Abstract
Paliperidone palmitate (PP) is a recently introduced long-acting atypical, or second-generation, antipsychotic. Published data on PP are currently limited to controlled trials and case reports. In this observational study, we followed up 200 consecutive patients prescribed PP in normal practice. After 1 year, 65% of patients were still receiving PP. The number of admissions to hospital in the year following PP initiation was 0.49/patient compared with 0.69/patient/year, 3 years before initiation (P=0.0001). The mean number of bed days fell from 38.78 to 23.09/patient/year over the corresponding period (P=0.0001). The median number of bed days 3 years before PP initiation was 21.50/year and in the year following PP initiation, it was 0. Outcomes were numerically but not statistically better in those continuing PP than in those who ceased PP within a year of initiation. PP was effective and well-tolerated and, given its positive effect on hospital bed days, broadly cost-effective.
Keywords: antipsychotic, long-acting injection, paliperidone, schizophrenia
Introduction
Depot or long-acting antipsychotics confer better protection against psychotic relapse than their oral equivalents (Hogarty et al., 1979; Tiihonen et al., 2011). Conventional, or first generation, antipsychotic depots are widely used and have a low acquisition cost, but are associated with a high risk of tardive dyskinesia (Novick et al., 2010).
In the last 10 years or so, several second-generation long-acting injections have been introduced. These formulations might be expected to provide at least as effective protection against relapse but with a lower risk of acute and chronic movement disorders (Leucht et al., 1999). However, there is only one controlled comparisons of conventional and atypical depots (Rubio et al., 2006) – a open randomized comparison of fairly high-dose risperidone with moderate doses of zuclopenthixol. A single randomized controlled trial against prescriber choice of oral medication found no benefit for risperidone long-acting injection on rehospitalization (Rosenheck et al., 2011). As a consequence, our understanding of the value of atypical depots is largely predicated on placebo-controlled regulatory studies and uncontrolled observational studies.
Observational studies of risperidone depot (Risperdal Consta) show that it is effective in some patient groups (Taylor et al., 2009b), but also that its effect on time spent in hospital can be beneficial (Niaz and Haddad, 2007; Taylor et al., 2008), neutral (Taylor et al., 2008), or adverse [in that some mirror-image studies show an increase in bed days after the initiation of risperidone depot (Young and Taylor, 2006; Taylor et al., 2009a)].
We are engaged in a large-scale observational study of the most recently introduced atypical depot, paliperidone palmitate (PP). Here, we report on 1-year hospitalization outcomes for the first cohort of patients prescribed PP in our unit.
Materials and methods
PP was approved for use in our NHS trust in April 2011. Its use was not restricted in any way but prescribers were required to complete data-gathering forms on initiation and cessation of PP. The study was approved by the trust's Drug and Therapeutics Committee. All patients prescribed PP were included in the study with the sole exception of ‘forensic’ patients (whose stay in medium secure provision was governed by their sentence). This being an observational study, no attempt was made to influence decisions regarding the use of PP and patients received care as usual.
Demographic details were obtained for each patient from data forms and the electronic patient record. All adverse effects recorded were reported anonymously to Janssen-Cilag Ltd (Buckinghamshire, UK), the manufacturer of PP. Details of the time spent in hospital as inpatients were also obtained from electronic records. Hospitalizations were compared using the ‘mirror image’ method. We recorded bed days for 3 years before starting PP and compared the mean yearly figure with the year following discharge from hospital having started PP (inpatients) or from the date of PP initiation (outpatients). We also conducted sensitivity analysis comparing (i) the mean yearly bed days 3 years before the admission when PP was started with the year after discharge (i.e. discarding all of the index admission), and (ii) the mean yearly bed days before and after PP initiation (i.e. including all of the index admission).
Staff collecting data were those directly involved in the care of the individual patients. Once entered on a communal database, all patient details were anonymized and the data files were secured.
Statistical analysis
Baseline patient and medication characteristics were summarized using descriptive statistics. Means and SDs were calculated for continuous data and frequencies and percentages for categorical data. Means and SDs were calculated as a summary measure for admissions into hospital and associated bed days in the 3-year period before initiating PP. For continuous data not normally distributed, comparisons for admission rates and bed days before and after PP were performed using the nonparametric Wilcoxon signed-rank test for paired data. Differences in outcomes between continuers and discontinuers of PP were compared with the Mann–Whitney U-test. Statistical significance was considered demonstrated if P value was less than 0.05. All tests were performed using STATA version 11.2 (StataCorp LP, College Station, Texas, USA).
