after the start of infusion. If the start of maintenance dosing was delayed for renal impairment, then additional samples were scheduled for 22–24 and 46–48 hours after the dose. For the maintenance dose on day 3, 4 or 5, sample collections were as for day 1, up to 12 hours after the dose.
Serum zanamivir concentrations were measured using a validated assay based on protein precipitation, followed by high-performance liquid chromatography–tandem mass spectrometry analysis. For a 50-µL aliquot of serum, the lower limit of quantification (LLQ) was 10 ng/mL, and the upper limit 10 000 ng/mL. Pharmacokinetic parameters were estimated from concentration-time data by standard noncompartmental analysis using WinNonlin software, professional version 5.2 (Pharsight).
Nasopharyngeal swab samples (Copan Diagnostics) to assess viral load and influenza subtype were collected on days 1–5 of treatment. Additional samples were taken on days 7 and 10 if dosing was continued beyond day 5, and on posttreatment assessment days if patients continued to be hospitalized and symptomatic. Optional endotracheal samples were collected at a single time point between days 3 and 5. RNA was isolated from nasopharyngeal and endotracheal samples, and 1-step qRT-PCR was used to quantify levels of influenza RNA. The LLQ for the assay was set at 2.7 log10 (500) copies/mL. Viral titers were also deduced by median tissue culture infectious dose (TCID50) calculation after serial dilution of samples, followed by adsorption onto Madin-Darby canine kidney cells. The number of plaque-forming units was used to calculate the TCID50, with an LLQ of 0.4 log10 TCID50/mL.
Statistical Analysis
The planned sample size for the study was chosen to provide enough patients to determine the safety and tolerability of intravenous zanamivir in the patient population. With 130 enrolled patients, we can exclude AEs with a frequency >2.8% with 95% confidence. Exploratory analyses of Cox regression and Pearson correlation were performed to investigate associations between various outcomes, such as mortality, clinical and virologic responses, hospital or ICU stay, clinical risk factors, and pharmacokinetic parameters. Wilcoxon signed rank test was performed to compare viral loads between endotracheal and nasopharyngeal samples.
RESULTS
Study Population
Between November 2009 and September 2011, 130 adult patients were enrolled from 30 centers. Three patients were pregnant (1 second trimester, 2 third trimester), and 1 patient was 1 day post partum. Patients were treated for a median of 5 days (range, 1–11 days): 87 patients (67%) received intravenous zanamivir for ≤5 days, and 43 (33%) received intravenous zanamivir for >5 days. Thirty patients (23%) were prematurely withdrawn from intravenous zanamivir treatment, 12 (9%) owing to (on-treatment) fatality, 11 (8%) at the discretion of the investigator (most because of clinical improvement and hospital discharge), 4 (3%) owing to AEs (cytolytic hepatitis, hepatic enzyme elevation, renal failure, and rash), 2 (2%) owing to withdrawal of consent, and 1 owing to protocol deviation. Twenty-three patients (18%) did not complete the study; 20 (15%) owing to death, and 1 each owing to withdrawal of consent, investigator discretion, or loss to follow-up.
Baseline patient characteristics and demographics are summarized in Table 1. The most common influenza symptoms at baseline were fever (82%), cough (81%), and dyspnea (72%); 77% of patients had ≥1 chronic underlying medical condition (Table 1). Ten patients (8%) had documentation of influenza vaccination in the 9 months before presentation. Chest radiographic evidence of pneumonia or pneumonitis was present at the baseline in 112 of 126 patients (89%). The median time from symptom onset to initiation of intravenous zanamivir was 4.5 days (range, 1–7 days), and 104 patients (80%) received oseltamivir before study entry (median exposure, 2 days). The most common influenza type/subtype was A/H1N1pdm09 (71%), followed by A/H3N2 (12%), influenza A subtype unknown (11%), and influenza B (2%). Four percent of isolates could not be typed.
Table 1.
Baseline Characteristics, Demographics, and Chronic Underlying Illnesses in 130 Patients
| Characteristic | Baseline Value |
|---|---|
| Age, median (range), y | 47.5 (18–94) |
| Male sex, No. (%) | 74 (57) |
| Race, No. (%) | |
| African American/African | 10 (8) |
| East Asian/Southeast Asian | 14 (11) |
| White/European | 97 (75) |
| Other | 9 (7) |
| Body mass index, median (range), kg/m2 | 25.5 (12–55) |
| Ventilation status at enrollment, No. (%) | |
| Extracorporeal membrane oxygenation | 3 (2) |
| Endotracheal mechanical ventilation | 60 (46) |
| Renal replacement therapy at enrollment | 6 (5) |
| Chronic underlying illnesses, summarized by organ system, No. (%) | |
| Any illness | 100 (77) |
| Respiratory | 42 (32) |
| Rheumatology and immunology | 32 (25) |
| Gastrointestinal | 28 (22) |
| Cardiovascular | 26 (20) |
| Endocrine | 24 (18) |
| Renal | 15 (12) |
| Neurology | 13 (10) |
| Oncology | 13 (10) |
Safety End Points
Overall AEs, SAEs, and grade 3 or 4 AEs were reported in 110 (85%), 44 (34%), and 57 (44%) patients, respectively. Summaries of SAEs and grade 3/4 AEs are presented in Table 2 and Table 3. A summary of all AEs is presented in Supplementary Table 2.
