Abstract
The objective of this study was to investigate the prevalence of acetylcholinesterase inhibitor (AChEI) and memantine use, duration of treatment, concomitant use of these drugs, and factors associated with the discontinuation of AChEI therapy during 2006–2009. We utilized data from a nationwide sample of community-dwelling individuals with a clinically verified Alzheimer’s disease diagnosed during the year 2005 (n=6858) as a part of the MEDALZ-2005 study. During the 4-year follow-up, 84% used AChEI and 47% used memantine. Altogether, 22% of the sample used both drugs concomitantly. The median duration of the first AChEI use period was 860 (interquartile range 295–1458) days and 1103 (interquartile range 489–1487) days for the total duration of AChEI use. Although 20% of the AChEI users discontinued the use during the first year, over half of them restarted later. The risk of discontinuation was higher for rivastigmine [hazard ratio 1.34 (confidence interval 1.22–1.48)] and galantamine users [hazard ratio 1.23 (confidence interval 1.15–1.37)] compared with donepezil users in the adjusted model. In conclusion, median time for AChEI use was over 3 years and every fifth Alzheimer’s disease patient used AChEI and memantine concomitantly during the follow-up. The low rate of discontinuation is consistent with the Finnish Care Guideline but in contrast to the results reported from many other countries.
Keywords: acetylcholinesterase inhibitor, Alzheimer’s disease, drug utilization, memantine, prevalence
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, accounting for 60–70% of all dementia cases (Fratiglioni et al., 2000; World Health Organization and Alzheimer’s Disease International, 2012). With population aging, the number of cases with AD is rapidly increasing, which poses challenges to the health care system (Alzheimer Association, 2011; World Health Organization and Alzheimer’s Disease International, 2012). AD is characterized by the progressive loss of cholinergic neurons in the central nervous system (Francis et al., 1999). Acetylcholinesterase inhibitors (AChEIs) increase acetylcholine levels by preventing degradation of acetylcholine (Martorana et al., 2010). AChEIs are used to enhance or stabilize cognition, enhance performance in the activities of daily living, improve the quality of life, and to reduce the behavioral symptoms (Geldmacher, 2003; Standridge, 2004). One important reason for their use is that AChEIs may delay institutionalization (Geldmacher, 2003; Beusterien et al., 2004; Standridge, 2004; Lopez et al., 2009), although their effectiveness for delaying admission to long-term care has been questionable (Courtney et al., 2004). Memantine reduces increased glutaminergic activation associated with AD and is used for behavioral and psychological symptoms of dementia (Ballard and Corbett, 2010; Martorana et al., 2010). In some studies, combination treatment with AChEIs and memantine has been reported to be more efficacious than AChEI use alone (Standridge, 2004; Lachaine et al., 2011; Gauthier and Molinuevo, 2013).
The efficacy of AChEIs has been demonstrated in randomized controlled trials (RCTs) (Ritchie et al., 2004; Standridge, 2004). However, real-life users may differ from the participants of RCTs, and thus be more likely to experience adverse drug events, low effectiveness, and challenges in drug dosing and adherence to therapy. In a Canadian study, fewer than half of the donepezil users would have been eligible to participate in RCTs (Gill et al., 2004). RCTs are often short in duration (3–6 months) and there is lack of evidence and guidelines on how long AChEIs should be used for and how to evaluate beneficial treatment effects in clinical practice (Thompson et al., 2004; Birks, 2006; Rodda et al., 2009). Thus, the effectiveness cannot be directly extrapolated from the efficacy estimates of the clinical trials.
The Finnish Clinical Care Guideline for cognitive disorders recommends that cognitive problems stated by the patient should be further examined and referred to specialist care (Finnish Medical Society Duodecim, Helsinki, 2010). Diagnostic procedure of cognitive disorders includes interview, memory and laboratory tests, and computed tomography or MRI scan. If the diagnosis is AD or the including features of AD, treatment with antidementia drugs should be initiated. AChEIs are recommended as the first-line treatment for AD and if there is a contraindication for AChEI use, the treatment may be started with memantine. The objective of antidementia drug use is the stabilization of cognitive function, maintenance of functioning in the activities of daily living, and relieving the behavioral symptoms. Concomitant use of AChEIs and memantine is suggested for the treatment of AD in severe stage.
There are differences between the Finnish system and the reimbursement policy or guidance in many other countries. The National Institute for Health and Clinical Excellence (NICE) in the UK recommends AChEI drugs only for the treatment of mild-to-moderate AD, and treatment of severe AD should be continued only if there is effect on the cognitive, functional, or behavioral symptoms (National Institute for Health and Clinical Excellence, 2011). Similarly, AChEIs are recommended for treatment of mild-to-moderate AD in Sweden (National Board of Health and Welfare, 2010). Some insurance schemes have restrictions for reimbursement of AChEIs (van den Bussche et al., 2011), whereas the French guideline does not recommend the concomitant use of AChEIs and memantine (French National Authority for Health, 2008; Tifratene et al., 2012). Thus, although the Finnish system requires exact diagnoses before reimbursement is granted, the use of antidementia drugs after this is less restricted than in many other countries.
The Finnish Special Reimbursement Register allows identification of individuals diagnosed with AD and linkage to data on prescription drug use and hospital admissions. Together with tax-supported public health service covering all citizens, Finland provides an excellent opportunity to describe the use of antidementia drugs among real-life drug users in a nationwide cohort.