Results
We followed 200 consecutive patients initiated on PP for 1 year. Demographic details of these patients are given in Table 1. For those who initiated PP as inpatients, mean (SD) duration of current episode was 23.8 (53.8) days. Sex, care setting and treatment responsiveness were not associated with admissions or hospital bed days following paliperidone initiation. Details of discontinuation of PP during the year’s follow-up are given in Table 2.
Table 1.
Baseline demographics
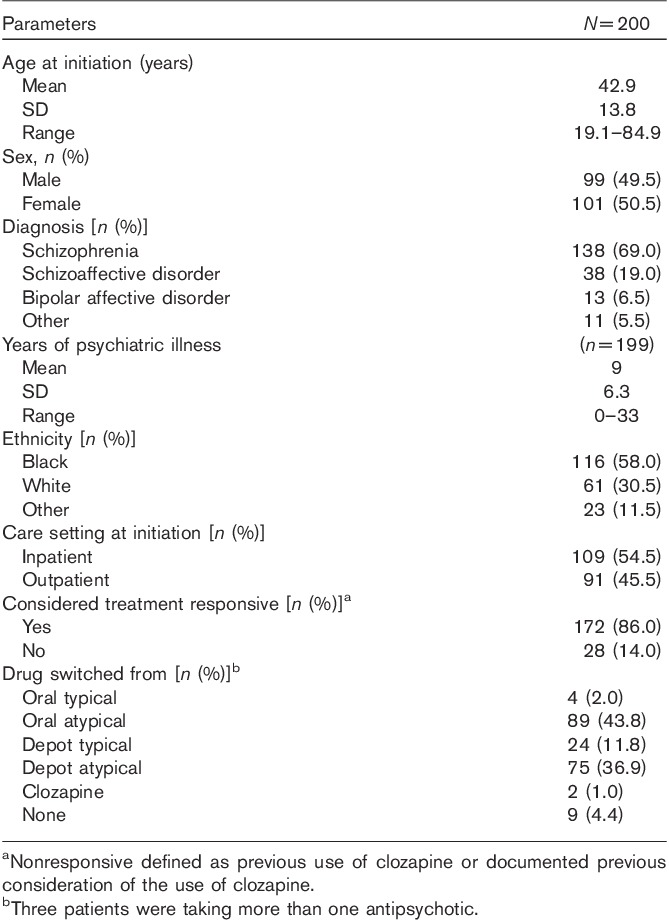
Table 2.
Clinical outcome at 1 year

In the primary analysis (Fig. 1), the mean number of admissions reduced from 0.67/patient/year to 0.49/patient in the year following initiation/discharge on PP (P=0.0001) (Fig. 2, Table 3). The mean bed days reduced from 38.78/patient/year to a mean of 23.09/patient/year (P=0.0001) (Fig. 3, Table 4). Median bed days in the year following initiation/discharge on PP was 0. Improvements were numerically greater in those continuing on PP compared with those who discontinued (Tables 3 and 4) but statistically nonsignificant (P=0.751 for change in admissions; P=0.708 for change in bed days).
Fig. 1.
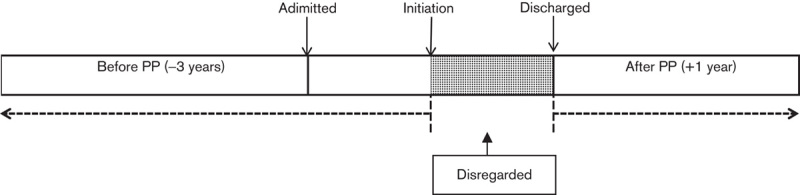
Schematic representation of the mirror image method for primary analysis (inpatients only). PP, paliperidone palmitate.
Fig. 2.
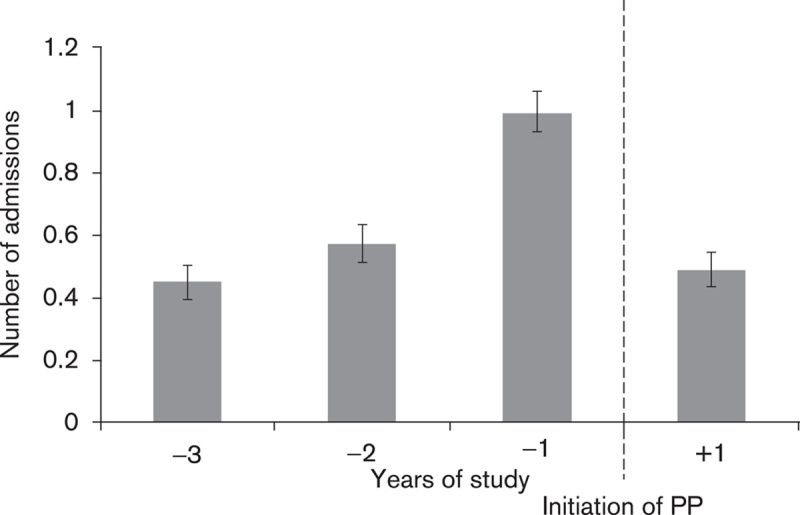
Primary analysis of the mean number of admissions (per patient/year). PP, paliperidone palmitate.
