Abstract
It is known that newer antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs), provide advantages in tolerability over antidepressants such as the tricyclics. However, even within the SSRI class, differences in efficacy or tolerability exist between the individual drugs. Among the three most widely prescribed SSRIs are paroxetine, sertraline, and escitalopram. Escitalopram is commonly referred to as an SSRI, but also has well-documented allosteric properties, and thus can be further classed as an allosteric serotonin reuptake inhibitor. All three antidepressants are efficacious compared with placebo, but there is evidence that escitalopram is more effective than a range of other antidepressants. There are no direct data to regard either paroxetine or sertraline as a superior antidepressant. Escitalopram is superior compared with paroxetine, which has a less favorable tolerability profile. Paroxetine is associated with cholinergic muscarinic antagonism and potent inhibition of CYP2D6, and sertraline has moderate drug interaction issues in comparison with escitalopram. Overall, as an allosteric serotonin reuptake inhibitor that is somewhat different from classical SSRIs, escitalopram is the first choice judged by combined efficacy and tolerability, and nonclinical data have offered possible mechanisms through which escitalopram could be more efficacious, based on its interaction with orthosteric and allosteric binding sites at the serotonin transporter.
Keywords: allosteric, escitalopram (S-citalopram), major depressive disorder, paroxetine, selective serotonin reuptake inhibitor, serotonin, serotonin transporter, sertraline
Introduction
Major depressive disorder (MDD) is among the most prevalent disabling diseases, affecting millions of people around the world. Pharmacotherapy for depression has evolved over the past 30 years. Initially, the main treatments were the tricyclic antidepressants and the monoamine oxidases. Newer antidepressants were approved for use from the late 1980s to the late 2000s, including the selective serotonin (5-HT) reuptake inhibitors (SSRIs) and the serotonin and norepinephrine (NE) reuptake inhibitors (SNRIs). Paroxetine and sertraline were among the first SSRIs to be approved for clinical use and have been available since the beginning of the 1990s (Grimsley and Jann, 1992; Johnson, 1992). Escitalopram, the S-enantiomer of the racemic SSRI citalopram, is the newest marketed SSRI, introduced in 2002. In general, newer antidepressants are better tolerated than the tricyclic antidepressants and monoamine oxidases owing in part to the reduced side effect burden (Gillman, 2007). Numerous direct comparisons in randomized double-blind, controlled clinical studies, pooled analyses, meta-analyses, and reviews have been published comparing the clinical efficacy and tolerability of antidepressants. The SSRIs share the same mechanistic target, the serotonin transporter (SERT), which is responsible for 5-HT uptake into serotonergic neurons (Blakely et al., 1991). Inhibition of 5-HT uptake by an SSRI results in higher extracellular levels of 5-HT and this is considered the basis of their antidepressant activity, although the exact antidepressant mechanism has yet to be elucidated. On the basis of its unique pharmacological characteristics, escitalopram is further classified as an allosteric serotonin reuptake inhibitor (ASRI), as described in the Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines (Lam et al., 2009; Nutt and Feetam, 2010).
According to the classical definition of an SSRI, the selectivity for inhibition of 5-HT uptake is defined relative to the ability of a given drug to inhibit the reuptake of norepinephrine, and SSRIs are often referred to as one drug class based on this definition. However, there is published evidence from preclinical in-vitro and in-vivo pharmacology studies (e.g. Sanchez and Meier, 1997) and clinical efficacy studies (Montgomery et al., 2007; Rao, 2007; Kasper et al., 2009b; Montgomery and Moller, 2009) that would support meaningful differences among SSRIs in their effects. Furthermore, the literature often provides within-discipline comparisons of drugs. This paper reviews potential differences between the clinical, clinical pharmacology, and nonclinical properties of the three most widely prescribed SSRIs, escitalopram, paroxetine, and sertraline, and discusses the potential link between the mechanistic data obtained in nonclinical settings and from clinical trials.
Clinical efficacy and tolerability
Data from placebo-controlled and/or head-to-head comparisons of the ASRI escitalopram versus the SSRIs sertraline and paroxetine are listed in Table 1 and are described below.
Table 1.
Summary of data from clinical studies and meta-analyses comparing the efficacy and tolerability profiles of escitalopram, paroxetine, and sertraline
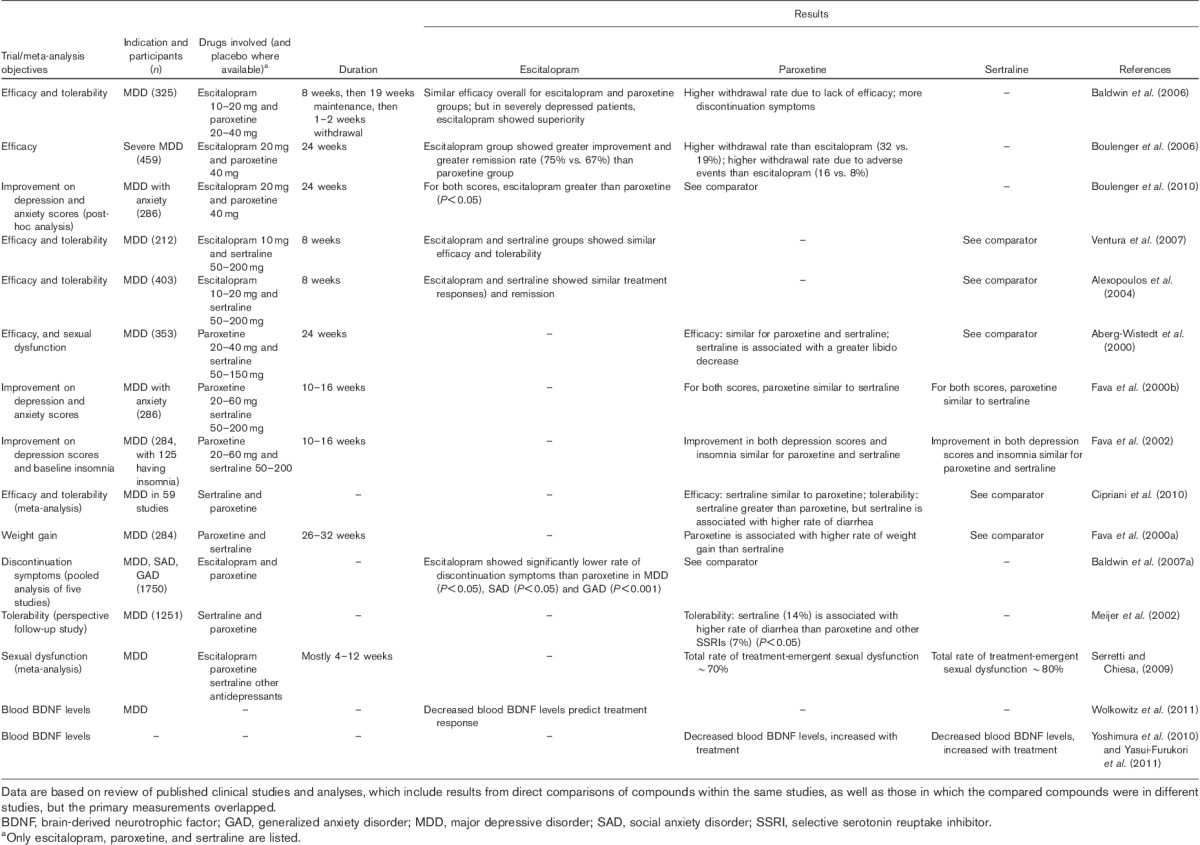
Efficacy: clinical studies with escitalopram and paroxetine
A relapse prevention study of 325 patients conducted with escitalopram and paroxetine included 8 weeks of initial treatment, followed by a 19-week maintenance treatment period and finally a 1–2 week tapered discontinuation period (Baldwin et al., 2006). Overall, withdrawal of patients for lack of efficacy (normally referred to as relapses) was significantly less common on escitalopram than paroxetine (Baldwin et al., 2006). In addition, the paroxetine treatment showed a higher rate of discontinuation symptoms, such as feeling tense, confusion, and nausea, than the escitalopram treatment (Baldwin et al., 2007b).
In a 24-week study with severely depressed patients, escitalopram was more effective than paroxetine at 24 weeks and at 8 weeks at a clinically relevant level as judged by the Montgomery–Åsberg Depression Rating Scale (MADRS) difference of two points as well as by the remitter analysis (Boulenger et al., 2006). In a post-hoc analysis of this study of patients with a high level of anxiety, identified as those with a baseline Hamilton Anxiety Rating Scale (HAM-A) score greater than 20 (n=280) using analysis of covariance, escitalopram treatment showed a significantly greater improvement in both anxiety symptoms (HAM-A score) and depression symptoms (MADRS score) than paroxetine treatment (Boulenger et al., 2010). In this study, the overall rate of withdrawal of patients in the paroxetine group was significantly higher than in the escitalopram group (Boulenger et al., 2010).