Table 2.
All Serious Adverse Events Reported by >1 Patienta
| Serious Adverse Event | Patients, No. (%) (N = 130) |
|---|---|
| All events | 44 (34) |
| Bacterial pulmonary infections (including pneumonia and bronchopneumonia) | 10 (8) |
| Respiratory failure | 9 (7) |
| Sepsis or septic shock | 7 (5) |
| Cardiogenic shock | 7 (5) |
| Acute kidney injury | 4 (3) |
| Bronchopulmonary aspergillosis | 3 (2) |
| Acute respiratory distress syndrome | 3 (2) |
| Pulmonary embolism | 3 (2) |
| Acute liver injuryb | 3 (2) |
| Multiorgan failure | 3 (2) |
| Encephalopathy | 2 (2) |
| Ventricular arrhythmia | 2 (2) |
| Hypoxia | 2 (2) |
| Bacteremiac | 2 (2) |
a Events were graded according to DAIDS toxicity criteria; all events recorded occurred after initiation of intravenous zanamivir treatment. Similar reported events were grouped together by organ system and mechanism. The following serious adverse events were reported by 1 patient: rash, thrombophlebitis or venous thrombosis, endocarditis, viral pericarditis, chronic obstructive pulmonary disease, hemothorax, pneumothorax, hemoptysis or pulmonary hemorrhage, atrioventricular block complete, hemorrhage, peripheral ischemia, shock hemorrhagic, ischemic stroke, hyponatremia, acute leukemia, and depression.
b Acute liver injury comprised all events of cytolytic hepatitis and increased alanine aminotransferase, hepatic enzyme, and transaminase levels.
c Including 1 case of Acinetobacter bacteremia; the organism in the second bacteremia case was not documented.
Table 3.
All Grade 3 or 4 Adverse Events Reported by >1 Patienta
| Adverse Event | Patients, No. (%) (N = 130) |
|---|---|
| Grade 3 or 4 adverse event | 57 (44) |
| Bacterial pulmonary infections (including pneumonia and bronchopneumonia) | 13 (10) |
| Acute liver injuryb | 11 (8) |
| Respiratory failure | 8 (6) |
| Hypotension | 8 (6) |
| Cardiogenic shock | 6 (5) |
| Sepsis or septic shock | 6 (5) |
| Neuropathy or neuromuscular disorder | 6 (5) |
| Acute kidney injury | 5 (4) |
| Acute respiratory distress syndrome | 4 (3) |
| Hypertension | 4 (3) |
| Anemia | 3 (2) |
| Thrombophlebitis or venous thrombosis | 3 (2) |
| Bronchopulmonary aspergillosis | 3 (2) |
| Pleural effusion | 3 (2) |
| Pulmonary embolism | 3 (2) |
| Multiorgan failure | 3 (2) |
| Atrial fibrillation | 3 (2) |
| Dyspnea | 2 (2) |
| Pneumothorax | 2 (2) |
| Encephalopathy | 2 (2) |
| Hypoalbuminemia | 2 (2) |
| Hypocalcemia | 2 (2) |
| Hypophosphatemia | 2 (2) |
| Pyrexia | 2 (2) |
| Rash | 2 (2) |
| Ventricular arrhythmia | 2 (2) |
| Hypoxia | 2 (2) |
| Bacteremiac | 2 (2) |
a Graded according to DAIDS toxicity criteria. All events recorded occurred after initiation of intravenous zanamivir treatment. Similar reported events were grouped together by organ system and mechanism. The following grade 3 or 4 adverse events were each reported by 1 patient: hemolytic anemia, bronchospasm, chronic obstructive pulmonary disease, hemothorax, lung disorder, hemoptysis or pulmonary hemorrhage, pulmonary edema, Clostridium difficile colitis, sinusitis, endocardisis, viral pericarditis, hemorrhage, peripheral ischemia, shock hemorrhagic, arrhythmia, cardiac failure congestive, left ventricular dysfunction, increased levels of aspartate aminotransferase, blood creatine phosphokinase, or blood creatine, electrocardiographic QT prolongation, ischemic stroke, agranulocytosis, lymphopenia, thrombocytopenia, hypokalemia, hyponatremia, hyperbilirubinemia, rhabdomyolysis, anxiety, depression, adrenal insufficiency, erosive gastritis, acute leukemia, and coagulopathy.
b Acute liver injury comprised all events of cytolytic hepatitis and increased levels of alanine aminotransferase, hepatic enzyme, and transaminases.
c Including 1 case of Acinetobacter bacteremia; the organism in the second bacteremia case was not documented.
