Abstract
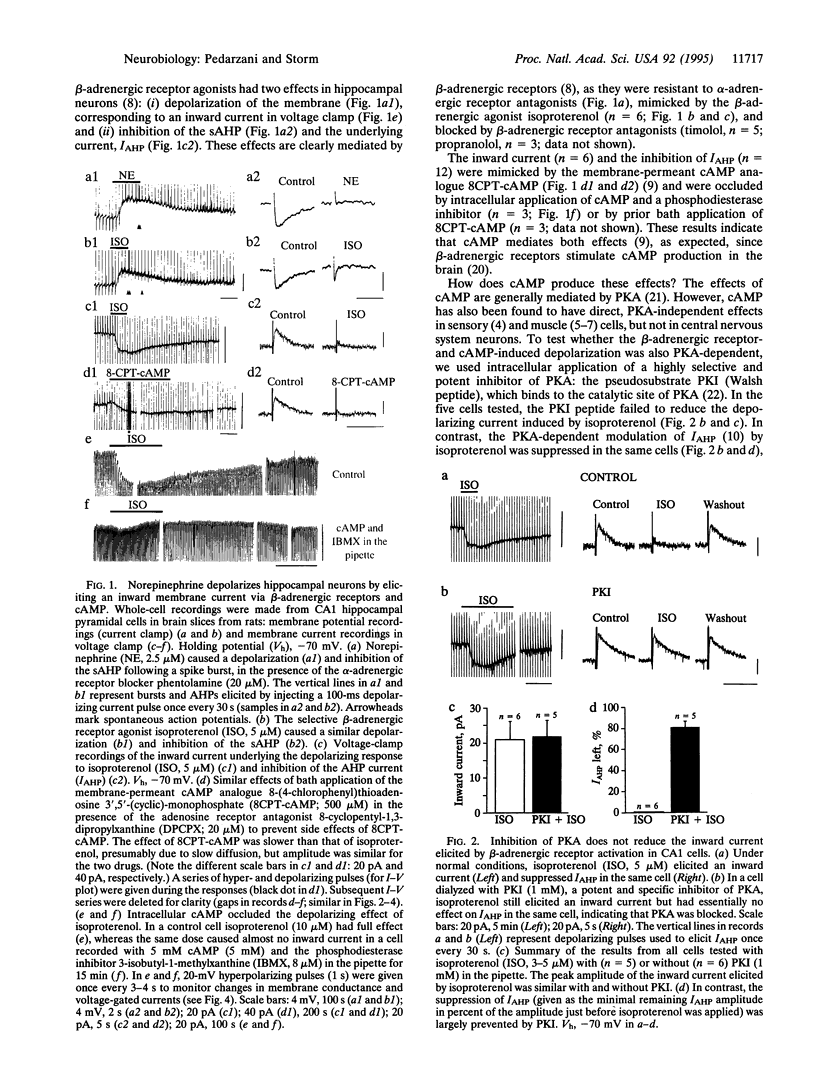
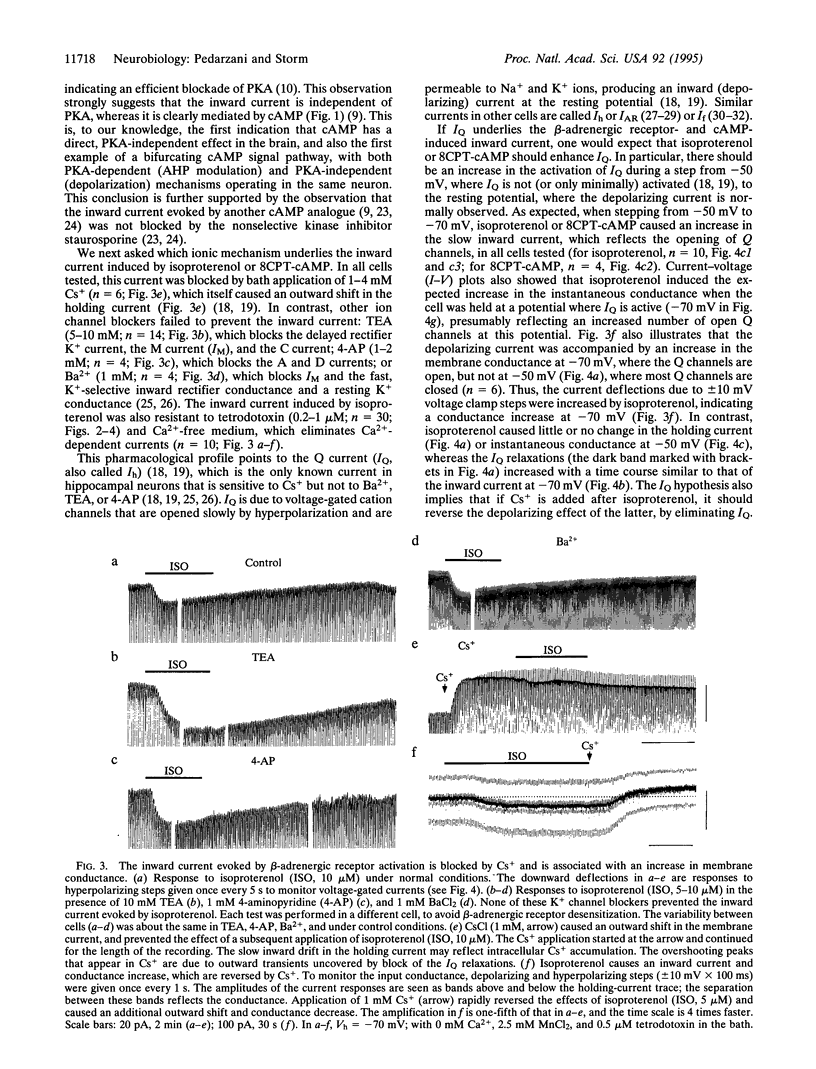
Ion channels underlying the electrical activity of neurons can be regulated by neurotransmitters via two basic mechanisms: ligand binding and covalent modification. Whereas neurotransmitters often act by binding directly to ion channels, the intracellular messenger cyclic AMP is thought usually to act indirectly, by activating protein kinase A, which in turn can phosphorylate channel proteins. Here we show that cyclic AMP, and transmitters acting via cyclic AMP, can act in a protein kinase A-independent manner in the brain. In hippocampal pyramidal cells, cyclic AMP and norepinephrine were found to cause a depolarization by enhancing the hyperpolarization-activated mixed cation current, IQ (also called Ih). This effect persisted even after protein kinase A activity was blocked, thus strongly suggesting a kinase-independent action of cyclic AMP. The modulation of this current by ascending monoaminergic fibers from the brainstem is likely to be a widespread mechanism, participating in the state control of the brain during arousal and attention.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Gähwiler B. H., Griffith W. H., Halliwell J. V. Membrane currents in hippocampal neurons. Prog Brain Res. 1990;83:141–160. doi: 10.1016/s0079-6123(08)61247-9. [DOI] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput Y., Araneda R. C., Andrade R. Pharmacological and functional analysis of a novel serotonin receptor in the rat hippocampus. Eur J Pharmacol. 1990 Jul 17;182(3):441–456. doi: 10.1016/0014-2999(90)90041-4. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Chu N., Bloom F. E. Norepinephrine-containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science. 1973 Mar 2;179(4076):908–910. doi: 10.1126/science.179.4076.908. [DOI] [PubMed] [Google Scholar]
- Delgado R., Hidalgo P., Diaz F., Latorre R., Labarca P. A cyclic AMP-activated K+ channel in Drosophila larval muscle is persistently activated in dunce. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):557–560. doi: 10.1073/pnas.88.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Mangoni M. Modulation of single hyperpolarization-activated channels (i(f)) by cAMP in the rabbit sino-atrial node. J Physiol. 