Abstract
Background. Highly pathogenic avian influenza A(H5N1) causes severe infections in humans. We generated 2 influenza A(H5N1) live attenuated influenza vaccines for pandemic use (pLAIVs), but they failed to elicit a primary immune response. Our objective was to determine whether the vaccines primed or established long-lasting immunity that could be detected by administration of inactivated subvirion influenza A(H5N1) vaccine (ISIV).
Methods. The following groups were invited to participate in the study: persons who previously received influenza A(H5N1) pLAIV; persons who previously received an irrelevant influenza A(H7N3) pLAIV; and community members who were naive to influenza A(H5N1) and LAIV. LAIV-experienced subjects received a single 45-μg dose of influenza A(H5N1) ISIV. Influenza A(H5N1)– and LAIV-naive subjects received either 1 or 2 doses of ISIV.
Results. In subjects who had previously received antigenically matched influenza A(H5N1) pLAIV followed by 1 dose of ISIV compared with those who were naive to influenza A(H5N1) and LAIV and received 2 doses of ISIV, we observed an increased frequency of antibody response (82% vs 50%, by the hemagglutination inhibition assay) and a significantly higher antibody titer (112 vs 76; P = .04). The affinity of antibody and breadth of cross-clade neutralization was also enhanced in influenza A(H5N1) pLAIV–primed subjects.
Conclusions. ISIV administration unmasked long-lasting immunity in influenza A(H5N1) pLAIV recipients, with a rapid, high-titer, high-quality antibody response that was broadly cross-reactive across several influenza A(H5N1) clades.
Clinical Trials Registration. NCT01109329.
Keywords: H5N1, avian influenza, live attenuated, vaccine
(See the editorial commentary by Falsey on pages 1857–9.)
Since the first reported human infections by avian influenza A(H5N1) in Hong Kong in 1997 [1], >600 cases of influenza A(H5N1) infection with >380 deaths have been reported to the World Health Organization from 15 countries [2]. Vaccines are the most effective option for the control and prevention of influenza virus infection. Since 2005, there has been a global effort to develop and evaluate vaccines against influenza A(H5N1) and other influenza viruses of pandemic potential [3], using traditional and novel strategies, with the goal of rapid vaccine development and deployment in the event of a pandemic.
Live attenuated influenza vaccines (LAIVs) bearing the 6 internal protein genes of the A/AA/6/60 cold-adapted (AA ca) donor virus and the hemagglutinin (HA) and neuraminidase (NA) genes from seasonal human influenza viruses are licensed in the United States for healthy individuals 2–49 years of age. LAIVs may have great potential for use during influenza pandemics by virtue of their high yield in eggs, ability to rapidly induce immunity, and ability to provide protection against antigenically drifted viruses [4, 5]. As part of our efforts to prepare vaccines for influenza viruses with pandemic potential, we generated candidate pandemic LAIVs (pLAIVs) against 2 influenza A(H5N1) strains that caused human infections, A/Hong Kong/213/2003 (HK 2003) and A/VietNam/1203/2004 (VN 2004), by plasmid-based reverse genetics on the AA ca backbone. On the basis of promising preclinical data in mice and ferrets [6], phase 1 clinical trials of the safety and immunogenicity of the vaccines for healthy adults were undertaken in 2007 [7].
In these trials, 2 doses of 107.5 50% tissue culture infectious dose were administered 4–8 weeks apart. The vaccines were generally well tolerated. Vaccine virus was detected by culture or real-time reverse-transcription polymerase chain reaction in few subjects. Serum antibody responses were not detected by the hemagglutination inhibition (HAI) assay or microneutralization (MN) assay in any subject after either dose of the HK 2003 vaccine and were only detected in 1 subject each following the first and second dose of the VN 2004 vaccine [7]. These data suggested that the influenza A(H5N1) HK 2003 and VN 2004 pLAIVs were poorly infectious and poorly immunogenic in humans.
Unadjuvanted inactivated influenza A(H5N1) subvirion vaccines (ISIVs) are also poorly immunogenic in humans, requiring up to 2 doses of 90 μg each to achieve an HAI titer of 1:40 in >50% of subjects [8]. However, studies of other influenza A(H5N1) vaccines led us to hypothesize that subclinical priming may have been achieved in the influenza A(H5N1) pLAIV studies and that these responses were undetectable by the traditional methods used to measure them. VN 2004 pLAIV primed for a robust antibody response to inactivated influenza A(H5N1) vaccine in ferrets [9], and clinical studies demonstrated that robust serum antibody responses were observed following booster vaccinations with influenza A(H5N1) ISIVs even in individuals who had no detectable antibody response to the initial vaccine [10–16]. On the basis of these reports, we hypothesized that previous receipt of an influenza A(H5N1) pLAIV would immunologically prime subjects for a robust response that could subsequently be unmasked by administration of a dose of influenza A(H5N1) ISIV [8].
MATERIALS AND METHODS
Ethics Statement
This open-label phase 1 study (clinical trials registration: NCT01109329) was performed under an investigational new drug application (BB-IND 14793) reviewed by the Food and Drug Administration and approved by the Western Institutional Review Board. Informed, witnessed, written consent was obtained from each subject.
Vaccine
Monovalent influenza A(H5N1) ISIV is a sterile split-virus suspension prepared from influenza virus propagated in embryonated chicken eggs manufactured by Sanofi Pasteur (Swiftwater, PA) that contains 90 μg/mL of influenza A(H5N1) VN 2004 HA. The clinical trial material for this study was lot number UD08916, prepared for investigational use. The vaccine was provided by the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services.