In total, 28 patients (22%) reported AEs considered by the investigator to have a possible causal relationship to zanamivir, the most common were acute liver injury in 13 patients (10%), rash in 4 (3%), and thrombophlebitis or venous thrombosis in 4 (3%). SAEs considered by the investigator to be possibly zanamivir related included 2 cases of ventricular arrhythmia, 2 of acute liver injury meeting laboratory criteria of Hy's law, 2 of encephalopathy, and 1 of renal failure. One event of ventricular arrhythmia (torsade de pointes) reported as possibly related to intravenous zanamivir occurred 16 days after completion of zanamivir treatment, and was confounded by haloperidol treatment.
Seventeen patients (13%) experienced protocol-defined liver AEs (n = 14; 11%) or SAEs (n = 3; 2%). The median time from initiation of intravenous zanamivir to onset of liver AEs was 9 days (range, 1–27 days), and the median time from the last dose of intravenous zanamivir to the onset of liver AEs was 1 day (range, 1–22 days). Except for 1 patient who died of an unrelated cause on day 3, all liver SAEs and AEs resolved or improved by the end of follow-up (about 3 weeks after the last dose of intravenous zanamivir). Eleven AEs and 2 SAEs were considered by the investigator to be potentially attributable to intravenous zanamivir. Of the 3 patients with liver SAEs (ALT ≥3 × ULN and total bilirubin ≥2 × ULN), 1 experienced an SAE that resolved by the end of the study, 1 died of cardiogenic shock (unrelated to intravenous zanamivir), and 1 (with confounding hepatitis C and a liver event not attributable to study drug) died of hemothorax and multiorgan failure. Most protocol-defined liver events were associated with A/H1N1pdm09 infection and multiorgan failure. In the overall study population, there were no changes in median ALT, aspartate aminotransferase, or total bilirubin levels during or after treatment.
Twenty-six patients died, for an overall cumulative mortality (including poststudy deaths) of 20%; 14- and 28-day cumulative mortality were 13% (n = 17) and 17% (n = 22), respectively. The most common causes of death were respiratory failure (n = 7; 5%), sepsis or septic shock (n = 5; 4%), cardiogenic shock (n = 4; 3%), and bacterial pulmonary infections, including pneumonia and bronchopneumonia (n = 4; 3%). None of the deaths was considered by the investigator to be attributable to zanamivir treatment. No other safety signals or clinically significant trends in laboratory values, vital signs or electrocardiographic findings were identified or considered attributable to zanamivir. All 3 pregnant patients survived and gave birth to healthy infants. No SAEs were reported in the pregnant or postpartum patients.
Serum Pharmacokinetics
Serum samples were obtained in 126 (97%) patients for pharmacokinetic analysis. Results are provided in Table 4 and Table 5. For the initial 600-mg dose on day 1 (Table 4), area under the serum concentration–time curve extrapolated to infinity (AUC(0-∞)) values typically increased with decreasing CLcr, from 82.9 h · µg/mL for patients with CLcr ≥80 mL/min to 950 h · µg/mL for patients with CLcr <15 mL/min No differences were observed in maximum plasma concentration (Cmax) between the renal function groups (range of group means, 32.8–47.1 µg/mL). During maintenance dosing, as expected, the maximum plasma concentration decreased and the trough plasma concentration (Cmin) increased with reduced doses for renal impairment, but similar AUCs were observed (Table 5).
Table 4.
Zanamivir Pharmacokinetic Parameter Estimates by Renal Function Group for Initial 600-mg Dose on Day 1a
| CLcr, mL/minb | Geometric Mean (%CV) [No. of Patients] |
||||
|---|---|---|---|---|---|
| Cmax, μg/mL | AUC(0-∞) , h · µg/mL | t1/2, h | CL, mL/min | Vss. L | |
| ≥80 | 32.8 (34) [67] | 82.9 (36) [63] | 2.39 (31) [67] | 121 (36) [63] | 22.0 (30) [63] |
| 50 to <80 | 34.2 (19) [15] | 120 (38) [15] | 3.47 (73) [18] | 83.4 (38) [15] | 21.3 (20) [15] |
| 30 to <50 | 37.7 (34) [13] | 244 (27) [12] | 6.11 (30) [13] | 40.9 (27) [12] | 20.7 (41) [12] |
| 15 to <30 | 36.9 (23) [5] | 729 (77) [5] | 19.0 (72) [5] | 13.7 (77) [5] | 22.6 (23) [5] |
| <15 | 47.1 (…) [2] | 950 (…) [2] | 18.4 (…) [2] | 10.5 (…) [2] | 16.3 (…) [2] |
Abbreviations: %CV, percent coefficient of variation; AUC(0-∞), area under the serum concentration-time curve extrapolated to infinity; CL, systemic clearance of zanamivir; CLcr, creatinine clearance; Cmax, peak concentration at end of infusion; t½, elimination half-life; Vss, steady-state volume of distribution.
a Because of missed samples, not all pharmacokinetic parameters could be estimated for all patients.
b Denotes renal function as either CLcr or clearance estimated for continuous renal replacement therapy modality (CLCRRT), n = 4.