Objectives
We investigated the duration of AChEI and memantine use during the 4-year follow-up period among all community-dwelling individuals diagnosed with AD in 2005 in Finland. We also analyzed the prevalence of AChEI use, concomitant use of AChEIs and memantine, and factors associated with discontinuation of AChEI therapy.
Methods
Cohort and data sources
This study utilized data from the MEDALZ-2005 (medication use and AD) cohort consisting of all community-dwelling individuals with verified diagnoses of AD residing in Finland and alive on 31 December 2005 (Tolppanen et al., 2013). For each individual with AD, a comparison individual matched with age (±1 year), sex, and region of residence, without AD was identified from the register including all residents of Finland who are entitled to benefits by the Social Insurance Institution (SII). The MEDALZ-2005 cohort consists of 28 093 individuals with AD and their 28 093 comparison individuals (n=56 186). Follow-up data are available until the end of 2009.
Individuals with probable AD need a verified diagnosis completed according to the predefined criteria and monitored by the SII to be entitled to reimbursed antidementia drugs (Tolppanen et al., 2013). The diagnosis is applied from the SII by the attending physician, and diagnosis is recorded in the Special Reimbursement Register maintained by the SII. This register includes data on entitlement to special reimbursement due to several chronic diseases that have been diagnosed by a physician. Diagnosis of AD was based on the NINCDS-ADRDA (McKhann et al., 1984) and DSM-IV (American Psychiatric Association, 1994) criteria for AD, including computed tomography or MRI scan and confirmation of the diagnosis by a neurologist or geriatrician. The special reimbursement for AD medication is not withdrawn when the disease progresses to a severe stage, and thus MEDALZ-2005 cohort includes individuals with all stages of AD.
Data of MEDALZ-2005 cohort have been linked to several nationwide registers (Tolppanen et al., 2013). Data have been collected from the Prescription Register, Special Reimbursement Register, Hospital Discharge Register (Hilmo), and Register of Care at Social Institutions. The Prescription Register, maintained by the SII, includes information on all prescription purchases of all reimbursed drugs, but drugs used in hospitals or nursing homes are not included. Drugs are classified according to the Anatomical Therapeutic Chemical classification system (ATC) (WHO Collaborating Centre for Drug Statistics Methodology, 2013). In addition, we have data on date of death and the date of institutionalization to a facility that provides drugs to the residents (instead of the SII).
Data from these registers are collected utilizing person identification numbers, which have been used since 1972 in Finland. The data were deidentified before submission to the research team, and no ethics committee approval was required as only deidentified data were used and the study participants were not contacted.
For the study of AChEI and memantine use, we analyzed data of individuals who received diagnosis of AD during the year 2005. Altogether, 7217 community-dwelling individuals were diagnosed with AD in 2005 and were alive on 31 December 2005. We excluded those who were in long-term hospital or institutional care on 1 January 2006 (n=359), and thus study population consisted of 6858 individuals diagnosed with AD. The beginning of follow-up was defined as the date of diagnoses for each participant. Follow-up ended on 31 December 2009 with long-term hospitalization/institutionalization or death, whichever came first. Long-term hospitalization/institutionalization was determined as at least 90 days stay because in hospital care and in public nursing homes drugs are provided by the hospital or institution and not recorded in the Prescription Register.
Duration of drug use
A previously utilized method was used to construct drug use periods from drug purchases recorded in the Prescription Register data (Tiihonen et al., 2009; Purhonen et al., 2012). Our method is based on mathematical modeling of drug use taking account of regularity of purchases as well as hospitalizations and nursing home stays because drug use during hospital or public nursing home stay is not recorded in the register. The method was used to calculate when continuous drug use started and ended (named drug use periods) for each individual and for each drug (ATC code). The drug use periods were defined from individual purchase histories utilizing purchased amount in defined daily doses, which is recorded for each purchase in the Prescription Register. Purchases were joined to the same drug use period, if the purchased amount of drug was enough to last to the next purchase with calculated personal daily dose allowing some stockpiling and taking into account the personal purchase regularity and possible hospitalizations. Thus, drug use was determined as discontinued if the purchased amount of drug was not enough to last to next purchase. When an individual had bought the drug only once during the follow-up, the typical refill time of the package was used. This refill time was calculated from drug use periods of individuals using the particular drug package. Thus, single purchase was deemed to last according to the typical purchasing behavior in the study population.
Drug use model is based on the calculation of drug use periods for each ATC code. First, the use of AChEIs was modeled separately for donepezil, rivastigmine, and galantamine (ATC codes N06DA02, N06DA03, N06DA04, respectively). Drug use periods for different AChEIs were combined to retrieve the use of ‘any AChEI’ (use of N06DA). During any AChEI use period, individuals were allowed to switch between different AChEIs (if there were no breaks in drug availability). All analyses of AChEI use were completed with combined drug use periods. The use of memantine was modeled as use of ATC code N06DX01. Concomitant use of AChEIs and memantine was studied as use of any AChEI (N06DA) and memantine (N06DX01). Concomitant use (instead of changing from one drug group to another) was defined as overlapping time of at least 60 days. Discontinuation of drug use was defined as the end of drug use on the basis of individual purchase histories or break in drug availability between two consecutive purchases, taking into account changing dose, adherence to drug use, and stockpiling of drugs.