Study Design
This study was conducted at the Center for Immunization Research at the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD). Subjects who had previously received 2 doses of the influenza A(H5N1) VN 2004 pLAIV (group 1) and HK 2003 pLAIV (group 2) were contacted and invited to participate in this study. As a control group, subjects who previously received 2 doses of the influenza A(H7N3) pLAIV (which had the same internal protein genes but irrelevant HA and NA; group 3) [17] were invited to participate.
In addition, a convenience sample of 40 healthy men and nonpregnant women between 22 and 54 years of age who were LAIV- and influenza A(H5)–naive were recruited and randomly assigned by a computer-generated randomization schedule to receive 1 (group 4) or 2 (group 5) doses of ISIV. Subjects were assigned study numbers reflecting their group allocation, based on the order they arrived on the day of vaccination. The study physician notified them of their group assignments at the time of the first vaccination.
To establish the health status of potential participants, Center for Immunization Research staff elicited medical histories, administered physical examinations, and obtained blood and urine specimens for standard health screening assays as previously described [7, 17–20]. Inclusion criteria included healthy adult males and nonpregnant females aged 22–54 years who were willing to forego seasonal LAIV for the duration of the trial. In addition, they must have received 2 doses of live attenuated influenza A(H5N1) or influenza A(H7N3) vaccine in a prior trial (groups 1, 2, and 3) or be LAIV and influenza A(H5) naive (groups 4 and 5, respectively).
Exclusion criteria included pregnancy or breast-feeding; evidence of clinically significant disease or cognitive impairment; history of anaphylaxis or Guillain-Barré syndrome; life-threatening reaction to prior influenza vaccine; egg allergy; current asthma; positivity for human immunodeficiency virus, hepatitis B virus, and/or hepatitis C virus; or current immunosuppression. Volunteers also could not have received a live vaccine within 4 weeks or a killed vaccine within 2 weeks prior to study vaccination or another investigational vaccine or drug within 30 days prior to study vaccination.
Additional exclusion criteria for the influenza A(H5)- and LAIV-naive groups were as follows:
previous enrollment in an influenza A(H5) vaccine trial or in any study of an avian influenza vaccine, seropositivity to influenza A(H5N1) (serum HAI titer >1:8), or previous receipt of any intranasal live attenuated influenza vaccine. Potential participants who were female were tested for pregnancy and were counseled to avoid becoming pregnant during the study.
On study day 0, each volunteer received 45 μg (0.5 mL) of the monovalent influenza A(H5N1) ISIV intramuscularly. Subjects in group 5 received a second 45-μg dose of ISIV 28 days later. Reactogenicity events and adverse events were recorded for 7 days after each vaccination; serious adverse events were recorded for 6 months after the last vaccination.
Study Objectives
The primary objective was to characterize the kinetics and magnitude of the antibody response, as measured by HAI and MN assays, in these 5 groups. These were assessed at baseline and on study days 7, 28, 56, and 180 for groups 1–4 and on study days 7, 28, 35, 56, 84, and 208 for group 5 (corresponding to −21, 0, 7, 28, 56, and 180 days from the second dose of ISIV, respectively). The secondary objective of this trial was to assess the reactogenicity of the ISIV in previous recipients of pLAIV. Reactogenicity was followed for 7 days.
Serum Antibody Assays
HAI and MN assays were performed at Southern Research Institute (Birmingham, AL), using wild-type influenza A/VN/1203/2004(H5N1), in an enhanced biosafety level 3 containment laboratory. HAI assays were performed according to standard procedures [21], using horse erythrocytes. MN assays were performed according to previously described methods [22, 23].
Cross-clade Reactivity Determined by the MN Assay
Neutralizing antibody activity against different clades of influenza A(H5N1) was analyzed by MN based on the methods of the pandemic influenza reference laboratory of the Centers for Disease Control and Prevention (CDC) [22]. Reverse genetics (RG) derived influenza A(H5N1) representing different clades of the highly pathogenic avian influenza A(H5N1)/goose/Guangdong/1/96 lineage, engineered to delete the virulence motif, were obtained from St. Jude's Children's Research Hospital (SJCRH; Memphis, TN), the CDC, and the National Institute for Biological Standards and Control (Potters Bar, United Kingdom): A/VietNam/1203/2004 (SJCRH, clade 1), A/Indonesia/5/2005 (PR8-IBCDC-RG2; clade 2.1.3.2), A/turkey/Turkey/1/05 (NIBRG-23; clade 2.2.1), A/Anhui/1/05 (IBCDC-RG5, clade 2.3.4) and A/Egypt/3072/2010 (IBCDC-RG29; clade 2.2.1). The assays were conducted with three replicates of each serum sample and were performed at least twice.
Affinity Measurements by Surface Plasmon Resonance
Steady-state equilibrium binding of postvaccination sera was monitored at 25°C, using a ProteOn surface plasmon resonance biosensor (BioRad) as previously described [24–26]. Details of the method are provided in the Supplementary Materials.
Statistical Analysis
Geometric mean titers (GMTs) of antibodies and their 95% confidence intervals, detected by HAI and MN assays, were computed by transforming the results to a logarithmic scale (base 2) and converting the values back to the original scale. The area under the curve (AUC) of the titers was compared between all 5 groups, using analysis of variance (ANOVA). Additionally, t tests were used to compare the GMTs between pairs of groups at specific times to identify significant ANOVA effects. P values, group means, and 95% CIs of the mean difference are reported.
