Abstract
Major depressive disorder (MDD) is characterized by increased rates of impaired function and disability. During antidepressant treatment, functional improvement often lags behind symptomatic resolution, and residual impairment is associated with an increased risk for relapse. When evaluating MDD treatments, it is important to assess not only depressive symptoms but also functional outcomes. In this post-hoc analysis, data from five studies were pooled to examine the effect of levomilnacipran extended-release (ER) versus placebo on functional impairment as measured using the Sheehan Disability Scale. The mean change in the Sheehan Disability Scale total score was significantly greater for levomilnacipran ER versus placebo in the overall pooled population, for both sexes, and across all ages. Statistically significantly higher rates of functional response, functional remission, combined (functional and symptomatic) response, and combined remission were achieved with levomilnacipran ER compared with placebo in the pooled population, as well as in the male, female, younger, and middle-aged population subgroups. The levomilnacipran ER group also showed significantly improved functional outcomes versus placebo regardless of baseline depression severity. Similarly, functional impairment was significantly improved and higher functional and combined response and remission rates were achieved with levomilnacipran ER compared with placebo regardless of the baseline level of functional impairment.
Keywords: depression, disability, functional impairment, levomilnacipran extended-release, major depressive disorder, Montgomery–Åsberg depression rating scale, Sheehan Disability Scale
Introduction
Major depressive disorder (MDD) is a prevalent and recurrent disorder that is characterized by emotional and physical symptoms, as well as increased rates of impaired occupational functioning, marital problems, and social isolation (Murray and Lopez, 1997; Kessler et al., 2003; Wang et al., 2003; Moussavi et al., 2007; Sheehan and Sheehan, 2008; Trivedi et al., 2010; Guico-Pabia et al., 2011). MDD-related symptoms and functional impairment are associated with decreased quality of life and increased disability; depressive disorders are among the leading causes of disability worldwide (Murray et al., 2012).
Patients who respond to treatment and experience improvement in depressive symptoms may have residual social and work impairment because functional changes often lag behind symptomatic improvement (Wells et al., 1989; Coryell et al., 1990, 1993; Judd et al., 2000, 2008; Hirschfeld et al., 2002; Israel, 2006; Kennedy et al., 2007; McKnight and Kashdan, 2009). Incomplete recovery from depressive symptoms and residual impairment in social functioning have been associated with increased risk for MDD relapse or recurrence (Judd et al., 2000; Solomon et al., 2004; Trivedi, 2009; Vittengl et al., 2009; Trivedi et al., 2010). When evaluating MDD treatments, it is important to assess not only depressive symptoms, but also the patient’s level of functional impairment (Langlieb and Guico-Pabia, 2010); from a patient’s perspective, return to a normal level of functioning is equally as important as symptom resolution (Zimmerman et al., 2006).
An extended-release (ER) formulation of levomilnacipran, a potent and selective serotonin and norepinephrine reuptake inhibitor (SNRI), is Food and Drug Administration-approved for the treatment of MDD in adults. In contrast to the SNRIs duloxetine, venlafaxine, and desvenlafaxine, which show preference for serotonin relative to norepinephrine reuptake inhibition, levomilnacipran has greater potency in vitro for inhibiting norepinephrine compared with serotonin reuptake (Auclair et al., 2013). Recently it has been postulated that antidepressants with a prominent noradrenergic component may be especially effective in improving functional impairment in depressed patients (Nutt, 2008; Briley and Moret, 2010; Moret and Briley, 2011).
The efficacy and safety of levomilnacipran ER in the treatment of MDD have been evaluated in five phase II/III studies, four of which have met the prespecified primary efficacy outcome (Asnis et al., 2013; Montgomery et al., 2013; Bakish et al., 2014; Gommoll et al., 2014; Sambunaris et al., 2014). In this post-hoc analysis, data from all five phase II and III studies (Fig. 1) have been pooled to examine the effect of levomilnacipran ER versus placebo on functional impairment as measured using the Sheehan Disability Scale (SDS; Sheehan et al., 1996). As the likelihood of achieving symptomatic remission during treatment has been associated with various patient characteristics such as age, sex, and depression severity (Bosworth et al., 2002; Trivedi et al., 2006; Mancini et al., 2012), these characteristics were evaluated in this study for their potential association with improvement of functional impairment.
Fig. 1.