Covariates
Diagnostic data were extracted from the Special Reimbursement Register. A modified Charlson’s comorbidity score (Charlson et al., 1987) was calculated for each participant at the index date (i.e. date of diagnosis of AD). Modified index was calculated using the following diseases with corresponding scores: heart failure, coronary artery disease, diabetes type I or type II, chronic asthma or chronic obstructive pulmonary disease, disseminated connective tissue diseases, rheumatoid arthritis, and other comparable conditions (score of 1); uremia requiring dialysis, severe anemia in connection with chronic renal failure, leukemia and other malignant diseases of blood and bone marrow including malignant diseases of the lymphatic system, and cancer including breast and prostate cancers, female genital tract cancer, and malignant neoplasms (score of 2). We also constructed variable ‘any cardiovascular disease’, which indicates diagnoses of coronary artery disease, chronic heart failure, hypertension, chronic arrhythmias, or hypercholesterolemia.
Analyses
Time to discontinuation of therapy from the first AChEI drug use period was assessed by Kaplan–Meier analysis. Factors associated with the first AChEI discontinuation event were investigated with Cox proportional hazards model. In both analyses, participants were censored at the end of follow-up (31 December 2009), death or long-term hospitalization/institutionalization whichever came first, and whichever event was the first discontinuation of AChEI use. Individuals who switched between AChEI drugs during the continuous treatment were excluded from these analyses. The beginning of follow-up for the Cox regression analyses was the first purchase of AChEI drug. Unadjusted analyses were conducted by studying each covariate and time to discontinuation separately. Adjusted multivariable Cox model included all covariates. Covariates were categorized as they did not present normal distribution, and included age (<80 vs. ≥80 years), sex, modified Charlson’s comorbidity index score (0 vs. 1 vs. ≥2), and initial choice of AChEI drug. Subanalysis was conducted to determine whether introduction of generic donepezil explained the differences in the risk of discontinuation. The first prescriptions of generic donepezil were dispensed during May 2008 and subanalysis was restricted to time period without generic AChEI drugs in market. All statistical analyses were carried out with SAS 9.3 (SAS Institute, Cary, North Carolina, USA).
Results
Baseline characteristics
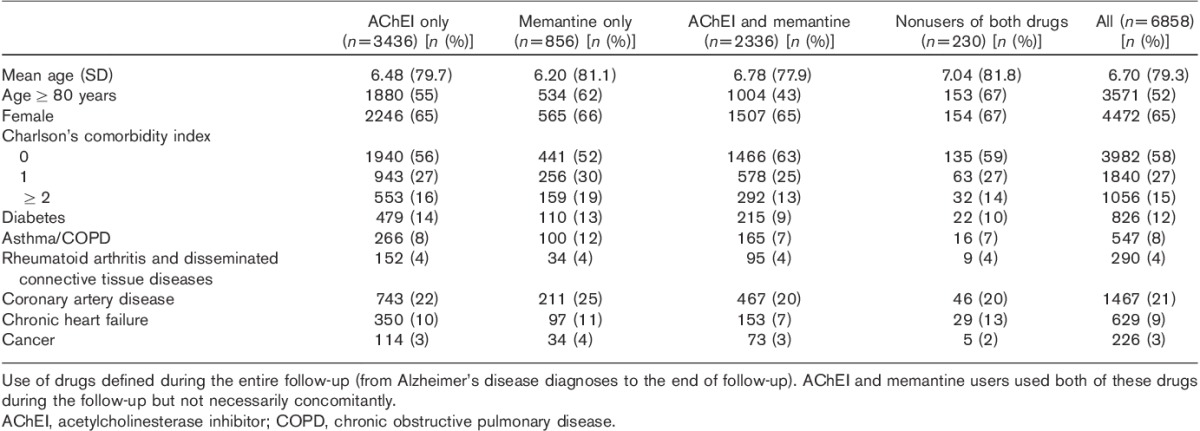
Individuals diagnosed with AD in 2005 were on average 79 years old and the majority of them were women (Table 1). Half of the individuals had diagnosed cardiovascular disease and 12% had diabetes.
Table 1.
Baseline characteristics of acetylcholinesterase inhibitor users, memantine users, users of both acetylcholinesterase inhibitor and memantine, and nonusers of both drugs among those diagnosed with Alzheimer’s disease in 2005 in the MEDALZ-2005 study
Acetylcholinesterase inhibitor drug use
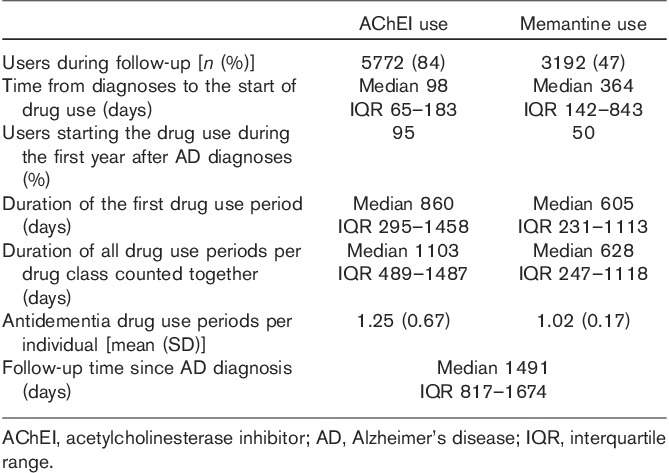
Of 6858 individuals diagnosed with AD, 84% (n=5772) used AChEI drug during the follow-up and 95% (n=5470) of them started the use during the first year after the diagnoses (Table 2). During the follow-up, 21% (n=1221) of users had several drug use periods – that is, there were breaks in drug availability. Majority (76%, n=930) of individuals with several drug use periods had two periods. When all drug use periods were counted together, the median duration of all AChEI use periods was 1103 days (Table 2).