In the analysis of the affinity measurements, between-group comparisons of the average off-rates were performed using the Student t test. Spearman correlations are reported for the calculation of correlations between off-rate and MN titers. All P values reported are 2 sided.
RESULTS
Enrollment and Demographic Characteristics
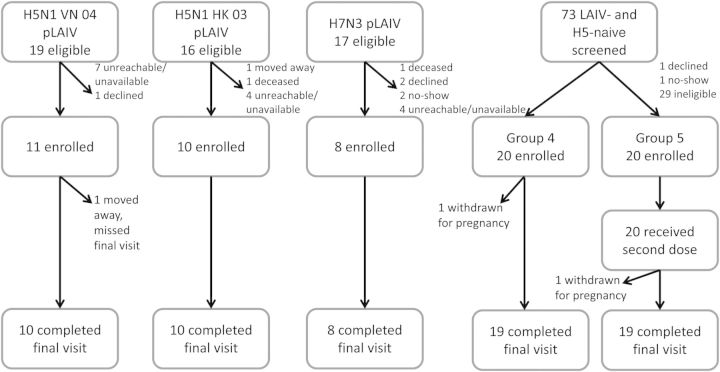
Figure 1 summarizes the study design and enrollment, including the number of eligible participants and the reasons for nonparticipation. Nineteen subjects received 2 doses of the influenza A(H5N1) VN 04 pLAIV in May–June 2007. Attempts were made to contact all subjects. Eleven subjects were screened, found to be eligible, and enrolled in this study in group 1. Of the 16 subjects who received 2 doses of the influenza A(H5N1) HK 03 pLAIV in July and August 2007, 10 were screened and enrolled in this study in group 2. Influenza A(H7N3) pLAIV was given as 2 doses to 17 subjects in September and October 2007. Eight of these individuals were enrolled as the LAIV-experienced, influenza A(H5)–naive subjects in group 3. Forty individuals were enrolled in the LAIV- and influenza A(H5)–naive groups: 20 were randomly assigned to receive 1 dose of the inactivated vaccine (group 4), and 20 were randomly assigned to receive 2 doses (group 5). Subjects were recruited in the summer and fall of 2011. The first vaccinations were given in October 2011, and the final, second-dose vaccinations for group 5 were given in March 2012. The last study visits concluded in August 2012.
Figure 1.
Study design. Abbreviations: H5, influenza A(H5N1); H5N1, influenza A(H5N1); H7N3, influenza A(H7N3); LAIV, live attenuated influenza vaccine.
Demographic data are shown in Table 1. The 5 groups were essentially comparable, with the exception of age. The subjects were mainly male (61%) and black (88%). Of the 69 subjects enrolled in the study, 66 completed the final study visit. One subject in group 1 relocated out of the area prior to the 6-month visit; 1 subject each in groups 4 and 5 were withdrawn because of unanticipated pregnancies. Both pregnancies were terminated for reasons unrelated to vaccine.
Table 1.
Demographic Characteristics, by Study Group
| Characteristic | 1 (n = 11) | 2 (n = 10) | 3 (n = 8) | 4 (n = 20) | 5 (n = 20) | Overall (n = 69) |
|---|---|---|---|---|---|---|
| Dose 1 | H5N1 VN04 pLAIV | H5N1 HK03 pLAIV | H7N3 pLAIV | None | ISIV | |
| Dose 2 | ISIV | ISIV | ISIV | ISIV | ISIV | |
| Age, y, median (range) | 44 (30–53) | 34 (24–49) | 35.5 (23–54) | 35 (23–47) | 34 (22–53) | 37 (22–54) |
| Female sex | 3 (27) | 5 (50) | 2 (25) | 9 (45) | 8 (40) | 27 (39) |
| Race | ||||||
| Black | 9 (82) | 9 (90) | 8 (100) | 18 (90) | 17 (85) | 61 (88) |
| White | 1 (9) | 1 (10) | 0 | 1 (5) | 2 (10) | 5 (7) |
| Multiracial | 1 (9) | 0 | 0 | 1 (5) | 1 (5) | 3 (4) |
Data are no. (%) of subjects, unless otherwise indicated.
Abbreviations: H5N1, influenza A(H5N1); H7N3, influenza A(H7N3); ISIV, inactivated subvirion influenza vaccine; pLAIV, pandemic live attenuated influenza vaccine.
Safety and Reactogenicity
The influenza A(H5N1) ISIV was well tolerated. No vaccine-related severe adverse events occurred. Almost all of the adverse events were mild (Supplementary Materials).