1994 Feb 1;474(3):473–482. doi: 10.1113/jphysiol.1994.sp020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991 May 9;351(6322):145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Gerber U., Sim J. A., Gähwiler B. H. Reduction of Potassium Conductances Mediated by Metabotropic Glutamate Receptors in Rat CA3 Pyramidal Cells Does Not Require Protein Kinase C or Protein Kinase A. Eur J Neurosci. 1992;4(9):792–797. doi: 10.1111/j.1460-9568.1992.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Greene R. W. Effects of histamine on hippocampal pyramidal cells of the rat in vitro. Exp Brain Res. 1986;62(1):123–130. doi: 10.1007/BF00237408. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Maccaferri G., Mangoni M., Lazzari A., DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol. 1993 Jun;69(6):2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol. 1986 Mar;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986 Mar;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Nicoll R. A. Dopamine decreases the calcium-activated afterhyperpolarization in hippocampal CA1 pyramidal cells. Brain Res. 1986 Aug 6;379(2):210–215. doi: 10.1016/0006-8993(86)90773-0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989 Jun;12(6):215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990 Dec;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Palmer G. C., Sulser F., Robison G. A. Effects of neurohumoral and adrenergic agents on cyclic AMP levels in various areas of the rat brain in vitro. Neuropharmacology. 1973 Apr;12(4):327–337. doi: 10.1016/0028-3908(73)90092-0. [DOI] [PubMed] [Google Scholar]
- Pedarzani P., Storm J. F. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993 Dec;11(6):1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Sim J. A., Gerber U., Knöpfel T., Brown D. A. Evidence Against a Role for Protein Kinase C in the Inhibition of the Calcium-activated Potassium Current IAHP by Muscarinic Stimulants in Rat Hippocampal Neurons. Eur J Neurosci. 1992;4(9):785–791. doi: 10.1111/j.1460-9568.1992.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Steriade M., McCormick D. A., Sejnowski T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993 Oct 29;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Torres G. E., Chaput Y., Andrade R. Cyclic AMP and protein kinase A mediate 5-hydroxytryptamine type 4 receptor regulation of calcium-activated potassium current in adult hippocampal neurons. Mol Pharmacol. 1995 Jan;47(1):191–197. [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991 Sep;43(3):299–349. [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]