In a pooled analysis of two studies, it was shown that at 6 months escitalopram was significantly more effective and had significantly fewer withdrawals than paroxetine (Kasper et al., 2009a). A review found that escitalopram was significantly more effective than citalopram, paroxetine, and duloxetine at a clinically relevant level as judged by the strict criteria of responder analysis difference of 10% or two or more points difference on the MADRS (Montgomery and Moller, 2009). The response rate for escitalopram (74%) was also significantly higher with escitalopram than for these comparators (63%) (Montgomery and Moller, 2009). For long-term treatment, escitalopram (n=394) showed a greater mean treatment difference from baseline than paroxetine (n=383) on the MADRS and Clinical Global Impression (CGI) scores in post-hoc analysis of two trials (Kasper et al., 2009a). In addition, in the subgroup of severely depressed patients, escitalopram demonstrated a significantly greater improvement in efficacy than paroxetine (Kasper et al., 2009a).
Efficacy: clinical studies with escitalopram and sertraline
In an 8-week head-to-head comparison study, escitalopram and sertraline showed similar efficacy, response rates (75 vs. 70%), and rates of withdrawn patients due to adverse events (2 vs. 4%) (Ventura et al., 2007). However, this study may have underestimated the efficacy of escitalopram due to the bias of allowing sertraline to be flexibly dosed compared with the low fixed dose of escitalopram at 10 mg/day. In a placebo-controlled trial of flexibly dosed escitalopram and sertraline in MDD patients, both drugs were well tolerated with similar treatment response (60 and 62%, respectively) and remission rates (46 and 46%, respectively) as compared with placebo (42% response, and 27% remission) after 8 weeks of treatment (Alexopoulos et al., 2004).
Efficacy: clinical studies with sertraline and paroxetine
In a 24-week MDD study of continuation therapy (n=353 patients), sertraline and paroxetine showed a similar very low recurrence rate, as assessed by the MADRS, the CGI, and the Battelle Quality of Life Questionnaire (Aberg-Wistedt et al., 2000). In another MDD study, the subgroup with at least moderate depressive severity and high anxiety (n=108) at baseline, treatment with paroxetine, sertraline, or fluoxetine for 10–16 weeks resulted in similar outcomes, as measured by improvement in Hamilton Depression Rating Scale (HAM-D) scores, response rates, and remission (Fava et al., 2000b). The efficacies of paroxetine and sertraline were also compared in a head-to-head study (fluoxetine was also included in the study; n=284 depressed patients) (Fava et al., 2002). After 10–16 weeks of treatment, improvement in depression and insomnia symptoms was similar for all three groups, as measured by the HAM-D (Fava et al., 2002). It should be noted that these two studies seem underpowered for a valid conclusion, and the study duration may not be ideal for observing either acute effects or long-term efficacy.
Efficacy: meta-analyses
Overall, escitalopram, sertraline, and paroxetine are all efficacious as compared with placebo, as found in the meta-analysis of 35 trials reported from 1980 to 2011 involving 142 drug–placebo comparisons, which showed computed relative response rate ratios to placebo of 1.33, 1.33, and 1.44, respectively (Undurraga and Baldessarini, 2012). Escitalopram has been compared with other antidepressants including paroxetine and sertraline extensively in meta-analyses. Based on an analysis of 10 studies involving a total of 2687 MDD patients up to 2004, escitalopram was found to have significantly higher overall treatment effect (estimated difference in treatment effect of 1.07 points), response rate (odds ratio 1.29), and remission rate (odds ratio 1.21) compared with all comparators including paroxetine and sertraline (Kennedy et al., 2006). In a follow-up meta-analysis comparing escitalopram with active controls including SSRIs (citalopram, fluoxetine, paroxetine, sertraline) and SNRIs (venlafaxine, duloxetine) involving 4549 patients in 16 randomized controlled trials, escitalopram was again found to be significantly more effective than comparators in treatment effect (measured as change from baseline in MADRS total score), as well as in the rates of response and remission (Kennedy et al., 2009). The results suggest the overall superior efficacy of escitalopram compared with paroxetine and sertraline as well as other SSRIs and SNRIs, though the superiority to other SSRIs was to the largest degree between escitalopram and citalopram (Kennedy et al., 2009), a difference that has been well established (Montgomery et al., 2011). In a recent meta-analysis of 10 antidepressants including paroxetine and sertraline for their remission rates, escitalopram was reported to have the most favorable treatment effect, with a remission probability of 0.47 after an 8- to 12-week treatment (Ramsberg et al., 2012). Another indirect (rather than using pooled raw data) meta-analysis of 12 newer-generation antidepressants involved in 117 randomized controlled trials concluded that the odds ratios on efficacy (escitalopram vs. paroxetine, 1.3; sertraline vs. paroxetine, 1.2) and tolerability (escitalopram vs. paroxetine, 1.3; sertraline vs. paroxetine, 1.25) profiles significantly favored escitalopram and sertraline compared with those of paroxetine (Cipriani et al., 2009). Meta-analyses for sertraline or paroxetine, however, did not find any superiority to each other or to escitalopram on efficacy (Thase et al., 2005; Cipriani et al., 2010). In the meta-analysis based on results reported from 234 studies between 1980 and 2011, Gartlehner et al. (2011) also found similar response rates for paroxetine and sertraline (odds ratio 1.02). In addition, a statistically significant odds ratio (1.49) for escitalopram compared with citalopram and numerical advantages for escitalopram in comparison with paroxetine (odds ratio 0.78) and sertraline (odds ratio 0.8) in treatment response rate were reported.
In general, results from individual well-designed and adequately powered randomized controlled trials should have priority in both scientific and regulatory settings, whereas meta-analyses are always post hoc and regarded as carrying less weight. An antidepressant is considered superior in efficacy if there are two or more double-blind studies where it is significantly better on the primary efficacy measure than a marketed antidepressant under conditions of fair comparison. Escitalopram has met this criterion with seven studies, but neither sertraline nor paroxetine was able to rely on a single study and therefore cannot be considered superior (Montgomery et al., 2007). For example, when the efficacies of the newer drugs were compared, escitalopram (23.7%) ranked higher than sertraline (20.3%) (Cipriani et al., 2009).
Tolerability: escitalopram, paroxetine, and sertraline
A comprehensive literature search of randomized controlled clinical studies found that about 60% of patients experienced at least one adverse event during treatment with an antidepressant. Overall, the newer-generation antidepressants had similar tolerability profiles, with the types of adverse events usually including diarrhea, dizziness, dry mouth, fatigue, headache, nausea, sexual dysfunction, sweating, tremor, and weight gain (Cipriani et al., 2010). As concluded by a meta-analysis reviewing 117 randomized controlled trials involving 25 928 participants and 12 newer-generation antidepressants, escitalopram and sertraline showed a superior profile of tolerability, with significantly fewer discontinuations of patients than other antidepressants, including paroxetine (Cipriani et al., 2009). In addition, a meta-analysis showed a considerably higher incidence of treatment-emergent sexual dysfunction for sertraline (∼80%) than for escitalopram (∼40%) (Serretti and Chiesa, 2009). This is in agreement with escitalopram having the highest cumulative probability of being among the four best treatments in terms of acceptability in a recent review: escitalopram (27.6%), sertraline (21.3%), and paroxetine (0.2%) (Cipriani et al., 2009).
Compared with other SSRIs, a higher incidence of adverse effects was indicated for paroxetine treatment, including sedation, constipation, sexual dysfunction, discontinuation syndrome, weight gain, and congenital malformations, in a review of head-to-head studies (Marks et al., 2008). A review of tolerability based on data from randomized controlled clinical trials involving about 4000 patients with short-term and long-term treatments indicated that paroxetine was associated with significantly higher incidence of adverse events related to sexual dysfunction, as well as more discontinuation symptoms, than escitalopram (Baldwin et al., 2007b). In general, these findings are consistent with a recent review on the overall profile of paroxetine (Gibiino and Serretti, 2012).