Table 5.
Zanamivir Pharmacokinetic Parameter Estimates by Renal Function Group for Maintenance Dose on Day 3, 4, or 5a
| CLcr, mL/minb | Geometric Mean (%CV) [No. of Patients] |
|||||
|---|---|---|---|---|---|---|
| Cmax, μg/mL | Cmin, μg/mL | AUC(0-τ), h · µg/mL | t1/2, h | CL, mL/min | Vss, L | |
| ≥80 | 35.3 (32) [72] | 0.82 (135) [76] | 90.3 (36) [65] | 2.56 (34) [68] | 111 (36) [65] | 21.6 (33) [65] |
| 50 to <80 | 29.3 (27) [7] | 2.19 (158) [7] | 90.7 (29) [6] | 3.69 (49) [6] | 73.5 (29) [6] | 21.4 (36) [6] |
| 30 to <50 | 20.9 (30) [6] | 6.10 (45) [7] | 136 (24) [5] | 8.50 (51) [6] | 30.7 (24) [5] | 22.3 (48) [5] |
| 15 to <30 | 23.6 (16) [5] | 16.2 (27) [5] | 217 (29) [4] | 36.0 (68) [4] | 11.5 (29) [4] | 35.6 (40) [4] |
| <15 | 7.45 (…) [2] | 5.30 (…) [2] | 80.1 (…) [2] | 61.1 (…) [2] | 12.5 (…) [2] | 66.5 (…) [2] |
Abbreviations: %CV, percent coefficient of variation; AUC(0-τ), area under the concentration-time curve during a 12-hour maintenance dosing interval; CL, systemic clearance of zanamivir; CLcr, creatinine clearance; Cmax, peak concentration at end of infusion; Cmin, trough concentration during 12-hour maintenance dosing interval; t½, elimination half-life; Vss, steady-state volume of distribution.
a Because of missed samples, not all pharmacokinetic parameters could be estimated for all patients.
b Denotes renal function as either CLcr or clearance estimated for continuous renal replacement therapy modality (CLCRRT, n = 4).
Virology
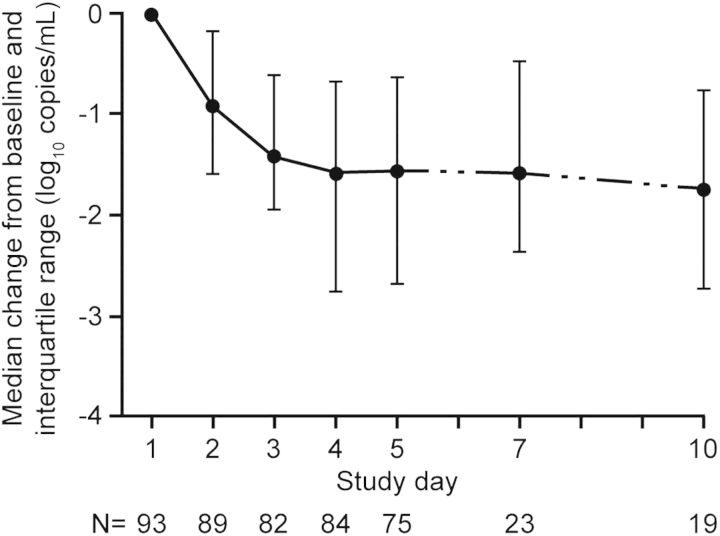
In 93 of 124 patients (75%) with influenza qRT-PCR–positive nasopharyngeal samples at baseline, the median viral load was 5.34 log10 copies/mL, which decreased at day 3 (2 days of treatment) by 1.42 log10 copies/mL (Figure 1). The median time to no detectable virus RNA by qRT-PCR was 3 days (range, 1–31; interquartile range, 1–5). Only 54 of 126 patients (43%) had positive qVC results from nasopharyngeal samples at baseline; thus, the median qVC result was below the LLQ. We found that qVC results in this study were less reliable than qRT-PCR results and were not used for analyses. We identified no baseline or emergent H275Y, I223R/V/K, or Q136K mutations. Detailed genotypic, phenotypic, and minority species analyses will be reported in a separate publication.
Figure 1.
Median change from baseline influenza A or B viral load by quantitative real-time polymerase chain reaction in patients with positive baseline results; interquartile ranges are also shown.