Serum Antibody Response
The AUCs of the HAI and MN titer curves differed significantly between groups (P < .001, by ANOVA; Supplementary Materials). The frequencies and magnitudes of the HAI and MN antibody responses among responders in each group who developed a ≥4-fold rise in titer (titer ≥20) on days 28 or 56 are presented in Table 2. On day 28, both the frequencies and magnitudes of the HAI and MN antibody responses in group 1 (recipients of influenza A(H5N1) VN 04 pLAIV) were greater than in group 3 (recipients of the influenza A[H7N3] pLAIV) and groups 4 and 5 (unprimed subjects; Supplementary Table 2). On day 56, when subjects in group 5 had received 2 doses of influenza A(H5N1) ISIV, 50% and 56% of responders had a GMT of 76 and 35, respectively, by the HAI and MN assays. In contrast, 82% and 55% of responders in group 1 had GMTs of 112 and 61, respectively, by the HAI and MN assays, and 40% and 60% in group 2 had GMTs of 120 and 43 by the HAI and MN assays, respectively. Furthermore, the t tests for comparison of group 1 to group 5, using data from days 28 and 56 (both of which were 28 days after the last ISIV), respectively, yielded P values of < .05 for HAI titers and < .10 for MN titers. Thus, subjects who were primed with either influenza A(H5N1) pLAIV developed HAI and MN responses of greater magnitude than subjects who received 1 or 2 doses of influenza A(H5N1) ISIV without prior influenza A(H5N1) pLAIV priming.
Table 2.
Serum Antibody (Ab) Responses on Days 28 and 56 Following Administration of Inactivated Subvirion Influenza A(H5N1) Vaccine (ISIV)
| Ab Assay, Study Group | pLAIV | VN04 ISIV Doses, No. | Subjects, No. | 28 d After ISIVa |
56 d After ISIVa |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | GMT | Subjects With 4-fold Ab Riseb |
Range | GMT | Subjects With 4-fold Ab Riseb |
||||||
| Percentage | GMT (95% CI) | Percentage | GMT (95% CI) | ||||||||
| HAI assay | |||||||||||
| 1 | H5N1 VN 04 | 1 | 11 | 5–1280 | 87c | 73 | 222 (115–426) | 5–960 | 66 | 82 | 112 (54–233) |
| 2 | H5N1 HK 03 | 1 | 10 | 5–480 | 29d | 50 | 146 (57–374) | 5–480 | 21 | 40 | 120 (20–727) |
| 3 | H7N3 | 1 | 8e | 5–160 | 8 | 14 | 160 (0f) | 5 | 5 | 0 | 5 |
| 4 | None | 1 | 20 | 5–640 | 8 | 10 | 277 | 5–240 | 8 | 10 | 120 |
| 5 | None | 2 | 20e | 5–640 | 15 | 40 | 81 (36–181) | 5–120 | 21c,d | 50 | 76 (43–133) |
| MN assay | |||||||||||
| 1 | H5N1 VN 04 | 1 | 11 | 5–1280 | 48g | 73 | 89 (31–253) | 5–1280 | 25 | 55 | 61 (12–318) |
| 2 | H5N1 HK 03 | 1 | 10 | 5–160 | 31h | 60 | 61 (29–127) | 5–160 | 22 | 60 | 43 (24–76) |
| 3 | H7N3 | 1 | 8e | 5–60 | 8 | 14 | 60 (0) | 5 | 5 | 0 | 5 |
| 4 | None | 1 | 20 | 5–60 | 7 | 10 | 35 | 5–40 | 4 | 10 | 35 (6–215) |
| 5 | None | 2 | 20e | 5–480 | 11 | 30 | 46 (11–190) | 5–160 | 19g,h | 56 | 35 (19–64) |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; HAI, hemagglutination inhibition; MN, microneutralization; pLAIV, pandemic live attenuated influenza vaccine
a Days are counted relative to the only ISIV dose for groups 1–4 and after the first of 2 ISIV doses for group 5.
b Serological response defined as a ≥4-fold rise in Ab titer (≥1:20).
c Group 1 day 28 vs group 5 day 56: P = .04 (t test).
d Group 2 day 28 vs group 5 day 56: P = .62 (t test).
e Serum samples were available from 7 subjects in group 3 on day 28 and from 18 subjects in group 5 on day 56.
f A single subject in this group had antibody detected.
g Group 1 day 28 vs group 5 day 56: P = .08 (t test).
h Group 2 day 28 vs group 5 day 56: P = .22 (t test).
Kinetics of the Antibody Response
Figure 2A demonstrates the kinetics of the HAI antibody response. Seven of 11 subjects (64%) in group 1 had 4-fold rises in HAI antibody titer by day 7 following receipt of the influenza A(H5N1) ISIV, with a GMT of 165 and a titer range of 20 to 1280 in responders. Of the pLAIV-naive individuals, 10% had 4-fold rises by day 7. A reverse cumulative frequency distribution curve of the HAI titers on day 7 for group 1 is presented in Figure 2B. Reverse cumulative frequency distribution curves on day 28 after receipt of the last dose of ISIV for groups 1, 2, 4, and 5 are presented in Figure 2C. Consistent with previous observations about the longevity of the antibody response to influenza A(H5) vaccines [27–30], the GMT of HAI antibodies in all groups declined 6 months after receipt of inactivated influenza A(H5) vaccine, and fewer subjects had detectable antibodies by 6 months after the last dose of ISIV (Figure 2A).
Figure 2.