On the basis of a head-to-head comparative study of MDD patients (n=284) treated with sertraline or paroxetine, the paroxetine group showed a significantly higher weight gain (measured as the proportion of patients with a weight increase of >7% from baseline) than the sertraline group (Fava et al., 2000a). A pooled analysis of five studies in MDD, social anxiety disorder, and generalized anxiety disorder patients showed that discontinuation of escitalopram treatment resulted in significantly lower rates of discontinuation symptoms than paroxetine and venlafaxine XR in MDD (P<0.05), and also showed lower rates than paroxetine in social anxiety disorder (P<0.05) and generalized anxiety disorder (P<0.001) (Baldwin et al., 2007a). Diarrhea is another common adverse event worth noting for antidepressants. In an earlier study, in which 659 patients were randomized to treatment with sertraline and 592 patients to other SSRIs (paroxetine, fluoxetine or fluvoxamine), the rates of other adverse events were similar for all four drugs, but the incidence of diarrhea was higher with sertraline (14%) than with the other SSRIs (7%) (Meijer et al., 2002). Consistent with this, a recent meta-analysis found that sertraline was indeed associated with a higher incidence of diarrhea than comparator drugs (including paroxetine) (Cipriani et al., 2010). The review by Gartlehner et al., (2011) further supports these differences by showing that paroxetine had a higher incidence of sexual dysfunction compared with escitalopram and sertraline, and sertraline was associated with higher incidence of diarrhea than paroxetine (average rates 16 vs. 8%).
Mechanisms related to efficacy and tolerability
Clinical pharmacokinetics
Some basic pharmacokinetic and pharmacodynamic properties of escitalopram, paroxetine, and sertraline are compared in Table 2. In general, the three antidepressants produce good absorption, distribution, and clearance profiles at their therapeutic doses. Escitalopram is approved at clinical dosages of 10 and 20 mg (with 5 mg in certain subpopulations or as starting dose), and when taken orally reaches Tmax in 5 h, is 56% protein bound, and reaches steady-state concentration in the blood within 1–2 weeks (Sogaard et al., 2005; Rao, 2007; Spina et al., 2012). Paroxetine is approved at clinical dosages of 12.5, 25, 37.5, and 50 mg daily, and when taken orally reaches Tmax in 6–10 h, is 95% protein bound, and reaches steady-state concentration in the blood within two weeks (Hiemke and Hartter, 2000; Bourin et al., 2001). Sertraline is approved at higher clinical dosages, that is with 50 mg daily up to 200 mg daily for certain subpopulations, and when taken orally sertraline reaches Tmax in 5–9 h, is highly protein bound (99%), and reaches steady-state concentration in the blood within 1 week (Hiemke and Hartter, 2000; MacQueen et al., 2001; DeVane et al., 2002). Frequently, treatment with sertraline or paroxetine needs to be titrated by the physician to obtain the optimal dose for the individual patient.
Table 2.
The pharmacokinetic and pharmacodynamic properties of escitalopram, paroxetine, and sertraline
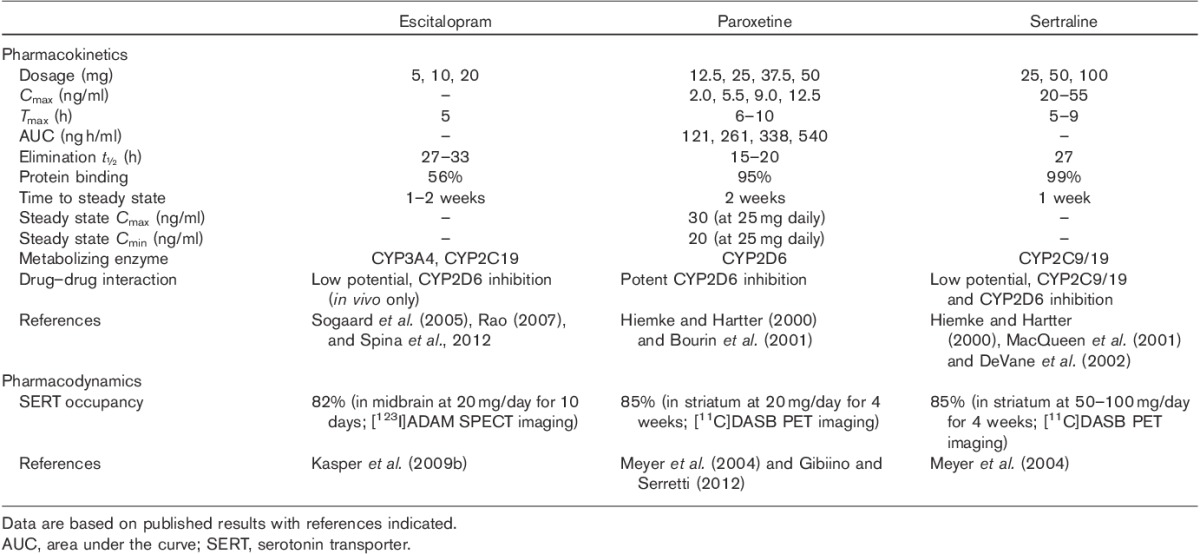
Aspects of drug–drug interactions provide clinically relevant differences between escitalopram, paroxetine, and sertraline (Hiemke and Hartter, 2000). The three cytochrome P450 (CYP) isoenzymes, CYP1A2, CYP2D6, and CYP3A4, are responsible for the metabolism of most drugs; thus, drugs with inhibitory activities at any of the three CYPs may be prone to drug–drug interactions. Escitalopram is metabolized in parallel by at least two CYP enzymes, CYP3A4 and CYP2C19 (and to a lesser extent by CYP2D6), and has little inhibitory action against other CYP enzymes or P-glycoprotein (Rao, 2007), thus having a low potential for drug–drug interactions. As shown in Table 2, paroxetine is a potent inhibitor of CYP2D6 and is the SSRI most likely to cause drug–drug interactions (Richelson, 2001). Sertraline can inhibit CYP2C9/19 and CYP2D6 but to a lesser degree than paroxetine, and thus has a lower likelihood of causing drug–drug interactions (Richelson, 2001). Thus, escitalopram may be superior to paroxetine and sertraline in this regard.
Pharmacological mechanisms related to clinical efficacy and tolerability
The primary target mediating the therapeutic actions of escitalopram, paroxetine, and sertraline is the SERT, and all three drugs have very high affinity at the SERT (Table 3). Paroxetine has the highest affinity at the SERT, whereas escitalopram has the highest degree of selectivity (i.e. >1000-fold relative to a large number of receptors and neurotransmitter transporters) as compared with paroxetine (>200-fold) and sertraline (>60-fold). In the clinical imaging studies mentioned above, the three antidepressants all bind to SERT at their therapeutic doses in humans, with occupancy of ∼80%.
Table 3.
The in-vitro pharmacological profiles of escitalopram, paroxetine, and sertraline
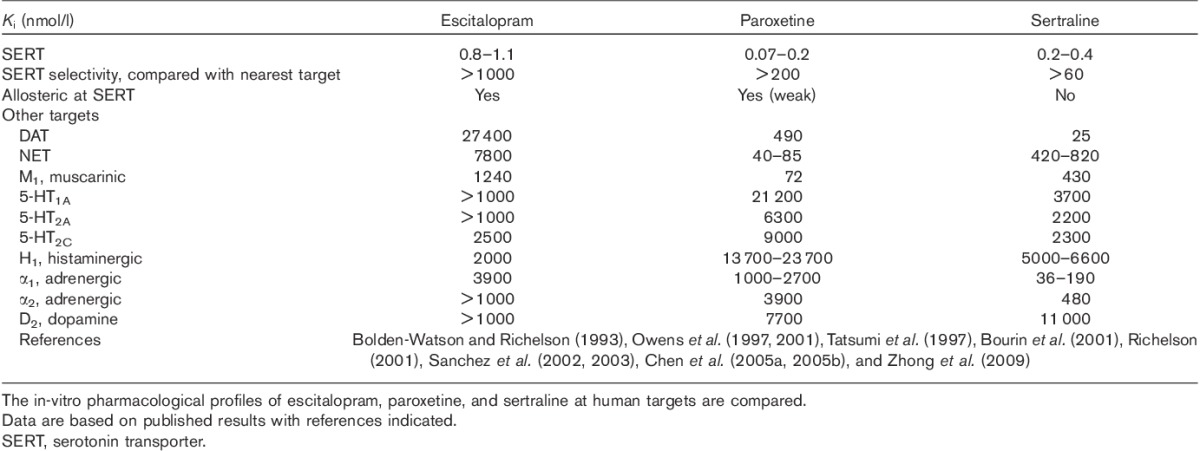
The clinical trial data, in head-to-head comparisons and in meta-analyses and as described in literature reviews, have shown higher efficacy for escitalopram and sertraline treatment of depression than paroxetine, with data also showing that escitalopram is associated with higher efficacy compared with other SSRIs. The efficacy of escitalopram may at least in part be ascribed to its actions at allosteric sites of the SERT (Chen et al., 2005a, 2005b; Sanchez, 2006; Nutt and Feetam, 2010; Zhong et al., 2009, 2012a, 2012b). The SERT has two types of binding site, the orthosteric binding site (also referred to as the primary site) to which escitalopram and other SSRIs bind, resulting in inhibition of its uptake function, and one or more allosteric sites (Chen et al., 2005a, 2005b; Sanchez, 2006). Many studies have led to the thorough characterization of the allosteric mechanism of escitalopram (Wennogle and Meyerson, 1982; Plenge and Mellerup, 1997; Chen et al., 2005a, 2005b), although other compounds have also been reported to have allosteric activities at the SERT but are less well characterized (Nandi et al., 2004; Nightingale et al., 2005; Boos et al., 2006).