Table 2.
Comparison of acetylcholinesterase inhibitor and memantine use during the 4-year follow-up among those diagnosed with Alzheimer’s disease in 2005 in the MEDALZ-2005 study (n=6858)
At the beginning of AChEI treatment, 49% (n=2851) started with donepezil, 29% (n=1685) with rivastigmine, and 22% (n=1236) with galantamine. There were 14% (n=788) users who used two AChEI drugs and 1% (n=73) who used all three AChEI drugs during the follow-up.
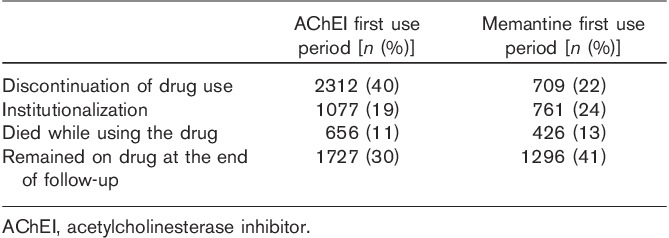
AChEI purchases ended in our data because of long-term hospitalization/institutionalization (no follow-up data on drug use), death, the end of study period, and discontinuation of drug use (Table 3). The most common reasons (over 50%) were the end of follow-up and long-term hospitalization/institutionalization. Altogether, 3277 individuals had follow-up time until the end of 2009. Of these individuals, 77% used AChEI at the end of the follow-up.
Table 3.
End of acetylcholinesterase inhibitor and memantine first use periods among those diagnosed with Alzheimer’s disease in 2005 in the MEDALZ-2005 study
When analyzing individuals with at least 1 year of follow-up time after starting AChEI use (n=5162), 20% of users discontinued drug use during the first year. However, 63% of these discontinuers restarted AChEI use later during the follow-up. Among those who used only one AChEI drug during the first drug use period (n=4682), discontinuation rates during the first year after starting AChEI therapy were significantly different according to the choice of AChEI drug. Rivastigmine (26%) and galantamine (25%) users were more likely to discontinue than donepezil (18%) users (χ2, P<0.0001).
In the unadjusted Cox model, factors associated with the discontinuation of AChEI use were the initial choice of AChEI drug, older age, and female sex, whereas Charlson’s comorbidity index score was not associated with discontinuation (Table 4, Fig. 1). These associations remained significant also in the adjusted model. Compared with donepezil users, rivastigmine [hazard ratio 1.34 (confidence interval 1.22–1.48)] and galantamine [hazard ratio 1.23 (confidence interval 1.15–1.37)] users were more likely to discontinue AChEI use. In subanalyses restricted to time period when generic AChEI drugs were not available on market, the risk of discontinuation remained about 30% higher in rivastigmine and galantamine users compared with donepezil users (results not shown).
Table 4.
Factors associated with risk of discontinuation of acetylcholinesterase inhibitor therapy among those diagnosed with Alzheimer’s disease in 2005 in the MEDALZ-2005 study
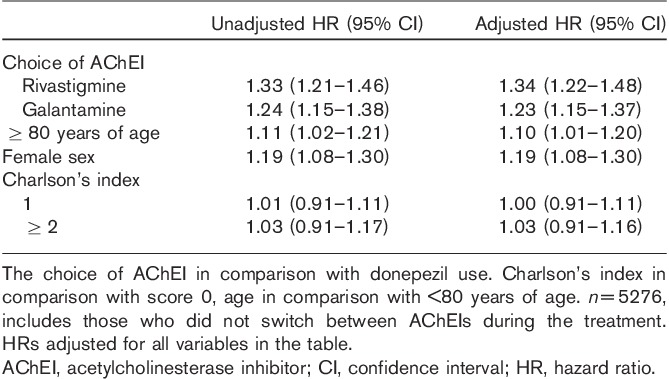
Fig. 1.
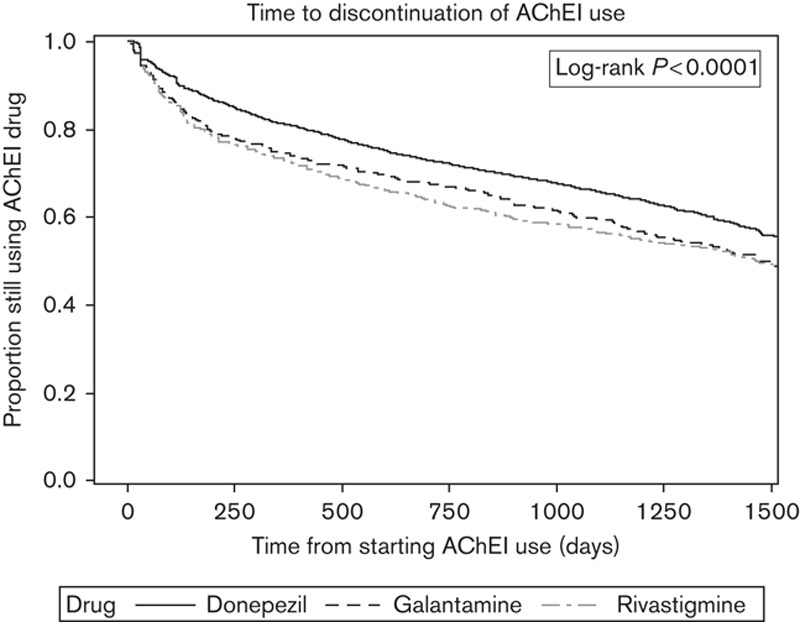
Risk of discontinuation of acetylcholinesterase inhibitor (AChEI) use according to AChEI drugs among those diagnosed with Alzheimer’s disease in 2005 in the MEDALZ-2005 study (unadjusted).