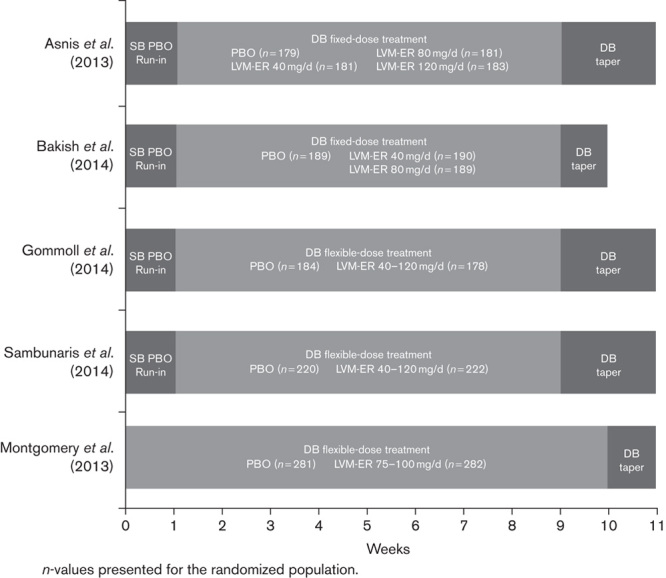
Levomilnacipran ER study designs. DB, double-blind; ER, extended-release; LVM ER, levomilnacipran extended-release; PBO, placebo; SB, single-blind.
Methods
Study design
Analyses were based on pooled data from two fixed-dose (40, 80, and 120 mg/day; 40 and 80 mg/day; Asnis et al., 2013; Bakish et al., 2014) and three flexible-dose [40–120 mg/day (two studies); 75–100 mg/day; Montgomery et al., 2013; Gommoll et al., 2014; Sambunaris et al., 2014], randomized, double-blind, placebo-controlled studies on levomilnacipran ER (Fig. 1). Study methods and design for the individual studies have been presented or published previously. The primary efficacy measure for all five studies was a change in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score from baseline, and all studies prespecified change in the SDS total score from baseline as a secondary efficacy measure. The studies were 8 weeks (Asnis et al., 2013; Bakish et al., 2014; Gommoll et al., 2014; Sambunaris et al., 2014) or 10 weeks (Montgomery et al., 2013) in duration and included patients 18–80 years of age who met Diagnostic and Statistical Manual of Mental Disorders, 4 ed. – text revision (APA, 2000) criteria for MDD. Patients were required to have met the following criteria for study inclusion: a clinician-rated MADRS (Montgomery and Åsberg, 1979) score of 30 or higher for three of the studies (Asnis et al., 2013; Gommoll et al., 2014; Sambunaris et al., 2014); a MADRS score of 26 or higher and a Clinical Global Impression-Severity score of 4 or higher (Guy, 1976) in one study (Bakish et al., 2014); a 17-item Hamilton Depression Rating Scale score of greater than 22 (Hamilton, 1960), an SDS score of 10 or higher, and at least one SDS subscale score of 6 or higher for the non-US study (Montgomery et al., 2013).
The key exclusion criteria included Diagnostic and Statistical Manual of Mental Disorders, 4 ed. – text revision Axis I disorders other than MDD, a history of nonresponse to two or more antidepressants after adequate treatment, and current risk of suicide. Each study was in full compliance with the guidelines for Good Clinical Practice and the ethical principles of the Declaration of Helsinki. The protocol for each study was approved by the Institutional Review Board at each study center, and all patients provided written informed consent.
Functional impairment efficacy outcomes
Functional impairment was assessed on the basis of the SDS total score and Work/School, Social Life, and Family Life subscales. Functional impairment in each subscale domain is scored from 0 (not at all) to 10 (extremely); SDS total score is the sum of the three domain scores (range 0–30; Sheehan et al., 1996).
Statistical analyses
Post-hoc analyses evaluated change from baseline to end of treatment (week 8 or 10 depending on study duration) in SDS total and subscale scores in the overall patient population and in patient subgroups categorized by sex; age [younger (<45 years), middle-aged (≥45 and <60 years), and older (≥60 years)]; severity of depressive symptoms at baseline (MADRS<30, MADRS≥30, MADRS≥35); and functional impairment at baseline [lower level of impairment (SDS total score<21) and higher level of impairment (SDS total score≥21); the SDS cutoff was based on the median SDS total score of 21 at baseline].
Analyses were based on the modified intent-to-treat population [all patients who received one or more doses of the study drug and had undergone one postbaseline MADRS total score assessment (primary efficacy parameter in the individual studies)]. With regard to SDS total score, only scores from patients with valid responses on all three subscales at baseline were included in the analyses. Some unemployed patients did not receive scores on the Work/School subscale. These patients did not have valid SDS total scores and were not included in the analyses (Sheehan et al., 1996).
Analyses were carried out using the mixed-effects model for repeated measures approach with treatment group, pooled study center (nested within study), visit, and the treatment group-by-visit interaction as factors, and the baseline SDS total score and baseline-by-visit interaction as covariates.
Rates of functional response (SDS total score≤12 and all subscale scores≤4) and functional remission (SDS total score≤6 and all subscale scores ≤2) were determined. Criteria for SDS functional response and remission were based on those proposed by Sheehan and Sheehan (2008). Rates of combined response (defined as functional and symptomatic response; SDS total score≤12, all subscale scores≤4, and ≥50% reduction in MADRS score from baseline) and combined remission (defined as functional and symptomatic remission; SDS total score≤6, all subscale scores≤2, and MADRS score≤10) were also determined. Functional and combined response and remission rates were analyzed using logistic regression, with treatment group and corresponding baseline SDS total score as explanatory variables.