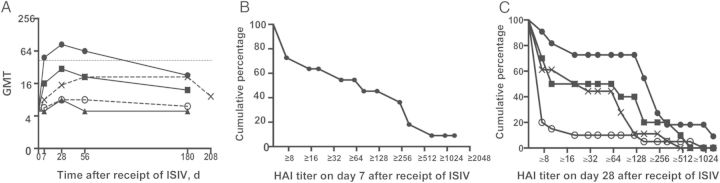
A, Kinetics of the hemagglutination inhibition (HAI) antibody response following vaccination with influenza A(H5N1) inactivated subvirion influenza vaccine (ISIV) in individuals primed with influenza A(H5N1) pandemic live attenuated influenza vaccine (pLAIV), in those primed with influenza A(H7N3) pLAIV, or in LAIV-naive individuals. HAI assay–based reciprocal geometric mean titers (GMTs) to wild-type A/VietNam/1203/2004(H5N1), using horse red blood cells. Dotted line indicates a HAI assay–based GMT of 1:40. Groups are represented by the following symbols: filled circle, group 1 (influenza A[H5N1] VN 04 pLAIV); square, group 2 (influenza A[H5N1] HK 03 pLAIV); triangle, group 3 (influenza A[H7N3] pLAIV); open circle, group 4 (single dose of influenza A[H5N1] ISIV); and X, group 5 (2 doses of influenza A[H5N1] ISIV). B, Influenza A(H5N1) pLAIV primes for a rapid antibody response to influenza A(H5N1) ISIV. Reverse cumulative frequency distribution of HAI titers for subjects in group 1 (primed with influenza A[H5N1] VN 2004 pLAIV) on day 7 after receipt of ISIV. C, Reverse cumulative frequency distribution of HAI titers from subjects in groups 1, 2, 4, and 5 on day 28 after receipt of the last dose of ISIV. Groups are represented by the following symbols: filled circle, group 1 (influenza A[H5N1] VN 04 pLAIV); square, group 2 (influenza A[H5N1] HK 03 pLAIV); open circle, group 4 (1 dose of ISIV); and X, group 5 (2 doses of influenza A[H5N1] ISIV).
Antibody Affinity and Breadth
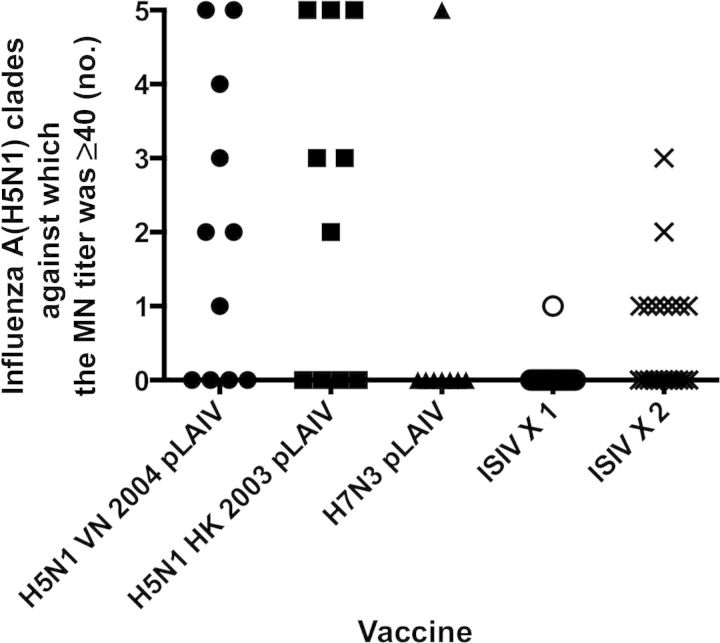
The breadth of the MN antibody response in sera from day 28 (and from day 56 for group 5, 28 days after a second dose of ISIV) is shown in Figure 3. Thirteen of 21 subjects in groups 1 and 2 had MN titers of ≥1:40. Of these 13, serum samples from all but 1 influenza A(H5N1) pLAIV–primed subject neutralized ≥2 clades of influenza A(H5N1). In contrast, serum from only 1 subject in group 5 neutralized >1 clade, and the receipt of a second dose of ISIV resulted in a higher GMT but did not increase the breadth of the antibody response.
Figure 3.
Influenza A(H5N1) pandemic live attenuated influenza vaccine (pLAIV) priming elicits neutralizing antibodies that show broad cross-reactivity against influenza A(H5N1) from antigenically distinct clades. The proportion of subjects from each group with a reciprocal microneutralization (MN) titer of ≥40 against any one of 5 influenza A(H5N1) viruses from 4 antigenically distinct clades of the Gs/gd lineage. MN assay was performed using reverse genetics–derived PR8 reassortant viruses bearing the hemagglutinin from the following viruses: A/VietNam/1203/2004 (clade 1), A/Indonesia/5/2005 (clade 2.1.3), A/Anhui/1/05 (clade 2.3.4), A/turkey/Turkey/1/05 (clade 2.2.1), and A/Egypt/3072/2101 (clade 2.2.1). Groups are represented by the following symbols: filled circle, group 1 (influenza A[H5N1] VN 04 pLAIV); square, group 2 (influenza A[H5N1] HK 03 pLAIV); triangle, group 3 (influenza A[H7N3] pLAIV); open circle, group 4 (single dose of influenza A[H5N1] inactivated subvirion influenza vaccine [ISIV]); and X, group 5 (2 doses of influenza A[H5N1] ISIV).
As can be seen in Figure 4A, the average off-rates for antibody bound to recombinant HA1 (rHA1; amino acids 1–330) were significantly slower in responders (titers of ≥1:40) than nonresponders (titers <1:40) in group 1 (P = .0083). A similar pattern was observed in group 2 but did not reach statistical significance. Importantly, the antibody affinity against rHA1 for responders among groups 1 and 2 was 2–3-fold stronger (slower off-rates) than in group 5 (P = .0249 and .0013, respectively). In contrast, antibody binding to recombinant HA2 (rHA2; amino acids 331–480; Figure 4B) did not differ among the groups or between responders and nonresponders.