Twenty-two of 23 patients had an endotracheal sample positive for influenza by qRT-PCR (influenza A/H1N1pdm09 in 19, A/no subtype in 2, and A/H3N2 in 1). The median viral load was 4.68 log10 copies/mL. A total of 21 patients had paired qRT-PCR data in both endotracheal and nasopharyngeal samples, with median viral loads of 4.89 and 3.60 log10 copies/mL, respectively (P = .004); of these, 17 (81%) had higher viral loads in endotracheal samples (Supplementary Figure 1; Supplementary Appendix).
Clinical End Points
The median duration of hospitalization was 15 days (range 1–133 days). One hundred eight patients (83%) had an ICU stay during the study; the median duration was 11.5 days (range, 1–104 days). The median time to return to predefined normal criteria for each vital sign was between 2 and 8 days, but data were highly variable (Table 6).
Table 6.
Time Until Return to Normal Vital Signs After Initiation of Intravenous Zanamivir Treatment
| Sign | Definition of Normal Criteria | Patients Who Reached Normal Criteria, No. (N = 130)a | Time Until Return to Normal Criteria, Median (Range), d |
|---|---|---|---|
| Afebrile status | ≤37.8°C | 120 | 3 (2–34) |
| Oxygen saturation | ≥95% | 87 | 8 (2–36) |
| Respiratory rate | Respiration rate ≤24/min or normal respiratory statusb | 89 | 8 (2–36) |
| Pulse rate | ≤100/min | 121 | 2 (2–22) |
| Systolic blood pressure | ≥90 mm/Hg | 128 | 2 (2–3) |
a Data were censored for patients who never reached normal criteria.
b Normal respiratory status was defined as (1) a return to premorbid oxygen requirement (2) a return to no need for supplemental oxygen, or (3) respiration rate ≤24/min without supplemental oxygen.
Sixty-four patients (49%) received systemic corticosteroids while receiving treatment with intravenous zanamivir. The median time to a qRT-PCR result <500 copies/mL was 3 days among patients who received corticosteroids (range, 1–31 days), compared with 2 days among those who did not (range, 1–27 days). The cumulative mortality rate was 22% (14 of 64 patients) among patients who received corticosteroids compared with 18% (12 of 66) among those who did not. The overall rate of infection-related SAEs was lower in the corticosteroid group (11% vs 18%), but 3 fatal cases of pulmonary aspergillosis occurred, all among patients who received corticosteroids.
Exploratory Analyses
The effect of antiviral treatment on mortality rates was explored using Cox modeling. Time-dependent exposure to intravenous zanamivir was associated with an adjusted hazard ratio (aHR) of 0.793 (95% confidence interval [CI], .230–2.736), accounting for prior or subsequent oseltamivir exposure (aHR, 0.731; 95% CI, .092–5.827) and other potential risk factors for increased mortality rates (Table 7). The univariate analysis showed that both H3N2 subtype and age were significantly associated with mortality rates, but corticosteroid use was not (hazard ratio, 1.17; 95% CI, .54–2.53). There was no correlation between higher zanamivir exposure and drug-related AEs or protocol-defined liver events. A weak but significant correlation (Pearson R = 0.28; P = .003) was noted between AUC(0-∞) and the occurrence of SAEs, but this was confounded by the presence of renal dysfunction at baseline. We found a weak correlation between AUC(0-∞) and decrease of nasopharyngeal qRT-PCR on day 3 of treatment (Pearson R = –0.23; P = .02).
Table 7.
Covariate Effects on Mortality Rates by Cox Modelinga
| Covariates | Univariate Model |
Multivariate model |
||
|---|---|---|---|---|
| HR | 95% CI | Adjusted HR | 95% CI | |
| Zanamivir on vs off | 0.651 | .202–2.102 | 0.793 | .230–2.736 |
| Oseltamivir on vs off | 0.754 | .096–5.933 | 0.731 | .092–5.827 |
| Female vs male sex | 0.942 | .428–2.073 | 1.153 | .513–2.590 |
| H3N2 vs other | 4.202 | 1.793–9.848 | 2.460 | .853–7.098 |
| Baseline mechanical ventilation vs none | 0.882 | .406–1.918 | 0.951 | .417–2.171 |
| Immunomodulator vs noneb | 0.566 | .076–4.194 | 0.488 | .063–3.792 |
| Age in years | 1.039 | 1.012–1.067 | 1.017 | .982–1.052 |
| Body mass index, kg/m2 | 0.962 | .909–1.018 | 0.985 | .928–1.046 |
| Baseline creatinine clearance, mL/min | 0.991 | .984–.998 | 0.995 | .986–1.004 |
| Baseline viral load, log10 copies/mL | 1.096 | .878–1.369 | 0.949 | .741–1.216 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Survival time starts from symptom onset date. Zanamivir and oseltamivir were modeled as daily time-dependent covariates.
b Immunomodulators (used during treatment with intravenous zanamivir) included cyclosporin, tacrolimus, azathioprine, capecitabine, cisplatin hydroxycarbamide, mycophenolate mofetil, mycophenolate sodium, mycophenolic acid, nilotinib, and sirolimus.