Table 3.
Comparison of number of hospital admissions before and after paliperidone palmitate (primary analysis)
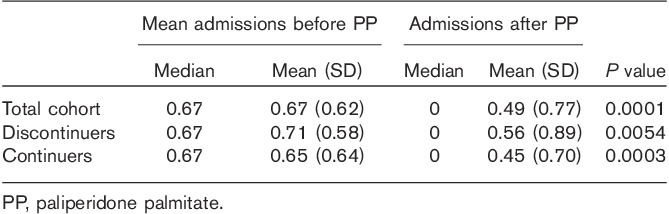
Fig. 3.
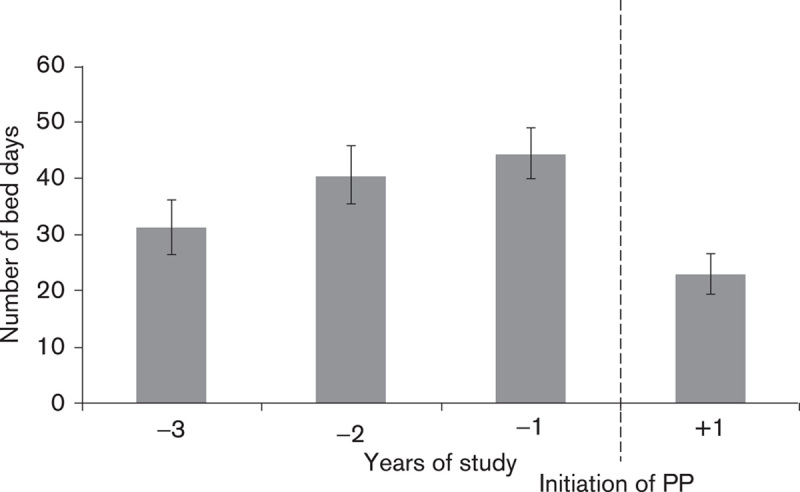
Primary analysis of the mean number of bed days spent in the hospital (per patient/year). PP, paliperidone palmitate.
Table 4.
Comparison of number of hospital bed days before and after paliperidone palmitate (primary analysis)
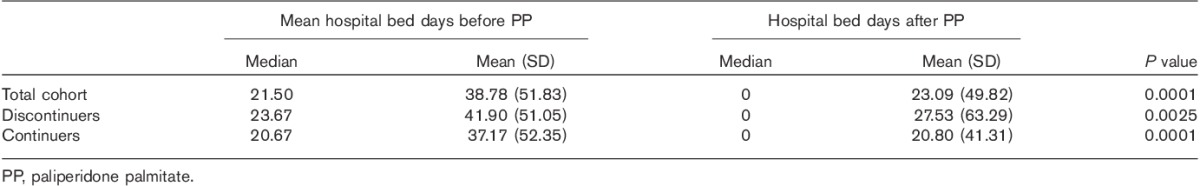
Sensitivity analyses generally suggested smaller benefits for PP. Disregarding all of the index admissions (Fig. 4), admissions for the total cohort fell from 0.53 (SD 0.58)/patient/year to 0.49 (0.77)/patient/year (P=0.014). Bed days reduced from a mean of 35.18 (50.41)/patient/year to 23.09 (49.82)/patient/year (P=0.0001). When the whole of the index admission was included and the ‘mirror’ set at the time of initiation of PP (Fig. 5), admissions fell from 0.67 (0.62)/patient/year to 0.51 (0.76)/patient/year (P=0.0001). Bed days increased from a mean of 38.78 (51.83)/patient/year to 56.75 (78.60)/patient/year (P=0.012).
Fig. 4.
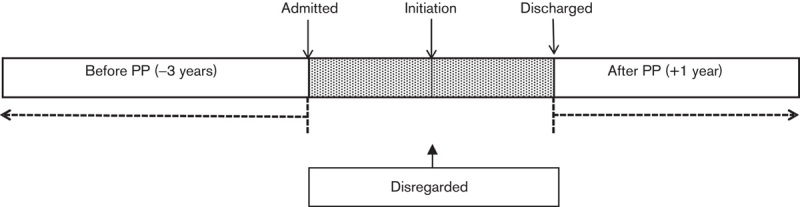
Schematic representation of the mirror image method sensitivity analysis – index admission ignored. PP, paliperidone palmitate.