In binding experiments with the SERT, the allosteric activity of escitalopram is characterized by its ability to prolong its own dissociation kinetics (Chen et al., 2005a, 2005b; Sanchez, 2006). By binding to both the orthosteric and allosteric binding sites, escitalopram elicits a more complete and sustained inhibition of 5-HT uptake, leading to higher extracellular 5-HT levels in vivo and faster 5-HT1A autoreceptor desensitization, as reviewed previously (Sanchez et al., 2004, 2006). Additional elucidation of this mechanism includes in-vitro as well as in-vivo studies demonstrating that specific mutations in the SERT disrupt the allosteric effect of escitalopram, and that R-citalopram, a less active enantiomer of citalopram (citalopram is also an antidepressant), inhibits the efficacy of escitalopram (Zhong et al., 2012a, 2012b). This makes escitalopram the only SERT-related antidepressant that shows dual allosteric and chiral advantages (El Mansari et al., 2007; Nutt and Feetam, 2010; Zhong et al., 2012a, 2012b). Thus, even though escitalopram was derived from the SSRI citalopram, it is further referred as an ASRI (Lam et al., 2009; Zhong et al., 2012a, 2012b), and these molecular interactions are depicted in Fig. 1. As noted in Table 3, paroxetine is also allosteric, but its allosteric effect is weaker (Chen et al., 2005b; Sanchez, 2006). In comparison, sertraline and many other antidepressants (e.g. fluoxetine, duloxetine, and venlafaxine) do not have allosteric activities at the SERT (Fig. 1b) (Chen et al., 2005a, 2005b).
Fig. 1.
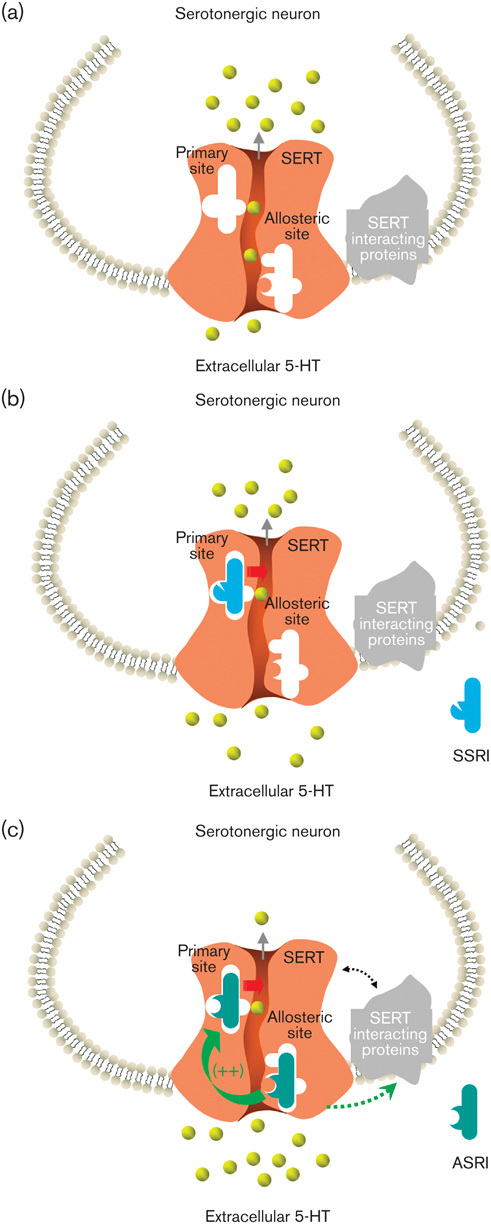
A putative model showing that escitalopram, paroxetine, and sertraline interact with the primary (orthosteric) and allosteric binding sites at the SERT leading to differential increases in extracellular 5-HT levels. In each diagram, the SERT is shown to be located at serotonergic neurons and to have the primary and allosteric binding sites. (a) In the absence of inhibitor drugs, the SERT performs its transport function, which removes extracellular 5-HT; (b) SSRIs such as sertraline are not able to bind to the allosteric site, and thus their action in increasing extracellular 5-HT levels is only mediated through the primary site; (c) ASRIs such as escitalopram bind to both the primary and the allosteric sites. Allosteric site binding enhances their binding to the primary site, resulting in more pronounced increases in extracellular 5-HT levels and potentially signaling through SERT-interacting proteins (SIPs) (Sanchez et al., 2004; Zhong et al., 2012a, 2012b). ASRI, allosteric serotonin reuptake inhibitor; SERT, serotonin transporter; SSRI, selective serotonin reuptake inhibitor. Drawing is based on previously published diagrams by Zhong et al. (2012a), with permission.
It is worth noting that for the SSRIs fluoxetine and paroxetine, enantiomers have also been studied. The different ability of escitalopram, paroxetine, and sertraline in increasing extracellular levels of 5-HT in relation to SERT occupancy in the rat brain has been demonstrated, which indicates that the allosteric property of escitalopram may translate to physiological conditions (Brennum et al., 2004). As shown in Fig. 2, extracellular 5-HT levels were measured in the ventral hippocampus of freely moving rats by means of microdialysis, and related to occupancy at the SERT using [3H]citalopram binding. At escitalopram, paroxetine, and sertraline doses of 0.5, 0.3, and 3.1 mg/kg, respectively, which corresponds to 88–92% SERT occupancy, the increase in extracellular 5-HT levels was the largest for escitalopram, followed by paroxetine and sertraline (Fig. 2a). From comparisons of the relationships between 5-HT level and SERT occupancy, it appears that escitalopram produces a higher extracellular 5-HT level than paroxetine and sertraline at the same SERT occupancy (Fig. 2b). For example, to achieve a 250% increase in extracellular levels of 5-HT, SERT occupancy needs to be 70, 83, and 95% for escitalopram, paroxetine, and sertraline, respectively (Fig. 2b). These differences do not reflect the in-vitro SERT inhibitory potency rank order (Table 3) and potentially support that there is an additional site of action (presumably an allosteric site) that mediates the more efficacious uptake inhibition by escitalopram, in addition to binding to the orthosteric site of the SERT.
Fig. 2.
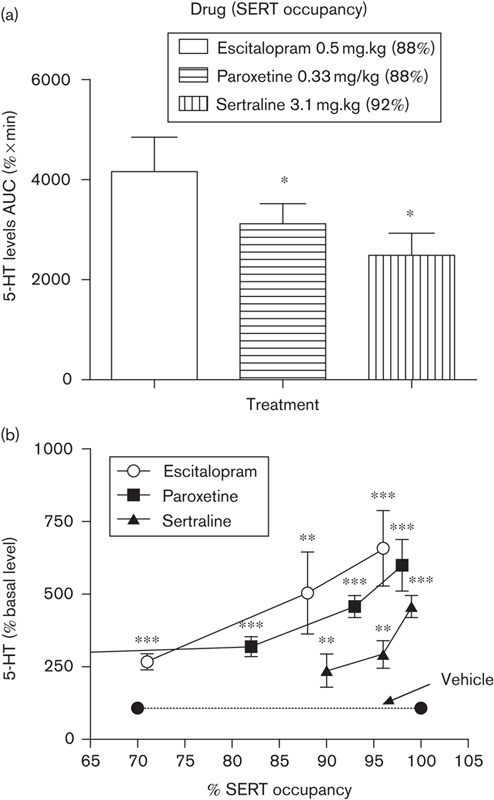
Increase in extracellular levels of 5-HT by escitalopram, paroxetine, and sertraline in relation to SERT occupancy in the rat. The ability of escitalopram, paroxetine, and sertraline to increase 5-HT levels in rat prefrontal cortex via SERT inhibition is shown. Rats in the microdialysis experiments were anesthetized and the drugs were administered by the subcutaneous route. SERT occupancy was measured by in-vivo binding using [3H]citalopram as radioligand. (a) Different 5-HT levels in the rat prefrontal cortex after treatment with escitalopram 0.5 mg/kg (n=8), paroxetine 0.33 mg/kg (n=7), and sertraline 3.1 mg/kg (n=6) to achieve 88–92% occupancies of the SERT. Data shown are averaged 5-HT levels by AUC (%×min); *P<0.05 compared with escitalopram. (b) Differential 5-HT level vs. SERT occupancy relationships for escitalopram, paroxetine, and sertraline. Data shown are averaged 5-HT levels as percentages of baseline; **P<0.01, ***P<0.001 compared with vehicle (Brennum et al., 2004). AUC, area under the curve; SERT, serotonin transporter.