Memantine use
Half of those diagnosed with AD in 2005 started memantine use during the follow-up (Table 1). When all memantine use periods were summed up, the median duration of use was 628 days (Table 2). During the first year after the diagnoses, 50% of memantine users started the use.
There were 285 (12%) individuals who discontinued memantine use during the first year of use (including those who had at least 1 year of follow-up time after starting memantine use, n=2334). Of these discontinuers, 23% restarted memantine use later during the follow-up. Over 40% of memantine users remained on memantine until the end of the study (Table 3).
Use of both acetylcholinesterase inhibitor and memantine
There were 2336 individuals who used both AChEI and memantine during the follow-up. Of these, 89% started AChEI use before memantine use. Among them, memantine use was started on average 504 days after AChEI use. Altogether, 22% (n=1506) of the sample used AChEI and memantine concomitantly. The median time of concurrent use was 450 (interquartile range 192–836) days.
Of the concomitant users of AChEIs and memantine, 54% (n=810) used donepezil, 26% (n=388) used rivastigmine, and 20% (n=308) used galantamine when concomitant use started. Discontinuation percents of AChEIs during concomitant use with memantine were 40% (n=322) for donepezil, 39% (n=151) for rivastigmine, and 35% (n=108) for galantamine (χ2, P=0.3508).
There were 1086 individuals who did not use any AChEI during follow-up. Of the nonusers of AChEI, 79% (n=856) used memantine during the follow-up. Thus, only 3% (230 of 6858 individuals diagnosed with AD in 2005) did not use any antidementia drug (AChEI or memantine) during the follow-up.
Discussion
In our nationwide sample of community-dwelling individuals diagnosed with AD within a calendar year, we found widespread utilization of antidementia drugs, and only 3% were nonusers of antidementia drugs during the 4-year follow-up period. The duration of AChEI use was one of the longest reported in the literature. We also found that the risk of discontinuation was 30% higher with rivastigmine and 20% higher with galantamine use compared with donepezil use.
In our study, 84% of the individuals with AD used AChEIs during the follow-up. This is a markedly higher percentage than that described in a previous study (van den Bussche et al., 2011). They reported that only 27% of the incident dementia cases received antidementia drugs during the first year after diagnoses in Germany, including both community-dwelling individuals and residents of nursing homes. In our study, 95% of AChEI users started the use during the first year after diagnoses. Our results are in line with a Swedish study reporting that 80% of the incident cases with AD started AChEI use (Johnell et al., 2013). Their study mainly included individuals referred to the specialist care, whereas our study describes nationwide treatment practice. Duration of the first AChEI use period (28 months) was comparable with a previous Canadian study, which reported that the length of AChEI treatment was 29 months with over about 3 years of follow-up (Herrmann et al., 2007). Furthermore, 77% of those who were included in our study on 31 December 2009, after 48–60 months of follow-up, used AChEI drug. Thus, prevalence at the end of follow-up is considerably higher than that reported by Amuah et al. (2010) who reported that, over 40 months, 84% of users had discontinued AChEI use. The duration of use and high prevalence at the end of follow-up indicate that long-term use of AChEIs is common in Finland.
Many of the differences between our study results and previous studies may be explained by the differences in treatment guidelines, reimbursement policies or health plan coverage of antidementia drugs, health care utilization, and the organization of care for individuals with AD. For example, to be eligible for reimbursed antidementia drugs in Finland, the diagnoses must be confirmed by a geriatrician or neurologist. In a study by Herrmann et al. (2007), 28% of AChEI users had seen a dementia specialist in 60 days before the first prescription in Canada. Van den Bussche et al. (2011) reported that contact with a specialist increased the likelihood of receiving antidementia drug prescription with an appropriate dose. The prevalence and duration of AChEI use in other studies may be different, if also other forms of dementia diseases were included. Johnell et al. (2013) reported that antidementia drug use was more common among individuals with AD compared with individuals with vascular dementia or frontotemporal dementia.
In Finland, AD is diagnosed according to a predefined protocol and completion of this process is prerequisite for obtaining the reimbursement for antidementia drugs (Finnish Medical Society Duodecim, Helsinki, 2010). All patients have equal access to public memory clinics where specialized physicians examine patients according to the care guideline. The guideline follows international diagnostic criteria for cognitive disorders. After proper diagnoses are made, the certificate describing the results is send to the SII and reimbursement is approved. Thus, the diagnostic process is not an obstacle in reimbursement. Proper diagnoses of cognitive disorders are essential in assuring the quality of care and appropriate treatment for all residents.