Figure 4.
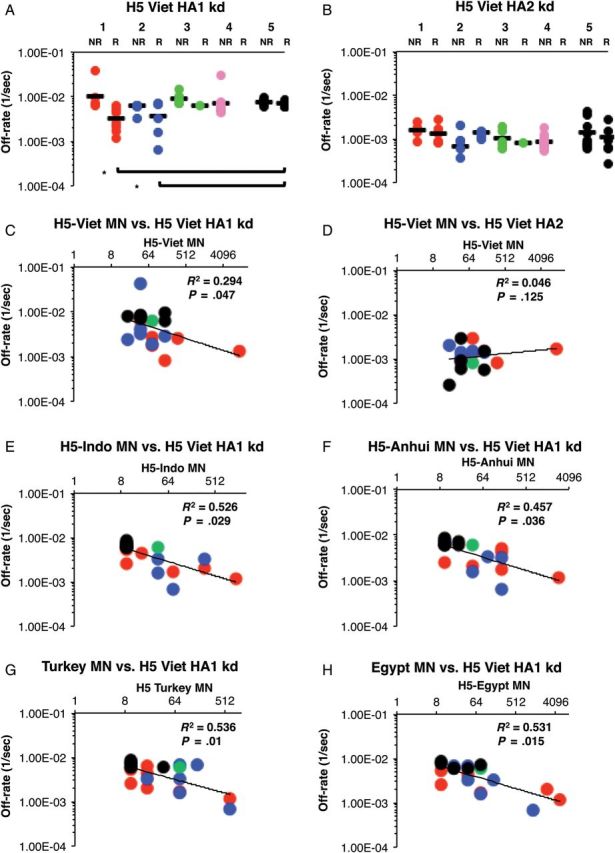
A and B, Live attenuated influenza vaccine (LAIV) priming enhances antibody affinity (slower off-rates) to influenza A(H5N1) hemagglutinin polypeptide 1 (HA1; but not HA2). Surface plasmon resonance (SPR) analysis of postvaccination sera from all responders (R; MN ≥1:40) and nonresponders (NR; MN <1:40) from all 5 study groups was performed with properly folded functional oligomeric influenza A(H5N1) rHA1 (A) and rHA2 (B) domains from influenza A/VN/1203/2004. Antibody off-rate constants that describe the fraction of antibody-antigen complexes decaying per second were determined directly from the serum sample interaction with rHA1 (1–330) protein and rHA2 (331–480), using surface plasmon resonance in the dissociation phase. Serum antibody off-rate constants (each symbol represents 1 individual) were determined as described in Materials and Methods. Differences in mean off-rate constants were statistically significant between different vaccine groups, with a P value of < .05 (by the t test). The off-rate constants were determined from 2 independent SPR runs. Groups are represented by the colored circles as follows: group 1, red (influenza A[H5N1] VN 04 pLAIV); group 2, blue (influenza A[H5N1] HK 03 pLAIV); group 3, green (influenza A[H7N3] pLAIV); group 4, pink (single dose of influenza A[H5N1] inactivated subvirion influenza vaccine [ISIV]); and group 5, black (2 doses of influenza A[H5N1] ISIV). C–H, Serum antibody off-rate to VN04 rHA1 (but not rHA2) in influenza A(H5N1) pLAIV–primed individuals correlates with the in vitro neutralizing capacity against the homologous and heterologous influenza A(H5N1) from different clades. Neutralizing titer is expressed as the standardized end-point neutralizing antibody titer of sera obtained after vaccination. Serum antibody off-rate constants (each symbol represents 1 individual) of responders against rHA1 (C); but not rHA2 (D) of VN04 correlated with the in vitro homologous neutralizing influenza A(H5N1) capacity as determined by MN titers. E–H, Antibody affinity of responders against VN04 rHA1 correlated strongly with heterologous cross-neutralizing titers against A/Indonesia/5/2005 (clade 2.1.3; E), A/Anhui/1/05 (clade 2.3.4; F), A/turkey/Turkey/1/05 (clade 2.2.1; G), and A/Egypt/3072/2101 (clade 2.2.1; H). Responders in each group are represented by the colored circles as follows: group 1, red (influenza A[H5N1] VN 04 pLAIV); group 2, blue (influenza A[H5N1] HK 03 pLAIV); group 3, green (influenza A[H7N3] pLAIV); group 4, pink (single dose of influenza A[H5N1] ISIV); group 5, black (2 doses of influenza A[H5N1] ISIV).
The possible contribution of antibody affinity to virus neutralization was evaluated. The antibody off-rates against rHA1 and rHA2 were plotted against the MN titer to the influenza A(H5N1) VN 2004 (vaccine strain) or heterologous influenza A(H5N1) clades and subclades mentioned above. Inverse correlations were observed between antibody off-rates against rHA1 (Figure 4C and 4E–H) but not rHA2 (Figure 4D) and MN titers against all the influenza A(H5N1) strains tested (R2 values and P values are given in Figure 4). Interestingly, group 5 responders had lower MN titers against heterologous strains, compared with responders in groups 1 and 2.
Thus, priming with influenza A(H5N1) pLAIV resulted in higher-affinity antibodies against the VN04 HA1 domain that correlated with cross-neutralization of antigenically diverse clades of influenza A(H5N1).