In clinical studies, the level of SERT occupancy during chronic SSRI treatment studied by PET using the radioligand [11C]N,N-dimethyl-2-(2-amino-4-cyanophenylthio) benzylamine ([11C]DASB) suggested that a SERT occupancy of ∼80% is necessary to achieve therapeutic effects of SSRI treatment and higher doses plateaued right above this range (Meyer et al., 2004). Thus, at higher doses of SSRIs, such as sertraline and citalopram, a maximal of 85% occupancy was achieved (Voineskos et al., 2007). Similar findings were seen with escitalopram using a selective radioligand 2-([2-([dimethylamino]methyl)phenyl]thio)-5-[123I]-iodophenylamine ([123I]ADAM) in single-photon emission computerized tomography studies, in which a maximal 82% SERT occupancy was identified (Kasper et al., 2009b). Thus, due to the plateau in SERT occupancy seen for these antidepressants, higher doses are thought to be unable to further increase efficacy, but rather to incur additional side effects, which may contribute to higher discontinuation rates (Preskorn, 2012). On the basis of the above preclinical observations, it may be hypothesized that the increase in extracellular 5-HT induced by escitalopram might be higher in humans than for paroxetine and sertraline, even though there is an ∼80% plateau of SERT occupancy.
Although it is clear that the primary target of escitalopram, paroxetine, and sertraline is the SERT, the precise cellular and physiological changes following uptake inhibition that mediate their antidepressant actions are poorly understood. It takes antidepressants, including the SSRIs, 1–2 weeks to produce their therapeutic effect, probably because slower neuroadaptive and neurochemical changes in the brain following the elevation of 5-HT levels are required for the therapeutic effect (Blier and de Montigny, 1999; Zhong et al., 2012a). For example, the recovery of raphe 5-HT neuronal firing after the desensitization of 5-HT1A autoreceptors is thought to reflect the neuroadaptive process underlying the delayed onset of antidepressant action (Blier and de Montigny, 1999; El Mansari et al., 2005). For escitalopram, it takes 2 weeks before 5-HT neuronal firing returns to control levels in rats, but for most SSRIs, it takes at least 3 weeks, suggesting a faster onset of action for escitalopram, possibly due to its action at the allosteric site (El Mansari et al., 2005; Mnie-Filali et al., 2007). This is consistent with the indication of escitalopram having a faster clinical onset than other SSRIs (Lepola et al., 2004; Kasper et al., 2006; Wade and Andersen, 2006). Among other neurochemical changes during antidepressant treatment, the neurotropin brain-derived neurotrophic factor (BDNF) was recently reviewed (Zhong et al., 2012a). As a potential biomarker, BDNF shows decreased levels in the blood of depressed patients and this can predict treatment response for escitalopram, paroxetine, and sertraline (Yoshimura et al., 2010; Wolkowitz et al., 2011; Yasui-Furukori et al., 2011). Thus, neurotropins such as BDNF might hold key insights associated with the neuroadaptive and neurochemical changes during antidepressant treatment, which may help differentiate the actions of SSRIs. Further studies in this area are warranted.
Pharmacological mechanisms beyond SERT inhibition and putative functional relevance
Although it is believed that the therapeutic effects of escitalopram, paroxetine, and sertraline are mediated through their actions at the SERT, some side effects also can be explained by their off-target effects at other transporters and receptors (Richelson, 2001, 2003). The activities of escitalopram, paroxetine, and sertraline at some of these targets, such as the adrenergic α1, histamine H1, and cholinergic muscarinic M1 receptors, and the dopamine (DA) transporter (DAT), are listed in Table 3.
It is worth mentioning the antagonistic activity of paroxetine at cholinergic M1 muscarinic receptors (Ki=72 nmol/l in comparison with Ki’s of 430 and 1240 nmol/l for sertraline and escitalopram), sertraline’s DAT inhibitory activity (Ki=25 nmol/l in comparison with Ki’s of 490 and 27 400 nmol/l for paroxetine and escitalopram), and paroxetine’s NE transporter (NET) inhibitory activity (Ki=40–85 nmol/l in comparison with Ki’s of 420–820 and 7800 nmol/l for sertraline and escitalopram) (Table 3). Potencies of this order of magnitude may be potentially meaningful at clinical exposure levels. Thus, commonly reported adverse effects of paroxetine are symptoms of sedation, constipation, and visual disturbance, which could be ascribed to anticholinergic activity (Pae and Patkar, 2007). Indeed, paroxetine has considerable potency for muscarinic receptors, allowing it to affect these receptors at the blood levels expected during treatment (Table 2). A study in mice, in which the anticholinergic effects of paroxetine were measured using oxotremorine-induced tremor, spontaneous defecation, and passive avoidance performance tests, also supports the notion of paroxetine having anticholinergic activity in vivo (Fujishiro et al., 2002). It was found that paroxetine induced more anticholinergic effects than fluvoxamine (another SSRI), although its effects were lower than those of a tricyclic clomipramine, as expected (Fujishiro et al., 2002). In a comparative study of escitalopram and paroxetine, the anticholinergic activity was assessed as blockade of hypothermia induced by the muscarinic agonist oxotremorine (Fig. 3a). Oxotremorine caused dose-dependent hypothermia, which was prevented by paroxetine but not escitalopram (Fig. 3a), demonstrating the anticholinergic activity of paroxetine. The role of dopamine reuptake inhibition (DAT activity) was also measured as stimulation of spontaneous locomotor activity (Fig. 3b and c). Sertraline produced a significant increase in the spontaneous locomotor activity compared with vehicle controls at doses close to those that produce 5-HT reuptake inhibition, that is, the minimal effective dose of 2.2 mg/kg corresponds to ∼89% SERT occupancy in mice (Sanchez, 2002; Larsen et al., 2004), whereas paroxetine and escitalopram were devoid of this effect, even at much higher doses (Fig. 3b and c). In line with these behavioral observations, Kitaichi et al. (2010) reported that sertraline, unlike paroxetine and fluvoxamine, increases extracellular DA in nucleus accumbens and striatum in freely moving rats (Kitaichi et al., 2010). It is difficult to predict the functional net effect of this combined SERT and DAT inhibition, as there is a high degree of functional connectivity between the monoaminergic neurotransmitter systems, but sertraline may potentially differ from an SSRI that is devoid of DAT inhibition. Thus, in the dorsal raphe nucleus, activation of dopaminergic D2 receptors increases whereas activation of serotonergic 5-HT1A receptors decreases the activity of 5-HT neurons. In the ventral tegmental area, activation of D2 receptors or 5-HT2C receptors decreases the activity of DA neurons (Alex and Pehek, 2007).
Fig. 3.