We found that 22% of AChEI users used both AChEI and memantine concomitantly. Although concomitant use has infrequently been reported, a similar prevalence has been described by Brewer et al. (2013) among users of antidementia drugs in Ireland. In a study by Tifratene et al. (2012) conducted in France, the prevalence of concomitant use was about 19% of AChEI users, although their national treatment guidelines did not recommend concomitant use. The Finnish Current Care Guideline states that concomitant use may be beneficial in moderate-to-severe AD (Finnish Medical Society Duodecim, Helsinki, 2010). Effectiveness and tolerability of concomitant use has been reported in the literature but is still under debate (Atri et al., 2008; Porsteinsson et al., 2008; Lopez et al., 2009). Our results indicate that memantine use is started later than AChEI use after diagnoses. This is in line with the indication of memantine for treatment of moderate-to-severe AD. Because of later timing of memantine use in terms of diagnoses, it seems that our 4-year follow-up was not long enough to evaluate duration of memantine treatment.
Discontinuation during the first year of use is one method to describe persistence. In our study, 20% of AChEI users discontinued the use during the first year. In previous studies, AChEI discontinuation during the first year has been reported to vary between 55 and 66% (Amuah et al., 2010; Pariente et al., 2010; van den Bussche et al., 2011). Memantine discontinuation during the first year of use was observed in 12% of memantine users, whereas a previous study reported 36% discontinuation rate during the first year (Vidal et al., 2008). MEDALZ-2005 cohort includes individuals with clinically diagnosed AD. Several pharmacoepidemiological studies reporting AChEI utilization have included individuals with all forms of dementia (Herrmann et al., 2007; Pariente et al., 2010; van den Bussche et al., 2011). In some cases, individuals have been identified from registers simply based on AChEI use, which may lead to misclassification bias if these drugs are prescribed imprecisely to various memory problems without diagnosing the underlying disease. Thus, higher discontinuation rates in other studies may be partly explained by the lack of effectiveness in individuals without a clinical dementia.
Similar to the study by Amuah et al. (2010), women were more likely to discontinue AChEI use in our study. We also found an association between older age and discontinuation, which may indicate more careful consideration of risks and benefits of drug use for the oldest old. The risk of discontinuation varied between AChEI drugs. Individuals starting with rivastigmine and galantamine were 20–30% more likely to discontinue AChEI use compared with donepezil users. Higher rate of discontinuation has previously been reported for rivastigmine compared with donepezil in studies with smaller or more heterogeneous sample (Abughosh and Kogut, 2008; Mucha et al., 2008; Brewer et al., 2013). Previous studies did not find a difference in the discontinuation rates between galantamine and donepezil. The difference between donepezil and rivastigmine discontinuation rates was not observed in the studies by Mauskopf et al. (2005) and Suh et al. (2005). Differences in the discontinuation rates between different AChEI drugs were not explained by lower price following generic substitution, as similar results were obtained from a sensitivity analysis restricted to time before introduction of the first generic AChEI drug (donepezil). The difference in the discontinuation rates may be because of different adverse effect profiles of AChEI drugs or with practical drug use issues such as dosing once or twice a day. We did not evaluate whether there was a difference in the discontinuation rates between immediate versus extended-release formulation of galantamine or between capsule and transdermal patch formulations of rivastigmine because users were allowed to change from one formulation to another.
About one-fifth of the first antidementia drug use periods ended in hospitalization/institutionalization. Thus, we do not have data on the continuation of antidementia drug use. There is a lack of current data on the prevalence and duration of antidementia drug use in nursing homes and long-term care facilities in Finland. However, the Finnish Care Guideline states that admission to long-term care is not an adequate reason to discontinue antidementia drug use, and discontinuation should always be on the basis of the patient condition (Finnish Medical Society Duodecim, Helsinki, 2010).
Clinical practice would benefit from further studies comparing tolerability of different AChEI drugs among real-life drug users with AD. Further, as the participants of RCTs evaluating the efficacy of these drugs may not be representative of the true user population (Gill et al., 2004), the risk–benefit ratio of these drugs should be investigated in the real-world settings. Finnish Clinical Care Guideline for cognitive disorders recommends antidementia drugs to support living at home, to reduce behavioral and psychological symptoms of dementia, and to support functioning in the activities in daily living (Finnish Medical Society Duodecim, Helsinki, 2010). Thus, effectiveness of antidementia drugs for achieving these goals should be investigated in future studies.
Besides clinically diagnosed AD, the strengths of our study include a sample of all community-dwelling individuals with diagnosed AD within a calendar year instead of participants of a particular health plan. We assessed the dispensing data, which are considered a more accurate estimate of drug exposure than medical records or other sources of prescribing data (Beardon et al., 1993). We also modeled drug use on the basis of moving averages of daily dose and variation in the purchase events for each participant. The limitations are related to the characteristics of data included in the registers. Prescription Register data include dispensing of reimbursed drugs instead of actual use. However, people tend to take drugs that they regularly purchase, and the validity of pharmacy records describing actual drug exposure compared with self-reported drug use has been confirmed (Haukka et al., 2007; Rikala et al., 2010). Although the year of diagnoses is an estimate of disease stage, the lack of disease severity is a limitation in our study. Drug exposure on individuals with frequent short periods in a facility providing all or part of the drugs (e.g. during caregiver holidays) complicates analyses of outpatient drug use. We adjusted analysis for comorbid diseases but residual confounding cannot be ruled out. We did not study AChEI use or discontinuation of use in terms of dose but in future studies, dose at the time of discontinuation should be examined.