We explored whether there was a relationship between baseline levels of influenza RNA by qRT-PCR and mortality rate by grouping patients with positive baseline qRT-PCR results (93 patients) in qRT-PCR tertiles and comparing them with those who had negative qRT-PCR resutls at baseline (31 patients; 6 had missing values at baseline). Patients in the 2 highest tertiles of influenza qRT-PCR had cumulative mortality rates of 23% and 22%, respectively; patients in the lower tertile (<4.57 log10 copies/mL) had a cumulative mortality rate of 17%, and this rate among patients with negative baseline qRT-PCR results was 13%. We also explored whether the change in nasopharyngeal influenza viral load from baseline to day 3 (n = 82 with paired samples) was associated with mortality rates. We found no association between change in viral load from baseline and mortality rate by Cox modeling (P = .92); deaths were evenly distributed between those who experienced changes from baseline greater than −1.42 log10 by treatment day 3 (8 subjects) and those who experienced smaller changes (7 subjects).
DISCUSSION
In this open-label, international, phase 2 trial we prospectively assessed the safety, tolerability, virologic effects, and pharmacokinetics of intravenous zanamivir (600 mg every 12 hours, adjusted for renal dysfunction) in 130 hospitalized adults with confirmed influenza, most of whom had associated pneumonia and were treated in an ICU. The all-cause cumulative mortality rate in this trial was 20%, and the 28-day mortality rate was 17%. Both intravenous zanamivir and oseltamivir use were associated with lower aHR for death when modeled as time-dependent exposures. The observed mortality rate was lower but consistent with retrospective cohort studies of critically ill hospitalized patients with influenza during this time period [20–25]. For example, in critically ill patients with 2009 influenza A/H1N1pdm09, a 50% ICU mortality rate has been reported [25], and a California-cohort study in 1950 patients reported a 25% mortality rate with neuraminidase treatment (median time from symptom onset, 4 days) and a 42% mortality rate without treatment [26]. Of the 26 deaths in this study, none was thought by the treating physician to be attributable to zanamivir. The small number of pregnant women did well clinically, with no observed adverse fetal effects.
Influenza viral load analyses suggested that intravenous zanamivir had a rapid antiviral effect, with a median decrease in viral load of 1.42 log10 copies/mL after 2 days of treatment, despite the presence of symptoms for a median of 4.5 days before study entry and prior use of oseltamivir in 80% of patients. This viral load reduction is consistent with findings of previous studies of inhaled zanamivir in acute uncomplicated seasonal influenza and limited data from hospitalized patients during the pandemic [27, 28]. In a Hong Kong study of 66 adults with influenza A/H1N1pdm09, patients with severe pneumonia experienced a longer duration of viral RNA positivity (by nasopharyngeal swab sample) after starting oseltamivir treatment (median, 6 days; range, 3–8) than those with milder illness (median, 2 days; range, 1–3) [29]. In our study, higher virus loads were observed in endotracheal samples than in simultaneous nasopharyngeal samples, reflecting the lower respiratory tract burden of influenza illness in this patient population [30]. In this study, we found a poor correlaton between the qVC and qRT-PCR results. The reason is not clear, but the qRT-PCR data were considered more reliable and have been presented to describe antiviral efficacy.
In most cases, the causal connection between AEs and zanamivir was confounded by influenza severity, underlying medical conditions, and numerous concomitant medications, including antibiotics in 92% (data not shown). Protocol-defined liver events were observed in 13% of the study population; in all patients except 1 who died imminently of cardiogenic shock, liver enzyme elevations resolved with resolution of influenza or critical illness. Intravenous zanamivir was not noted to increase liver chemistry values in phase I healthy volunteer studies or preclinical animal safety studies [31, 32]. In our study, liver events did not correlate with higher zanamivir exposure, and there were no overall increases or discernible pattern in liver enzyme results, with many patients experiencing liver enzyme elevations at baseline, during treatment, or after treatment. Liver enzyme elevation has also been described in critically ill patients with influenza A/H1N1pdm09 [33]. It is unclear whether intravenous zanamivir had a role in causing or exacerbating underlying liver inflammation. To address this uncertainty further, a current phase 3 trial of intravenous zanamivir in hospitalized adults with influenza is testing 2 dose levels of intravenous zanamivir (600 or 300 mg twice daily) and comparing them with oral oseltamivir (clinicaltrials.gov, NCT01231620).
Zanamivir pharmacokinetic parameters in this severely ill population were generally consistent with results of previous studies in healthy volunteers [34], although variability was greater. Dose adjustments for patients with renal impairment resulted in AUCs similar to those in patients with normal renal function. Pharmacokinetic parameters for patients receiving continuous renal replacement therapy or extracorporeal membrane oxygenation seemed similar to those for patients not undergoing these procedures (data not shown). However, few patients underwent continuous renal replacement therapy (n = 14) or extracorporeal membrane oxygenation (n = 4), and only 4 had simultaneous pharmacokinetic parameters. Thus, additional data are required to confirm these findings.