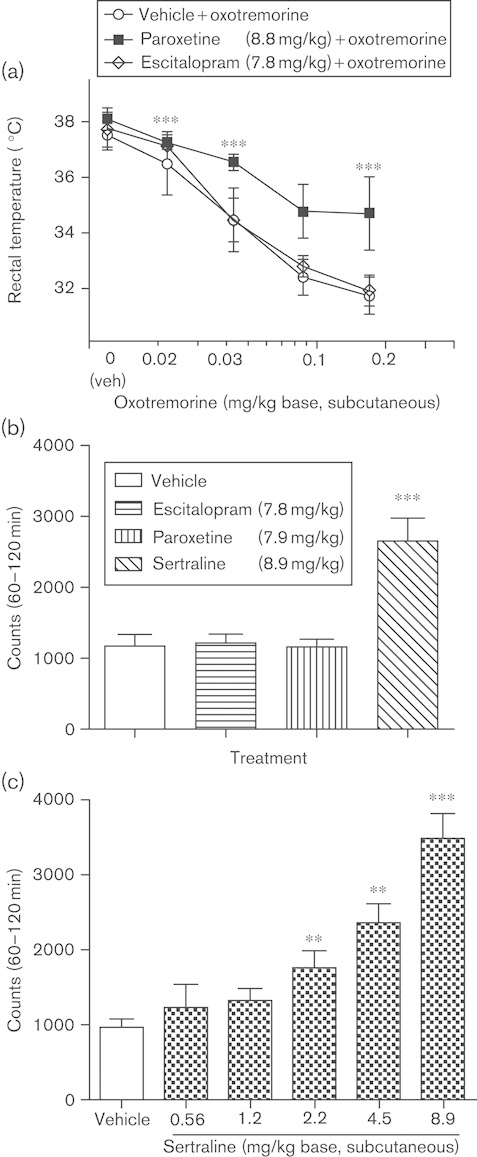
In-vivo measurements of the effects of escitalopram, paroxetine, and sertraline on muscarinic cholinergic and DAT activities in mice. The anticholinergic and DAT-inhibiting effects of escitalopram, paroxetine, and sertraline are shown in oxotremorine-induced hypothermia (a) and spontaneous locomotor activity (b, c) in mice. (a) The role of muscarinic cholinergic antagonism is assessed as antagonism of hypothermia induced by the muscarinic agonist oxotremorine. The test was conducted at room temperature and started at 11 a.m. Drug or vehicle was injected subcutaneously 30 min before oxotremorine. The rectal temperature was measured before drug and oxotremorine administration and after 30 min. Data were analyzed by analysis of variance; ***P<0.001 compared with vehicle+oxotremorine. (b) The role of dopamine reuptake inhibition by a single dose of escitalopram, paroxetine, or sertraline was assessed as stimulation of spontaneous locomotor activity. The test was conducted in cages equipped with infrared light sources and photocells and the number of light beam interruptions was used as measure of locomotor activity. The mice were placed individually in the test cages and were habituated for 30 min before administration of drug. The accumulated number of light beam interruptions recorded 60–120 min after drug administration was used as the measure of drug effect; ***P<0.001 compared with vehicle. (c) Multiple doses of sertraline were assessed for stimulation of spontaneous locomotor activity as in (b). Data were analyzed by analysis of variance; **P<0.01, ***P<0.001 compared with vehicle (Sanchez, 2002).
NE reuptake inhibition was assessed as antagonism of tetrabenazine-induced ptosis in mice, and paroxetine showed NE reuptake-inhibiting activity at doses close to those that produced 5-HT reuptake inhibition (Sanchez, 2002). Tetrabenazine is a monoamine-depleting agent producing immobility and ptosis. The latter effect is mediated by alpha adrenoceptors and has been shown to be reversed by compounds with NE reuptake inhibitory activities (Arnt et al., 1985). Despite inhibiting NET activities, paroxetine is still grouped in the class of SSRIs, as no clinical data support any comparable or superior SNRI features with paroxetine. In contrast, even though escitalopram does not have any noticeable activity on the NET, it seems to have advantages when compared with SNRIs in the treatment of MDD patients. For example, escitalopram was found to be associated with significantly lower duration of sick leave compared with duloxetine during treatment (Wade et al., 2008), and it may also have a better efficacy and tolerability profile than the SNRIs venlafaxine and duloxetine as second-step treatment for MDD (Lam et al., 2010).
Extrapyramidal side effects (EPS) have been discussed in association with SSRI treatment. A literature review of 89 cases of EPS associated with antidepressant treatment, including tremor, akathisia, dystonia, dyskinesia, and tardive dyskinesia, suggests relatively low occurrence rates for escitalopram (7%) and sertraline (10%) in comparison with other antidepressants (Madhusoodanan et al., 2010). Direct comparison analyses in clinical trials do not indicate a risk of EPS for escitalopram or sertraline (Baldwin et al., 2007b; Ventura et al., 2007; Cipriani et al., 2010). The exact mechanism for development of EPS is not fully understood, although it is generally accepted that dysfunction in dopaminergic transmission of the nigrostriatal pathway plays a key role (Glazer, 2000; Tuppurainen et al., 2010). Reduced DA transmission in the form of DA receptor blockade by antipsychotic treatment in schizophrenia is frequently manifested by the side effects of EPS and hyperprolactinemia, since dopamine exerts a potent and tonic inhibition of prolactin secretion under normal conditions (Kane, 2011). In a study of 159 patients on different medications, 27 cases (17%) of hyperprolactinemia were reported after SSRI treatment, and the occurrence was the highest for sertraline followed by paroxetine and other antidepressants (Petit et al., 2003). However, a more recent review of spontaneous reports suggested that paroxetine, but not sertraline or escitalopram, was associated with a higher risk of hyperprolactinemia (Trenque et al., 2011). An earlier analysis with the identification of 61 spontaneous reports concluded that SSRI use seems to be only moderately associated with EPS compared with other antidepressants, and suggests that patients with an already compromised dopaminergic function may be more susceptible (Schillevoort et al., 2002).
Conclusion
Escitalopram, paroxetine, and sertraline have well-established efficacy and tolerability profiles based on decades of clinical use as some of the most widely prescribed antidepressants. Although these antidepressants belong to the same general class (SSRIs) and all have demonstrated therapeutic efficacy, differences exist with respect to efficacy and tolerability, as shown by head-to-head comparisons and meta-analyses. There are studies demonstrating the superiority of escitalopram compared with paroxetine as well as a combined group of various SSRIs including paroxetine and sertraline. Paroxetine’s cholinergic muscarinic antagonism and potent inhibition of CYP2D6 may have an impact on its tolerability. Although sertraline has moderate drug–drug interaction issues, its DAT inhibitory properties may result in a different pharmacodynamic profile. Therefore, when compared with paroxetine and sertraline, escitalopram as an ASRI different from classical SSRIs consistently shows advantages in efficacy and tolerability profiles, and nonclinical data have offered possible mechanisms through which escitalopram could be more efficacious based on its interaction with orthosteric and allosteric binding sites at the SERT.
Acknowledgements
The authors thank Dr Huailing Zhong of U-Pharm Laboratories LLC (Parsippany, NJ) for insightful and valuable help with writing the manuscript and Dr David Simpson for helpful insights and comments.
Conflicts of interest
Connie Sanchez and Elin H. Reines are full-time employees of H. Lundbeck A/S. Stuart A. Montgomery has received consulting fees or honoraria from AstraZeneca, Bionevia, Bristol–Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Lilly, H. Lundbeck A/S, Merck & Co. Inc., M’s Science, Merz Pharmaceuticals, Neurim Pharmaceuticals, Otsuka, Pfizer Inc., Pierre Fabre, Roche Pharmaceuticals, Sanofi-Aventis, Sepracor Inc., Servier Laboratories, Synosis, Takeda, Theracos, Transcept, UBC, Xytis, and Wyeth.