DISCUSSION
The high-titer, rapid antibody response following a single dose of unadjuvanted influenza A(H5N1) ISIV in a majority of subjects who had received an influenza A(H5N1) pLAIV almost 5 years earlier is clear evidence that influenza A(H5N1) pLAIV priming induced long-lasting B-cell memory. The magnitude and frequency of the neutralizing antibody response in recipients of the influenza A(H5N1) pLAIVs is remarkable given the paucity of such responses in these participants after the priming doses of pLAIV, as well as the highly restricted replication of influenza A(H5N1) pLAIV in these individuals. These results demonstrate the utility of using an inactivated vaccine to probe for the immune response generated by the pLAIV.
The influenza A(H5N1) ISIV used in this study was previously demonstrated to be only moderately immunogenic; 2 doses of 45 μg of HA—the dose used in this study—resulted in seroconversion in only 41%–56% of study subjects in phase 1 clinical trials [8, 31]. We used this suboptimal dose to determine whether prior priming with influenza A(H5N1) pLAIV would result in an enhanced antibody response. Although limited by relatively small sample sizes in our previously vaccinated groups, our findings that 82% of subjects primed with an antigenically matched influenza A(H5N1) pLAIV responded to 45 μg of ISIV suggest that lower doses of ISIV may suffice for individuals who were primed with pLAIV.
We also evaluated the quality of the antibody response and found that priming with influenza A(H5N1) pLAIV induced higher-affinity antibody with a greater breadth of reactivity against different clades of influenza A(H5N1) than that observed in individuals who only received ISIV. The affinity of binding correlated with the breadth of cross-reactivity rather than with the magnitude of the response. Cross-reactivity is important because 10 distinct clades of influenza A(H5N1) have been identified in avian species since 2003. Influenza A(H5N1) continues to evolve in nature, and we do not know whether some clades pose a greater pandemic threat than others. Therefore, a vaccination strategy that induces a broadly cross-reactive antibody response is highly desirable. We did not specifically measure antibody against the conserved stem region of the HA protein because the antibodies detected after receipt of ISIV in pLAIV-primed individuals had robust HAI activity, which anti-HA stem antibodies lack [32–34], and the HAI and MN antibody responses were equally robust.
It will be important to elucidate the immunologic mechanism(s) that result in the induction of a robust antibody response, using prime-boost strategies with different vaccine platforms. Similar results have been reported following priming with an influenza A(H5) DNA vaccine and a recombinant adenovirus vaccine expressing the influenza A(H5) HA followed by boost with influenza A(H5N1) ISIV [11–13, 24]. In our study, because the neutralizing antibody response was detected as early as 7 days following administration of ISIV, it is unlikely that the antibodies measured were elicited by the ISIV; rather, immune memory was established after priming by pLAIV. A recent report identified a subset of ICOS+CXCR3+CXCR5+ T-helper cells that played a critical role in the induction of antibodies to inactivated influenza vaccine in previously primed individuals but not in naive subjects [35]. It will be interesting to determine whether this subset of CD4+ T-helper cells is detected in pLAIV-primed subjects. An immune correlate of protection for seasonal LAIV has yet to be identified; further studies with pLAIV followed by ISIV administration may lead to the identification of a correlate of immunity that could apply to seasonal LAIV, as well.
Although this study was conducted in a small number of subjects and the groups were not randomized, our findings suggest that pLAIV effectively primes the immune system and that the administration of a single dose of an ISIV of the same subtype is an effective way to reveal the immunological priming by pLAIV. These observations have implications for evaluating the immunogenicity of pLAIV. pLAIV priming occurred even in the absence of significant vaccine virus shedding and immunogenicity measured by traditional end points in the initial phase 1 clinical trials of the influenza A(H5N1) pLAIV [7]. Although pLAIV would not be recommended for widespread use unless a pandemic was imminent, 2 scenarios can be envisioned when a pLAIV could be used to prime a population. First, if an antigenically mismatched pLAIV seed virus is available, it could be used to prime and could be followed with the matched inactivated vaccine. Another scenario would occur if pLAIV becomes available sooner than inactivated vaccine, as in 2009, such that the LAIV can be used for priming. The rapid response to injected inactivated antigen observed in pLAIV-primed individuals also suggests that pLAIV recipients would likely be protected from severe illness in the event of an influenza A(H5) pandemic. Future studies will be designed to determine how optimal priming can be achieved. The nature of priming offered by different vaccine platforms, including DNA vaccines, vectored vaccines, and pLAIV, can be compared [9]. Some of the key variables to be explored are the number of pLAIV priming doses needed, the optimal interval between prime and boost, whether an ISIV can be used as a prime and a pLAIV as the boost, and the benefit of an exact antigenic match between the virus strains used to prime and boost.
The high-titer and high-quality, broadly cross-reactive antibody response seen in our study, in which pLAIV priming was unmasked by an ISIV boost, should raise interest in unconventional vaccination schedules that combine different types of vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Robert Huebner and Armen Donabedian of the Biomedical Advanced Research and Development Authority, Department of Health and Human Services, for providing inactivated vaccine; Diana Noah of Southern Research; staff of the Regulatory Compliance and Human Subjects Protection Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health; the study coordinators; the study nurses; and the study volunteers. The pLAIVs were generated under a cooperative research and development agreement between NIH and MedImmune.