Fig. 5.
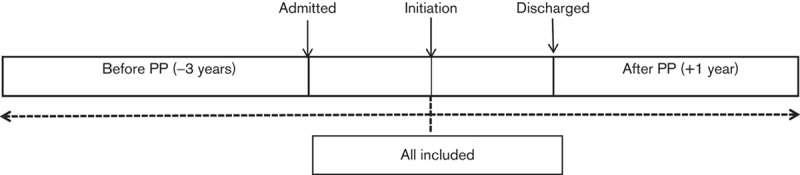
Schematic representation of the mirror image method sensitivity analysis – ‘mirror’ at paliperidone palmitate (PP) initiation for all patients.
Discussion
In this 1-year follow-up of patients prescribed PP in normal clinical practice, the use of PP was associated with an important reduction in hospitalizations and days spent in hospital. PP was continued for a year after initiation in nearly two-thirds of patients and discontinuations because of adverse effects were uncommon. These broadly favorable clinical outcomes are important in determining the place of PP in therapy – a branded product with a much higher purchase cost than generic conventional depots. Our observation that the use of PP reduces bed days by around 16/patient/year strongly supports the view that PP is likely to be cost-effective: its purchase cost (close to £5000/year at maximum dose) is outweighed by savings made on reduced hospitalizations (estimated cost/day of UK psychiatric inpatient care is £338; 16 days is £5408) (Department of Health, 2013).
Our results in this study are in some contrast with our previous observations of outcomes associated with risperidone depot. In a similar study in our unit (Young and Taylor, 2006; Taylor et al., 2009a) the use of risperidone depot was associated with a very substantial increase in bed days in the first year after initiation. After 3 years, those initiated on risperidone depot had spent nearly double the time in hospital than they had spent 3 years before. Only 14% of patients continued for 3 years. In both studies, included patients were broadly representative of our catchment area – including roughly equal numbers of men and women and the majority of patients being of African or African-Caribbean descent.
The improved outcome observed with PP here is unlikely to be a result of pharmacodynamic effects: paliperidone and risperidone have near identical pharmacological properties and risperidone is partly metabolized to paliperidone even after parenteral admission (Castberg and Spigset, 2005; Bowskill et al., 2012). It is more likely that the better outcomes are due to a number of factors combined: the facility to give loading doses of PP; the use of relatively higher equivalent doses with PP (the equivalent of 150 mg a month PP cannot be given with risperidone); and the broadly treatment-responsive nature of the patient cohort in this study [in contrast to the risperidone study (Taylor et al., 2006)]. It is also notable that the lengthy oral supplementation period necessary with risperidone long-acting injection is likely to have a negative effect on bed stay. Another factor might be that prescribers have, over time, become more adept at prescribing atypical long-acting injections and now choose those patients most likely to do well.
Also important in comparing the different results is the method of calculating ‘before’ and ‘after’ outcomes. In the previous risperidone study, we placed the ‘mirror’ at the point of prescription for both outpatients and inpatients. This is the same method as our second sensitivity analysis in this study – an analysis that also showed an increase in bed days, albeit a much smaller one than with risperidone. Our (a priori) primary analysis is, we feel, more clinically realistic. It assumes that the period of hospitalization before PP initiation is a result of failure of the previously prescribed antipsychotic. So, these bed days are allotted to the ‘before’ period. The period between starting PP and discharge from hospital is the time during which PP begins to exert its therapeutic effect after the failure of prior therapy. These bed days are disregarded as it seems unreasonable to allot them to either ‘before’ or ‘after’. Although some might argue that one of our sensitivity analyses might be a better or farer method, it is notable that following discharge on PP the median bed days in the year after this is 0. That is, at least half of the cohort spent no time in hospital in the year after discharge on PP – an important and somewhat unusual finding.
A clear limitation of our observational method is the absence of a control group. We cannot therefore assume or otherwise that PP is any better than any other drug. All we can conclude is that hospitalization outcomes improve after PP initiation. It also follows that improvement in outcomes observed after PP cannot with certainty be attributed to the treatment as baseline trends in bed days and admissions were not established: something a control group would help to clarify. It is possible that changes in hospitalization rates were at least in part linked to changes in admission policies or in the availability of beds. Also, without randomization we cannot discount the likely positive effects of choosing PP for particular patients (presumably responsive patients with a history of poor compliance). Nonetheless, this does mean that our results are a sound reflection of practice, at least in our trust.