References
- Aberg-Wistedt A, Agren H, Ekselius L, Bengtsson F, Akerblad AC.Sertraline versus paroxetine in major depression: clinical outcome after six months of continuous therapy.J Clin Psychopharmacol 2000;20:645–652 [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA.Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission.Pharmacol Ther 2007;113:296–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos G, Gordon J, Zhang D.A placebo-controlled trial of escitalopram and sertraline in the treatment of major depressive disorder.Neuropsychopharmacology 2004;29SupplS87 [Google Scholar]
- Arnt J, Christensen AV, Hyttel J.Pharmacology in vivo of the phenylindan derivative, Lu 19-005, a new potent inhibitor of dopamine, noradrenaline and 5-hydroxytryptamine uptake in rat brain.Naunyn Schmiedebergs Arch Pharmacol 1985;329:101–107 [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Cooper JA, Huusom AK, Hindmarch I.A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder.Int Clin Psychopharmacol 2006;21:159–169 [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Montgomery SA, Nil R, Lader M.Discontinuation symptoms in depression and anxiety disorders.Int J Neuropsychopharmacol 2007a;10:73–84 [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Reines EH, Guiton C, Weiller E.Escitalopram therapy for major depression and anxiety disorders.Ann Pharmacother 2007b;41:1583–1592 [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC.Cloning and expression of a functional serotonin transporter from rat brain.Nature 1991;354:66–70 [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C.Serotonin and drug-induced therapeutic responses in major depression, obsessive–compulsive and panic disorders.Neuropsychopharmacology 1999;21:91S–98S [DOI] [PubMed] [Google Scholar]
- Bolden-Watson C, Richelson E.Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes.Life Sci 1993;52:1023–1029 [DOI] [PubMed] [Google Scholar]
- Boos TL, Greiner E, Calhoun WJ, Prisinzano TE, Nightingale B, Dersch CM, et al. Structure-activity relationships of substituted N-benzyl piperidines in the GBR series: synthesis of 4-(2-(bis(4-fluorophenyl)methoxy)ethyl)-1-(2-trifluoromethylbenzyl)piperidine, an allosteric modulator of the serotonin transporter.Bioorg Med Chem 2006;14:3967–3973 [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Huusom AK, Florea I, Baekdal T, Sarchiapone M.A comparative study of the efficacy of long-term treatment with escitalopram and paroxetine in severely depressed patients.Curr Med Res Opin 2006;22:1331–1341 [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Hermes A, Huusom AK, Weiller E.Baseline anxiety effect on outcome of SSRI treatment in patients with severe depression: escitalopram vs paroxetine.Curr Med Res Opin 2010;26:605–614 [DOI] [PubMed] [Google Scholar]
- Bourin M, Chue P, Guillon Y.Paroxetine: a review.CNS Drug Rev 2001;7:25–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennum LT, Sanchez C, Larsen AK, Cremers TI, Kreilgaard M, Thomsen C.Escitalopram, paroxetine and sertraline, do they differ in their ability to inhibit serotonin transport? What can we learn from pre-clinical models?Eur Neuropsychopharmacol 2004;14Suppl 3S385 [Google Scholar]
- Chen F, Larsen MB, Neubauer HA, Sanchez C, Plenge P, Wiborg O.Characterization of an allosteric citalopram-binding site at the serotonin transporter.J Neurochem 2005a;92:21–28 [DOI] [PubMed] [Google Scholar]
- Chen F, Larsen MB, Sanchez C, Wiborg O.The S-enantiomer of R,S-citalopram, increases inhibitor binding to the human serotonin transporter by an allosteric mechanism. Comparison with other serotonin transporter inhibitors.Eur Neuropsychopharmacol 2005b;15:193–198 [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis.Lancet 2009;373:746–758 [DOI] [PubMed] [Google Scholar]
- Cipriani A, La FT, Furukawa TA, Signoretti A, Nakagawa A, Churchill R, et al. Sertraline versus other antidepressive agents for depression.Cochrane Database Syst Rev 2010CD006117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVane CL, Liston HL, Markowitz JS.Clinical pharmacokinetics of sertraline.Clin Pharmacokinet 2002;41:1247–1266 [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sanchez C, Chouvet G, Renaud B, Haddjeri N.Effects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: an in vivo electrophysiological study in rat brain.Neuropsychopharmacology 2005;30:1269–1277 [DOI] [PubMed] [Google Scholar]
- El Mansari M, Wiborg O, Mnie-Filali O, Benturquia N, Sanchez C, Haddjeri N.Allosteric modulation of the effect of escitalopram, paroxetine and fluoxetine: in-vitro and in-vivo studies.Int J Neuropsychopharmacol 2007;10:31–40 [DOI] [PubMed] [Google Scholar]
- Fava M, Judge R, Hoog SL, Nilsson ME, Koke SC.Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long-term treatment.J Clin Psychiatry 2000a;61:863–867 [DOI] [PubMed] [Google Scholar]
- Fava M, Rosenbaum JF, Hoog SL, Tepner RG, Kopp JB, Nilsson ME.Fluoxetine versus sertraline and paroxetine in major depression: tolerability and efficacy in anxious depression.J Affect Disord 2000b;59:119–126 [DOI] [PubMed] [Google Scholar]
- Fava M, Hoog SL, Judge RA, Kopp JB, Nilsson ME, Gonzales JS.Acute efficacy of fluoxetine versus sertraline and paroxetine in major depressive disorder including effects of baseline insomnia.J Clin Psychopharmacol 2002;22:137–147 [DOI] [PubMed] [Google Scholar]
- Fujishiro J, Imanishi T, Onozawa K, Tsushima M.Comparison of the anticholinergic effects of the serotonergic antidepressants, paroxetine, fluvoxamine and clomipramine.Eur J Pharmacol 2002;454:183–188 [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, Van NM, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis.Ann Intern Med 2011;155:772–785 [DOI] [PubMed] [Google Scholar]
- Gibiino S, Serretti A.Paroxetine for the treatment of depression: a critical update.Expert Opin Pharmacother 2012;13:421–431 [DOI] [PubMed] [Google Scholar]
- Gillman PK.Tricyclic antidepressant pharmacology and therapeutic drug interactions updated.Br J Pharmacol 2007;151:737–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer WM.Extrapyramidal side effects, tardive dyskinesia, and the concept of atypicality.J Clin Psychiatry 2000;61Suppl 316–21 [PubMed] [Google Scholar]
- Grimsley SR, Jann MW.Paroxetine, sertraline, and fluvoxamine: new selective serotonin reuptake inhibitors.Clin Pharm 1992;11:930–957 [PubMed] [Google Scholar]
- Hiemke C, Hartter S.Pharmacokinetics of selective serotonin reuptake inhibitors.Pharmacol Ther 2000;85:11–28 [DOI] [PubMed] [Google Scholar]
- Johnson AM.Paroxetine: a pharmacological review.Int Clin Psychopharmacol 1992;6Suppl 415–24 [PubMed] [Google Scholar]
- Kane JM.Addressing side effects from antipsychotic treatment in schizophrenia.J Clin Psychiatry 2011;72:e07. [DOI] [PubMed] [Google Scholar]
- Kasper S, Spadone C, Verpillat P, Angst J.Onset of action of escitalopram compared with other antidepressants: results of a pooled analysis.Int Clin Psychopharmacol 2006;21:105–110 [DOI] [PubMed] [Google Scholar]
- Kasper S, Baldwin DS, Larsson LS, Boulenger JP.Superiority of escitalopram to paroxetine in the treatment of depression.Eur Neuropsychopharmacol 2009a;19:229–237 [DOI] [PubMed] [Google Scholar]
- Kasper S, Sacher J, Klein N, Mossaheb N, Attarbaschi-Steiner T, Lanzenberger R, et al. Differences in the dynamics of serotonin reuptake transporter occupancy may explain superior clinical efficacy of escitalopram versus citalopram.Int Clin Psychopharmacol 2009b;24:119–125 [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Andersen HF, Lam RW.Efficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: a meta-analysis.J Psychiatry Neurosci 2006;31:122–131 [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Andersen HF, Thase ME.Escitalopram in the treatment of major depressive disorder: a meta-analysis.Curr Med Res Opin 2009;25:161–175 [DOI] [PubMed] [Google Scholar]
- Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, Koyama T.Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats.Eur J Pharmacol 2010;647:90–96 [DOI] [PubMed] [Google Scholar]
- Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy.J Affect Disord 2009;117Suppl 1S26–S43 [DOI] [PubMed] [Google Scholar]
- Lam RW, Lonn SL, Despiegel N.Escitalopram versus serotonin noradrenaline reuptake inhibitors as second step treatment for patients with major depressive disorder: a pooled analysis.Int Clin Psychopharmacol 2010;25:199–203 [DOI] [PubMed] [Google Scholar]
- Larsen AK, Brennum LT, Egebjerg J, Sanchez C, Halldin C, Andersen PH.Selectivity of (3)H-MADAM binding to 5-hydroxytryptamine transporters in vitro and in vivo in mice; correlation with behavioural effects.Br J Pharmacol 2004;141:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepola U, Wade A, Andersen HF.Do equivalent doses of escitalopram and citalopram have similar efficacy? A pooled analysis of two positive placebo-controlled studies in major depressive disorder.Int Clin Psychopharmacol 2004;19:149–155 [DOI] [PubMed] [Google Scholar]
- MacQueen G, Born L, Steiner M.The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders.CNS Drug Rev 2001;7:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusoodanan S, Alexeenko L, Sanders R, Brenner R.Extrapyramidal symptoms associated with antidepressants – a review of the literature and an analysis of spontaneous reports.Ann Clin Psychiatry 2010;22:148–156 [PubMed] [Google Scholar]