This study was limited by its single-arm, uncontrolled, open-label design and the critically ill nature of the patient population. Although a potential clinical benefit is difficult to assess without a control group, the safety and clinical outcomes observed in this study are consistent with those expected in patients with severe influenza and reflect a real-life clinical setting.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the patients who participated in this study, the study site staff, and Erica Bullock and Dawn Raimonde (both of GlaxoSmithKline) for study management oversight. Stuart Wakelin of Fishawack Scientific Communications provided editorial assistance in the preparation of the manuscript
Study investigators. In addition to the authors, participating study investigators included the following: Australia: A. Allworth, A. Bersten, M. Chapman, J. Fraser, J. Torresi, and S. Webb; Brazil: P. Barroso; Canada: F. Aoki, D. Grimard, F. Marquis, and A. McGeer; France: J. B. Amiel, P. E. Bollaert, T. Boulain, M. Clavel, J. Y. Lefrant, J. P. Mira, N. Pichon, J. F. Timsit, P. Vignon, and M. Wolff; Russia: R. Kozlov and A. Sabitov; Spain: C. Barros, E. Bouza, A. Esteban De La Torre, V. Falcó Ferrer, M. Jimenez Lendinez, E. Mesalles Sanjuán, J. C. Montejo, E. Navas Elorza, and A. Soriano Viladomiu; Thailand: V. Udompanich; United Kingdom: J. Bewley, A. Binning, M. Grocott, A. Mallick, L. Morrison, S. Shah, M. Singer, and R. Wenstone; United States: M. W. Buckley, V. Bandi, R. Chemaly, R. Dretler, C. Hurt, P. Kumar, M. Parry, J. Pullman, A. Reboli, J. Romero, M. Sherman, L. Waldman, G. Weinstein, and E. Wong.
Financial support. This work, including the conduct of the study, data analysis and interpretation, was supported by GlaxoSmithKline. Editorial assistance was provided by Fishawack Scientific Communications. C. v. d. H. was supported in part by the UNC Center for AIDS Research (grant P30-AI50410-14).
Potential conflicts of interest. F. M. M.'s institution has received grants from GlaxoSmithKline (GSK) and Alnylam; F. M. M. has received honoraria from GSK. C. v. d. H.'s institution has received grants from GSK. B. F. has received consultancy fees from Sanofi, Talecris, and GSK and is a board member at Kenta Biotech. D. G.'s institution has received grants from GSK. R. M. is a board member of Covidien and Hospal and has received speaker fees from both organizations. V. T.'s institution has received grants from GSK, and V. T. has received travel grants from GSK. J. A. L. has no financial or other conflicts of interest to declare. F. A. L. has received consultancy fees and grants from Novartis, Astellas, and Gilead, honoraria from Novartis, Astellas, Gilead, MSD, Pfizer, and Teva, and travel grants from Novartis, Astellas, and Pfizer. D. B.'s institution has received grants from GSK. C. Y. M., A. F. P., P. J. Y., H. H. Z., and S. W. are employees of and own shares and stock in GSK.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.US Department of Health and Human Services. HHS pandemic influenza plan. 2005. http://www.flu.gov/planning-preparedness/federal/hhspandemicinfluenzaplan.pdf/ Accessed December 2012. [Google Scholar]
- 2.Schanzer DL, Zheng H, Gilmore J. Statistical estimates of absenteeism attributable to seasonal and pandemic influenza from the Canadian Labour Force Survey. BMC Infect Dis. 2011;11:90. doi: 10.1186/1471-2334-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods JM, Bethell RC, Coates JA, et al. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993;37:1473–9. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GlaxoSmithKline. Prescribing information for zanamivir. 2011. http://us.gsk.com/products/assets/us_relenza.pdf/ Accessed February 2013.
- 5.Genentech. US Food and Drug Administration. Prescribing information for oseltamivir. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021087s056_021246s039lbl.pdf . Accessed May 2013.