Financial support. This work was supported by the Intramural Research Program of National Institute of Allergy and Infectious Diseases, National Institutes of Health; the Influenza Vaccine Manufacturing Improvement Initiative of the Biomedical Advanced Research and Development Authority, US Department of Health and Human Services; PATH Vaccine Solutions; and Pandemic Flu Funding from the Center for Biologics Evaluation and Research, Food and Drug Administration.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Subbarao K, Klimov A, Katz J, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/ . Accessed 8 October 2013. [Google Scholar]
- 3.Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. H5N1 vaccines in humans. Virus Res. 2013;178:78–98. doi: 10.1016/j.virusres.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168–75. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 5.Mendelman PM, Rappaport R, Cho I, et al. Live attenuated influenza vaccine induces cross-reactive antibody responses in children against an A/Fujian/411/2002-like H3N2 antigenic variant strain. Pediatr Infect Dis J. 2004;23:1053–5. doi: 10.1097/01.inf.0000143643.44463.b1. [DOI] [PubMed] [Google Scholar]
- 6.Suguitan AL, Jr., McAuliffe J, Mills KL, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3:e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karron RA, Talaat K, Luke C, et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine. 2009;27:4953–60. doi: 10.1016/j.vaccine.2009.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 9.Suguitan AL, Jr., Cheng X, Wang W, Wang S, Jin H, Lu S. Influenza H5 hemagglutinin DNA primes the antibody response elicited by the live attenuated influenza A/Vietnam/1203/2004 vaccine in ferrets. PLoS One. 2011;6:e21942. doi: 10.1371/journal.pone.0021942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goji NA, Nolan C, Hill H, et al. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis. 2008;198:635–41. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 11.Gurwith M, Lock M, Taylor EM, et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis. 2013;13:238–50. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledgerwood JE, Wei CJ, Hu Z, et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11:916–24. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledgerwood JE, Zephir K, Hu Z, et al. Prime-boost interval matters: a randomized phase I study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J Infect Dis. 2013;208:418–22. doi: 10.1093/infdis/jit180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 15.Stephenson I, Nicholson KG, Colegate A, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21:1687–93. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 16.Treanor JJ, Wilkinson BE, Masseoud F, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–7. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 17.Talaat KR, Karron RA, Callahan KA, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine. 2009;27:3744–53. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talaat KR, Karron RA, Liang PH, et al. An open-label phase I trial of a live attenuated H2N2 influenza virus vaccine in healthy adults. Influenza Other Respi Viruses. 2012;7:66–73. doi: 10.1111/j.1750-2659.2012.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talaat KR, Karron RA, Luke CJ, et al. An open label phase I trial of a live attenuated H6N1 influenza virus vaccine in healthy adults. Vaccine. 2011;29:3144–8. doi: 10.1016/j.vaccine.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karron RA, Callahan K, Luke C, et al. A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J Infect Dis. 2009;199:711–6. doi: 10.1086/596558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–5. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walls HH, Harmon MW, Slagle JJ, Stocksdale C, Kendal AP. Characterization and evaluation of monoclonal antibodies developed for typing influenza A and influenza B viruses. J Clin Microbiol. 1986;23:240–5. doi: 10.1128/jcm.23.2.240-245.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurana S, Wu J, Dimitrova M, et al. DNA priming prior to H5N1 inactivated influenza vaccination expands antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. J Infect Dis. 2013;208:413–17. doi: 10.1093/infdis/jit178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khurana S, Verma S, Verma N, et al. Properly folded bacterially expressed H1N1 hemagglutinin globular head and ectodomain vaccines protect ferrets against H1N1 pandemic influenza virus. PLoS One. 2010;5:e11548. doi: 10.1371/journal.pone.0011548. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Khurana S, Verma S, Verma N, et al. Bacterial HA1 vaccine against pandemic H5N1 influenza virus: evidence of oligomerization, hemagglutination, and cross-protective immunity in ferrets. J Virol. 2011;85:1246–56. doi: 10.1128/JVI.02107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrlich HJ, Muller M, Fritsch S, et al. A cell culture (Vero)-derived H5N1 whole-virus vaccine induces cross-reactive memory responses. J Infect Dis. 2009;200:1113–8. doi: 10.1086/605608. [DOI] [PubMed] [Google Scholar]
- 28.Lin JT, Li CG, Wang X, et al. Antibody persistence after 2-dose priming and booster response to a third dose of an inactivated, adjuvanted, whole-virion H5N1 vaccine. J Infect Dis. 2009;199:184–7. doi: 10.1086/595832. [DOI] [PubMed] [Google Scholar]
- 29.Nolan TM, Richmond PC, Skeljo MV, et al. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine. 2008;26:4160–7. doi: 10.1016/j.vaccine.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 30.Zangwill KM, Treanor JJ, Campbell JD, Noah DL, Ryea J. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J Infect Dis. 2008;197:580–3. doi: 10.1086/526537. [DOI] [PubMed] [Google Scholar]
- 31.Patel SM, Atmar RL, El Sahly HM, Cate TR, Keitel WA. A phase I evaluation of inactivated influenza A/H5N1 vaccine administered by the intradermal or the intramuscular route. Vaccine. 2010;28:3025–9. doi: 10.1016/j.vaccine.2009.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corti D, Suguitan AL, Jr., Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–73. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekiert DC, Bhabha G, Elsliger MA, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–51. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui J, Hwang WC, Perez S, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra32. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.