- Marks DM, Park MH, Ham BJ, Han C, Patkar AA, Masand PS, Pae CU.Paroxetine: safety and tolerability issues.Expert Opin Drug Saf 2008;7:783–794 [DOI] [PubMed] [Google Scholar]
- Meijer WE, Heerdink ER, van Eijk JT, Leufkens HG.Adverse events in users of sertraline: results from an observational study in psychiatric practice in the Netherlands.Pharmacoepidemiol Drug Saf 2002;11:655–662 [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study.Am J Psychiatry 2004;161:826–835 [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, Faure C, El Mansari M, Lambas-Senas L, Berod A, Zimmer L, et al. R-citalopram prevents the neuronal adaptive changes induced by escitalopram.Neuroreport 2007;18:1553–1556 [DOI] [PubMed] [Google Scholar]
- Montgomery S, Hansen T, Kasper S.Efficacy of escitalopram compared to citalopram: a meta-analysis.Int J Neuropsychopharmacol 2011;14:261–268 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Baldwin DS, Blier P, Fineberg NA, Kasper S, Lader M, et al. Which antidepressants have demonstrated superior efficacy? A review of the evidence.Int Clin Psychopharmacol 2007;22:323–329 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Moller HJ.Is the significant superiority of escitalopram compared with other antidepressants clinically relevant?Int Clin Psychopharmacol 2009;24:111–118 [DOI] [PubMed] [Google Scholar]
- Nandi A, Dersch CM, Kulshrestha M, Ananthan S, Rothman RB.Identification and characterization of a novel allosteric modulator (SoRI-6238) of the serotonin transporter.Synapse 2004;53:176–183 [DOI] [PubMed] [Google Scholar]
- Nightingale B, Dersch CM, Boos TL, Greiner E, Calhoun WJ, Jacobson AE, et al. Studies of the biogenic amine transporters. XI. Identification of a 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine (GBR12909) analog that allosterically modulates the serotonin transporter.J Pharmacol Exp Ther 2005;314:906–915 [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Feetam CL.What one hand giveth the other taketh away: some unpredicted effects of enantiomers in psychopharmacology.J Psychopharmacol 2010;24:1137–1141 [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB.Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites.J Pharmacol Exp Ther 1997;283:1305–1322 [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB.Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine.Biol Psychiatry 2001;50:345–350 [DOI] [PubMed] [Google Scholar]
- Pae CU, Patkar AA.Paroxetine: current status in psychiatry.Expert Rev Neurother 2007;7:107–120 [DOI] [PubMed] [Google Scholar]
- Petit A, Piednoir D, Germain ML, Trenque T.Drug-induced hyperprolactinemia: a case-non-case study from the national pharmacovigilance database.Therapie 2003;58:159–163 [DOI] [PubMed] [Google Scholar]
- Plenge P, Mellerup ET.An affinity-modulating site on neuronal monoamine transport proteins.Pharmacol Toxicol 1997;80:197–201 [DOI] [PubMed] [Google Scholar]
- Preskorn SH.The use of biomarkers in psychiatric research: how serotonin transporter occupancy explains the dose–response curves of SSRIs.J Psychiatr Pract 2012;18:38–45 [DOI] [PubMed] [Google Scholar]
- Ramsberg J, Asseburg C, Henriksson M.Effectiveness and cost-effectiveness of antidepressants in primary care: a multiple treatment comparison meta-analysis and cost-effectiveness model.PLoS One 2012;7:e42003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N.The clinical pharmacokinetics of escitalopram.Clin Pharmacokinet 2007;46:281–290 [DOI] [PubMed] [Google Scholar]
- Richelson E.Pharmacology of antidepressants.Mayo Clin Proc 2001;76:511–527 [DOI] [PubMed] [Google Scholar]
- Richelson E.Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance.J Clin Psychiatry 2003;64Suppl 135–12 [PubMed] [Google Scholar]
- Sanchez C.Escitalopram, a super selective serotonin reuptake inhibitor – in vivo.Nord J Psychiatry 2002;56:115–115 [Google Scholar]
- Sanchez C.The pharmacology of citalopram enantiomers: the antagonism by R-citalopram on the effect of S-citalopram.Basic Clin Pharmacol Toxicol 2006;99:91–95 [DOI] [PubMed] [Google Scholar]
- Sanchez C, Meier E.Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike?Psychopharmacology (Berl) 1997;129:197–205 [DOI] [PubMed] [Google Scholar]
- Sanchez C, Larsen AK, Gupta S.Escitalopram, the most selective serotonin reuptake inhibitor – in vitro data.Nord J Psychiatry 2002;56:115 [Google Scholar]
- Sanchez C, Bergqvist PB, Brennum LT, Gupta S, Hogg S, Larsen A, Wiborg O.Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities.Psychopharmacology (Berl) 2003;167:353–362 [DOI] [PubMed] [Google Scholar]
- Sanchez C, Bogeso KP, Ebert B, Reines EH, Braestrup C.Escitalopram versus citalopram: the surprising role of the R-enantiomer.Psychopharmacology (Berl) 2004;174:163–176 [DOI] [PubMed] [Google Scholar]
- Schillevoort I, van Puijenbroek EP, de BA, Roos RA, Jansen PA, Leufkens HG.Extrapyramidal syndromes associated with selective serotonin reuptake inhibitors: a case–control study using spontaneous reports.Int Clin Psychopharmacol 2002;17:75–79 [DOI] [PubMed] [Google Scholar]
- Serretti A, Chiesa A.Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis.J Clin Psychopharmacol 2009;29:259–266 [DOI] [PubMed] [Google Scholar]
- Sogaard B, Mengel H, Rao N, Larsen F.The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects.J Clin Pharmacol 2005;45:1400–1406 [DOI] [PubMed] [Google Scholar]
- Spina E, Trifiro G, Caraci F.Clinically significant drug interactions with newer antidepressants.CNS Drugs 2012;26:39–67 [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E.Pharmacological profile of antidepressants and related compounds at human monoamine transporters.Eur J Pharmacol 1997;340:249–258 [DOI] [PubMed] [Google Scholar]
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, et al. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials.J Clin Psychiatry 2005;66:974–981 [DOI] [PubMed] [Google Scholar]
- Trenque T, Herlem E, Auriche P, Drame M.Serotonin reuptake inhibitors and hyperprolactinaemia: a case/non-case study in the French pharmacovigilance database.Drug Saf 2011;34:1161–1166 [DOI] [PubMed] [Google Scholar]
- Tuppurainen H, Kuikka JT, Viinamaki H, Husso M, Tiihonen J.Extrapyramidal side-effects and dopamine D(2/3) receptor binding in substantia nigra.Nord J Psychiatry 2010;64:233–238 [DOI] [PubMed] [Google Scholar]
- Undurraga J, Baldessarini RJ.Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review.Neuropsychopharmacology 2012;37:851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura D, Armstrong EP, Skrepnek GH, Haim EM.Escitalopram versus sertraline in the treatment of major depressive disorder: a randomized clinical trial.Curr Med Res Opin 2007;23:245–250 [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Wilson AA, Boovariwala A, Sagrati S, Houle S, Rusjan P, et al. Serotonin transporter occupancy of high-dose selective serotonin reuptake inhibitors during major depressive disorder measured with [11C]DASB positron emission tomography.Psychopharmacology (Berl) 2007;193:539–545 [DOI] [PubMed] [Google Scholar]
- Wade A, Andersen HF.The onset of effect for escitalopram and its relevance for the clinical management of depression.Curr Med Res Opin 2006;22:2101–2110 [DOI] [PubMed] [Google Scholar]
- Wade AG, Fernandez JL, Francois C, Hansen K, Danchenko N, Despiegel N.Escitalopram and duloxetine in major depressive disorder: a pharmacoeconomic comparison using UK cost data.Pharmacoeconomics 2008;26:969–981 [DOI] [PubMed] [Google Scholar]
- Wennogle LP, Meyerson LR.Serotonin modulates the dissociation of [3H]imipramine from human platelet recognition sites.Eur J Pharmacol 1982;86:303–307 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Wolf J, Shelly W, Rosser R, Burke HM, Lerner GK, et al. Serum BDNF levels before treatment predict SSRI response in depression.Prog Neuropsychopharmacol Biol Psychiatry 2011;35:1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui-Furukori N, Tsuchimine S, Nakagami T, Fujii A, Sato Y, Tomita T, et al. Association between plasma paroxetine concentration and changes in plasma brain-derived neurotrophic factor levels in patients with major depressive disorder.Hum Psychopharmacol 2011;26:194–200 [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Ikenouchi-Sugita A, Hori H, Umene-Nakano W, Hayashi K, Katsuki A, et al. Blood levels of brain-derived neurotrophic factor (BDNF) in major depressive disorder.Seishin Shinkeigaku Zasshi 2010;112:982–985 [PubMed] [Google Scholar]
- Zhong H, Hansen KB, Boyle NJ, Han K, Muske G, Huang X, et al. An allosteric binding site at the human serotonin transporter mediates the inhibition of escitalopram by R-citalopram: kinetic binding studies with the ALI/VFL-SI/TT mutant.Neurosci Lett 2009;462:207–212 [DOI] [PubMed] [Google Scholar]
- Zhong H, Haddjeri N, Sanchez C.Escitalopram, an antidepressant with an allosteric effect at the serotonin transporter – a review of current understanding of its mechanism of action.Psychopharmacology (Berl) 2012a;219:1–13 [DOI] [PubMed] [Google Scholar]
- Zhong H, Sanchez C, Caron MG.Consideration of allosterism and interacting proteins in the physiological functions of the serotonin transporter.Biochem Pharmacol 2012b;83:435–442 [DOI] [PubMed] [Google Scholar]