Overall, the use of PP in this cohort was broadly successful – the majority completed 1 year on PP and there was a reduction in both hospitalizations and the number of days spent in hospital. PP may thus be cost-effective in practice; its use bringing about cost reductions greater than its purchase cost. Three-year outcomes in our full cohort of over 500 patients will be essential in confirming or otherwise these observations.
Acknowledgements
This study was funded by an unrestricted grant from Janssen UK.
Conflicts of interest
David Taylor received research funding from Eli Lilly, Servier and Janssen and consultancy and/or lecturing honoraria from Servier, Janssen, Roche, AstraZeneca, Lundbeck and Otsuka. For Olubanke Olofinjana there are no conflicts of interest.
References
- Bowskill SV, Handley SA, Fisher DS, Flanagan RJ, Patel MX.Risperidone and total 9-hydroxyrisperidone in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 2002–2010.Ther Drug Monit 2012;34:349–355 [DOI] [PubMed] [Google Scholar]
- Castberg I, Spigset O.Serum concentrations of risperidone and 9-hydroxyrisperidone after administration of the long-acting injectable form of risperidone: evidence from a routine therapeutic drug monitoring service.Ther Drug Monit 2005;27:103–106 [DOI] [PubMed] [Google Scholar]
- Department of Health 2013. Reference costs guidance for 2012–13. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/217040/2012-13-reference-costs-guidance.pdf. [Accessed 9 December 2013]
- Hogarty GE, Schooler NR, Ulrich R, Mussare F, Ferro P, Herron E.Fluphenazine and social therapy in the aftercare of schizophrenic patients: relapse analyses of a two-year controlled study of fluphenazine decanoate and fluphenazine hydrochloride.Arch Gen Psychiatry 1979;36:1283–1294 [DOI] [PubMed] [Google Scholar]
- Leucht S, Pitschel-Walz G, Abraham D, Kissling W.Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials.Schizophr Res 1999;35:51–68 [DOI] [PubMed] [Google Scholar]
- Niaz OS, Haddad PM.Thirty-five months experience of risperidone long-acting injection in a UK psychiatric service including a mirror-image analysis of in-patient care.Acta Psychiatr Scand 2007;116:36–46 [DOI] [PubMed] [Google Scholar]
- Novick D, Haro JM, Bertsch J, Haddad PM.Incidence of extrapyramidal symptoms and tardive dyskinesia in schizophrenia: thirty-six-month results from the European schizophrenia outpatient health outcomes study.J Clin Psychopharmacol 2010;30:531–540 [DOI] [PubMed] [Google Scholar]
- Rosenheck RA, Krystal JH, Lew R, Barnett PG, Fiore L, Valley D, et al. Long-acting risperidone and oral antipsychotics in unstable schizophrenia.N Engl J Med 2011;364:842–851 [DOI] [PubMed] [Google Scholar]
- Rubio G, Martinez I, Ponce G, Jimenez-Arriero MA, Lopez-Munoz F, Alamo C.Long-acting injectable risperidone compared with zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity.Can J Psychiatry 2006;51:531–539 [DOI] [PubMed] [Google Scholar]
- Taylor DM, Young C, Patel MX.Prospective 6-month follow-up of patients prescribed risperidone long-acting injection: factors predicting favourable outcome.Int J Neuropsychopharmacol 2006;9:685–694 [DOI] [PubMed] [Google Scholar]
- Taylor D, Fischetti C, Sparshatt A, Thomas A, Bishara D, Cornelius V.Risperidone long-acting injection: a 6-year mirror-image study of healthcare resource use.Acta Psychiatr Scand 2009a;120:97–101 [DOI] [PubMed] [Google Scholar]
- Taylor DM, Fischetti C, Sparshatt A, Thomas A, Bishara D, Cornelius V.Risperidone long-acting injection: a prospective 3-year analysis of its use in clinical practice.J Clin Psychiatry 2009b;70:196–200 [PubMed] [Google Scholar]
- Taylor M, Currie A, Lloyd K, Price M, Peperell K.Impact of risperidone long acting injection on resource utilization in psychiatric secondary care.J Psychopharmacol 2008;22:128–131 [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P.A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia.Am J Psychiatry 2011;168:603–609 [DOI] [PubMed] [Google Scholar]
- Young CL, Taylor DM.Health resource utilization associated with switching to risperidone long-acting injection.Acta Psychiatr Scand 2006;114:14–20 [DOI] [PubMed] [Google Scholar]