Conclusion
The median time for AChEI use was over 3 years and every fifth patient with AD used AChEI and memantine concomitantly during the 4-year follow-up period. The prevalence of antidementia drug use was high and the duration of therapy was one of the longest reported in the literature. The results are in line with the Finnish Care Guideline regarding the treatment of dementia disorders. Our results describe real-life antidementia drug utilization in a nationwide level. From a societal view, data on whether and how long antidementia drug use is effective in delaying admission to a nursing home and institutionalization would be crucial to be able to evaluate the benefits and harms as well as cost-effectiveness of antidementia drug use. Our results are in line with the previous reports that donepezil may be associated with lower risk of discontinuation than rivastigmine and galantamine. Clinical practice would benefit from further studies comparing tolerability of different AChEI drugs among real-life drug users with AD.
Acknowledgements
The authors thank the maintainers of Finnish health care registers for enabling this research.
Conflicts of interest
J.T. has served as a consultant to Lundbeck, Organon, Janssen-Cilag, Eli Lilly, AstraZeneca, F. Hoffman-La Roche, and Bristol-Myers Squibb. He has received fees for giving expert opinions to Bristol-Myers Squibb and GlaxoSmithKline, lecture fees from Janssen-Cilag, Bristol-Myers Squibb, Eli Lilly, Pfizer, Lundbeck, GlaxoSmithKline, AstraZeneca and Novartis; and a grant from Stanley Foundation. J.T. is a member of advisory board in AstraZeneca, Janssen-Cilag, and Otsuka. For the remaining authors there are no conflicts of interest.
References
- Abughosh SM, Kogut SJ.Comparison of persistence rates of acetylcholine-esterase inhibitors in a state Medicaid program.Patient Prefer Adherence 2008;2:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer Association 2011 Alzheimer’s disease facts and figures.Alzheimers Dement 2011;7:208–244 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and statistical manual of mental disorders 1994:4th ed.Washington, DC:American Psychiatric Association [Google Scholar]
- Amuah JE, Hogan DB, Eliasziw M, Supina A, Beck P, Downey W, Maxwell CJ.Persistence with cholinesterase inhibitor therapy in a population-based cohort of patients with Alzheimer’s disease.Pharmacoepidemiol Drug Saf 2010;19:670–679 [DOI] [PubMed] [Google Scholar]
- Atri A, Shaughnessy LW, Locascio JJ, Growdon JH.Long-term course and effectiveness of combination therapy in Alzheimer disease.Alzheimer Dis Assoc Disord 2008;22:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Corbett A.Management of neuropsychiatric symptoms in people with dementia.CNS Drugs 2010;24:729–739 [DOI] [PubMed] [Google Scholar]
- Beardon PH, McGilchrist MM, McKendrick AD, McDevitt DG, MacDonald TM.Primary non-compliance with prescribed medication in primary care.BMJ 1993;307:846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beusterien KM, Thomas SK, Gause D, Kimel M, Arcona S, Mirski D.Impact of rivastigmine use on the risk of nursing home placement in a US sample.CNS Drugs 2004;18:1143–1148 [DOI] [PubMed] [Google Scholar]
- Birks J.Cholinesterase inhibitors for Alzheimer’s disease.Cochrane Database Syst Rev 2006;25:CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer L, Bennett K, McGreevy C, Williams D.A population-based study of dosing and persistence with anti-dementia medications.Eur J Clin Pharmacol 2013;69:1467–1475 [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial.Lancet 2004;26:2105–2115 [DOI] [PubMed] [Google Scholar]
- Finnish Medical Society Duodecim, Helsinki 2010. Current care: memory disorders [in Finnish with English summary]. Available at: http://www.kaypahoito.fi [Accessed 30 August 2012]
- Francis PT, Palmer AM, Snape M, Wilcock GK.The cholinergic hypothesis of Alzheimer’s disease: a review of progress.J Neurol Neurosurg Psychiatry 1999;66:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartiques JF, et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts.Neurology 2000;54:S10–S15 [PubMed] [Google Scholar]
- French National Authority for Health 2008. Diagnostic and management of Alzheimer’s disease and related disorders. Professional guidelines. Haute Autorité de Santé. Available at: http://www.has-sante.fr. [Accessed 21 December 2013]
- Gauthier S, Molinuevo JL.Benefits of combined cholinesterase inhibitor and memantine treatment in moderate–severe Alzheimer’s disease.Alzheimers Dement 2013;9:326–331 [DOI] [PubMed] [Google Scholar]
- Geldmacher DS.Alzheimer’s disease: current pharmacotherapy in the context of patient and family needs.J Am Geriatr Soc 2003;51:S289–S295 [DOI] [PubMed] [Google Scholar]
- Gill SS, Bronskill SE, Mamdani M, Sykora K, Li P, Shulman KI, et al. Representation of patients with dementia in clinical trials of donepezil.Can J Clin Pharmacol 2004;11:e274–e285 [PubMed] [Google Scholar]
- Haukka J, Suvisaari J, Tuulio-Henriksson A, Lonnqvist J.High concordance between self-reported medication and official prescription database information.Eur J Clin Pharmacol 2007;63:1069–1074 [DOI] [PubMed] [Google Scholar]
- Herrmann N, Gill SS, Bell CM, Anderson GM, Bronskill SE, Shulman KI, et al. A population-based study of cholinesterase inhibitor use for dementia.J Am Geriatr Soc 2007;55:1517–1523 [DOI] [PubMed] [Google Scholar]