- 6.Collins PJ, Haire LF, Lin YP, et al. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature. 2008;453:1258–61. doi: 10.1038/nature06956. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen HT, Sheu TG, Mishin VP, Klimov AI, Gubareva LV. Assessment of pandemic and seasonal influenza A (H1N1) virus susceptibility to neuraminidase inhibitors in three enzyme activity inhibition assays. Antimicrob Agents Chemother. 2010;54:3671–7. doi: 10.1128/AAC.00581-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Influenza Update No. 158. 2012. http://www.who.int/influenza/surveillance_monitoring/updates/2012_04_27_surveilance_update_158.pdf/ Accessed January 2013. [Google Scholar]
- 9.Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis. 2012;206:148–57. doi: 10.1093/infdis/jis337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Clinical management of human infection with pandemic (H1N1) 2009. revised guidance. 2009 http://www.who.int/csr/resources/publications/swineflu/clinical_management_h1n1.pdf/ Accessed February 2013. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. 2009. http://www.cdc.gov/h1n1flu/recommendations.htm/ Accessed February 2013. [Google Scholar]
- 14.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 15.Joy MS, Matzke GR, Armstrong DK, Marx MA, Zarowitz BJ. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother. 1998;32:362–75. doi: 10.1345/aph.17105. [DOI] [PubMed] [Google Scholar]
- 16.Calfee DP, Peng AW, Cass LM, Lobo M, Hayden FG. Safety and efficacy of intravenous zanamivir in preventing experimental human influenza A virus infection. Antimicrob Agents Chemother. 1999;43:1616–20. doi: 10.1128/aac.43.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shelton MJ, Lovern M, Ng-Cashin J, et al. Zanamivir pharmacokinetics and pulmonary penetration into epithelial lining fluid following intravenous or oral inhaled administration to healthy adult subjects. Antimicrob Agents Chemother. 2011;55:5178–84. doi: 10.1128/AAC.00703-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health. Division of AIDS table for grading the severity of adult and pediatric adverse events. 2004. http://www.niaid.nih.gov/labsandresources/resources/daidsclinrsrch/documents/daidsaegradingtable.pdf/ Accessed February 2013. [Google Scholar]
- 19.Center for Drug Evaluation and Research (CDER). Food and Drug Administration, US Department of Health and Human Services. Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation. 2009. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM174090.pdf? Accessed February 2013. [Google Scholar]
- 20.Kopel E, Amitai Z, Grotto I, Kaliner E, Volovik I. Patients with pandemic (H1N1) 2009 in intensive care units, Israel. Emerg Infect Dis. 2010;16:720–1. doi: 10.3201/eid1604.091696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 22.Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–7. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez JE, Adiga R, Armstrong R, et al. Clinical experience in adults and children treated with intravenous peramivir for 2009 influenza A (H1N1) under an Emergency IND program in the United States. Clin Infect Dis. 2011;52:695–706. doi: 10.1093/cid/cir001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S, Benoit SR, Skarbinski J, Bramley AM, Finelli L 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Influenza-associated pneumonia among hospitalized patients with 2009 pandemic influenza A (H1N1) virus—United States, 2009. Clin Infect Dis. 2012;54:1221–9. doi: 10.1093/cid/cis197. [DOI] [PubMed] [Google Scholar]
- 25.Nin N, Soto L, Hurtado J, et al. Clinical characteristics and outcomes of patients with 2009 influenza A(H1N1) virus infection with respiratory failure requiring mechanical ventilation. J Crit Care. 2011;26:186–92. doi: 10.1016/j.jcrc.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Louie JK, Yang S, Acosta M, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis. 2012;55:1198–204. doi: 10.1093/cid/cis636. [DOI] [PubMed] [Google Scholar]
- 27.Meschi S, Selleri M, Lalle E, et al. Duration of viral shedding in hospitalized patients infected with pandemic H1N1. BMC Infect Dis. 2011;11:140. doi: 10.1186/1471-2334-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puhakka T, Lehti H, Vainionpää R, et al. Zanamivir: a significant reduction in viral load during treatment in military conscripts with influenza. Scand J Infect Dis. 2003;35:52–8. doi: 10.1080/0036554021000026981. [DOI] [PubMed] [Google Scholar]
- 29.Lee N, Chan PK, Wong CK, et al. Viral clearance and inflammatory response patterns in adults hospitalized for pandemic 2009 influenza A(H1N1) virus pneumonia. Antivir Ther. 2011;16:237–47. doi: 10.3851/IMP1722. [DOI] [PubMed] [Google Scholar]
- 30.Kilander A, Rykkvin R, Dudman SG, Hungnes O. Observed association between the HA1 mutation D222G in the 2009 pandemic influenza A(H1N1) virus and severe clinical outcome, Norway 2009–2010. Euro Surveill. 2010;4:15. doi: 10.2807/ese.15.09.19498-en. [DOI] [PubMed] [Google Scholar]
- 31.Freund B, Gravenstein S, Elliott M, Miller I. Zanamivir: a review of clinical safety. Drug Saf. 1999;21:267–81. doi: 10.2165/00002018-199921040-00003. [DOI] [PubMed] [Google Scholar]
- 32.Dines GD, Bethell R, Daniel M. Preclinical development of low toxicity drugs: focus on zanamivir, an anti-influenza drug. Drug Saf. 1998;19:233–41. doi: 10.2165/00002018-199819030-00006. [DOI] [PubMed] [Google Scholar]
- 33.Sević BJ, Obradović D, Batranović U, Stojanović M, Gmizić SS, Bosković T. Influenza A (H1N1)—past season's wonder flu in Vojvodina. Vojnosanit Pregl. 2012;69:951–5. [PubMed] [Google Scholar]
- 34.Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999;36(Suppl 1):1–11. doi: 10.2165/00003088-199936001-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.