- Johnell K, Religa D, Eriksdotter M.Differences in drug therapy between dementia disorders in the Swedish dementia registry: a nationwide study of over 7,000 patients.Dement Geriatr Cogn Disord 2013;35:239–248 [DOI] [PubMed] [Google Scholar]
- Lachaine J, Beauchemin C, Legault M, Bincau S.Economic evaluation of the impact of memantine on time to nursing home admission in the treatment of Alzheimer disease.Can J Psychiatry 2011;56:596–604 [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Wahed AS, Saxton RA, Wolk DA, Klunk W, Dekosky ST.Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease.J Neurol Neurosurg Psychiatry 2009;80:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorana A, Esposito Z, Koch G.Beyond the cholinergic hypothesis: do current drugs work in Alzheimer’s disease?CNS Neurosci Ther 2010;16:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauskopf JA, Paramore C, Lee WC, Snyder EH.Drug persistency patterns for patients treated with rivastigmine or donepezil in usual care settings.J Manag Care Pharm 2005;11:231–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM.Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease.Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- Mucha L, Wang SS, Cuffel B, McRae T, Mark TL, del Valle M.Comparison of cholinesterase inhibitor utilization patterns and associated health care costs in Alzheimer’s disease.J Manag Care Pharm 2008;14:451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Board of Health and Welfare National guidelines for care in cases of dementia 2010Stockholm:Socialstyrelsen [Google Scholar]
- National Institute for Health and Clinical Excellence 2011. NICE Technology Appraisal Guidance III: donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. Available at: http://www.nice.org.uk/nicemedia/live/13419/53619/53619.pdf. [Accessed 21 December 2013]
- Pariente A, Pinet M, Moride Y, Merlière Y, Moore N, Fourrier-Règlat A.Factors associated with persistence of cholinesterase inhibitor treatments in the elderly.Pharmacoepidemiol Drug Saf 2010;19:680–686 [DOI] [PubMed] [Google Scholar]
- Porsteinsson AP, Grossberg GT, Mintzer J.Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial.Curr Alzheimer Res 2008;5:83–89 [DOI] [PubMed] [Google Scholar]
- Purhonen M, Koponen H, Tiihonen J, Tanskanen A.Outcome of patients after market withdrawal of thioridazine: a retrospective analysis in a nationwide cohort.Pharmacoepidemiol Drug Saf 2012;21:1227–1231 [DOI] [PubMed] [Google Scholar]
- Rikala M, Hartikainen S, Sulkava R, Korhonen MJ.Validity of the Finnish Prescription Register for measuring psychotropic drug exposures among elderly Finns: a population-based intervention study.Drugs Aging 2010;27:337–349 [DOI] [PubMed] [Google Scholar]
- Ritchie CW, Ames D, Clayton T, Lai R.Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease.Am J Geriatr Psychiatry 2004;12:358–369 [DOI] [PubMed] [Google Scholar]
- Rodda J, Morgan S, Walker Z.Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer’s disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine.Int Psychogeriatr 2009;21:813–824 [DOI] [PubMed] [Google Scholar]
- Standridge JB.Pharmacotherapeutic approaches to the treatment of Alzheimer’s disease.Clin Ther 2004;26:615–630 [DOI] [PubMed] [Google Scholar]
- Suh DC, Thomas SK, Valiyeva E, Arcona S, Vo L.Drug persistency of two cholinesterase inhibitors: rivastigmine versus donepezil in elderly patients with Alzheimer’s disease.Drugs Aging 2005;22:695–707 [DOI] [PubMed] [Google Scholar]
- Thompson S, Lanctot KL, Herrmann N.The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer’s disease.Expert Opin Drug Saf 2004;3:425–440 [DOI] [PubMed] [Google Scholar]
- Tifratene K, Duff FL, Pradier C, Quetel J, Lafay P, Schuck S, et al. Use of drug treatments for Alzheimer’s disease in France: a study on a national level based on the National Alzheimer’s Data Bank (Banque Nationale Alzheimer).Pharmacoepidemiol Drug Saf 2012;21:1005–1012 [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J.11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study).Lancet 2009;374:620–627 [DOI] [PubMed] [Google Scholar]
- Tolppanen AM, Taipale H, Koponen M, Lavikainen P, Tanskanen A, Tiihonen J, Hartikainen S.Use of existing data sources in clinical epidemiology: Finnish health care registers in Alzheimer’s disease research – the Medication use among persons with Alzheimer’s disease (MEDALZ-2005) study.Clin Epidemiol 2013;5:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bussche H, Kaduszkiewicz H, Koller D, Eisele M, Steinmann S, Glaeske G, Wiese B.Antidementia drug prescription sources and patterns after the diagnosis of dementia in Germany: results of a claims data-based 1-year follow-up.Int Clin Psychopharmacol 2011;26:225–231 [DOI] [PubMed] [Google Scholar]
- Vidal JS, Lacombe JM, Dartigues JF, Pasquier F, Robert P, Tzourio C, Alpèrovitch A.Memantine therapy for Alzheimer disease in real-world practice: an observational study in a large representative sample of French patients.Alzheimer Dis Assoc Disord 2008;22:125–130 [DOI] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology 2013. The anatomical therapeutic chemical classification system. Available at: http://www.whocc.no/atc_ddd_index/. [Accessed 22 May 2013]
- World Health Organization and Alzheimer’s Disease International Dementia: a public health priority 2012Geneva, Switzerland:World Health Organization [Google Scholar]