All statistical tests were two-sided hypothesis tests performed at the 5% level of significance; confidence intervals were two-sided 95% confidence intervals.
Results
Sheehan Disability Scale total score change in individual trials
In all five studies, the SDS total score change from baseline was a prospectively defined secondary efficacy outcome. In four of five studies (Asnis et al., 2013; Montgomery et al., 2013; Bakish et al., 2014; Sambunaris et al., 2014), patients treated with levomilnacipran ER experienced significantly greater improvement relative to placebo in SDS total scores (Fig. 2). In one of the flexible-dose studies, the numeric improvement in SDS total score of the levomilnacipran ER group did not reach statistical significance relative to the placebo group (Gommoll et al., 2014); this was also true for the primary efficacy measure (MADRS) in that study.
Fig. 2.
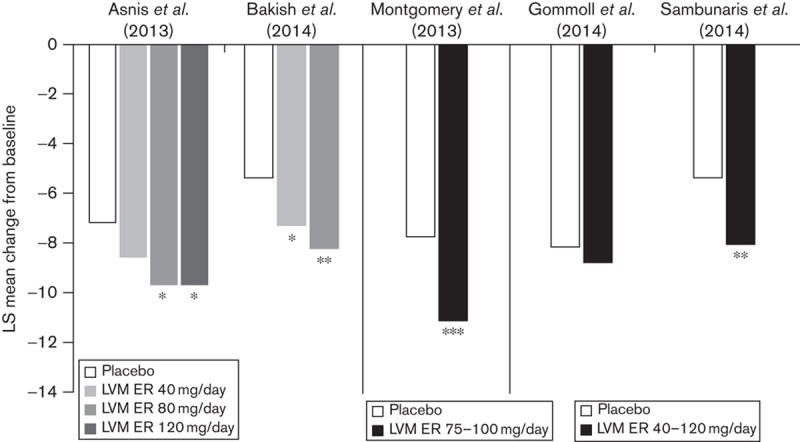
SDS total score: LS mean change from baseline to end of treatment in individual studies (MMRM, ITT population). *P<0.05, **P<0.01, ***P<0.001 vs. placebo. ITT, intent to treat; LS, least squares; MMRM, mixed-effects model for repeated measures; SDS, Sheehan Disability Scale.
Post-hoc analyses of pooled data
Baseline characteristics
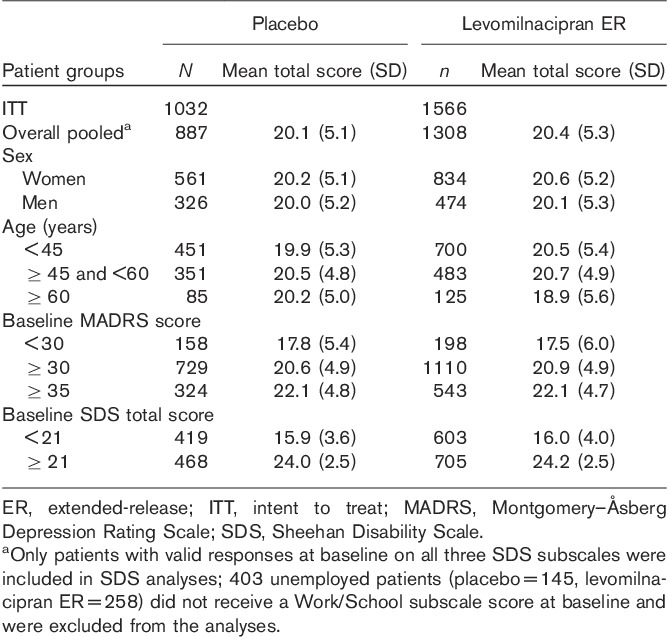
Baseline characteristics of the pooled population were similar between treatment groups; the majority of patients were women, less than 60 years of age, moderately to severely depressed, and functionally impaired (Table 1; Montgomery and Åsberg, 1979; Sheehan et al., 1996; Nemeroff, 2007). In both treatment groups, the severity of depressive symptoms at baseline correlated with the level of functional impairment; patients with less severe depression had lower SDS total scores, indicating lesser functional impairment, and more severely depressed patients had higher SDS scores, indicating increased functional impairment.
Table 1.
Baseline Sheehan Disability Scale total scores
Sheehan Disability Scale outcomes in the overall population and age and sex subgroups
The mean change in SDS total score from baseline to end of treatment was significantly greater for levomilnacipran ER versus placebo in the overall pooled population [least squares mean difference (LSMD)=−2.2; Fig. 3]. Significantly greater improvement relative to placebo was also seen at the end of treatment in the overall population for all three SDS subscales: Work/School (LSMD=−0.8), Social Life (LSMD=−0.7), and Family Life (LSMD=−0.6; Fig. 3).
Fig. 3.
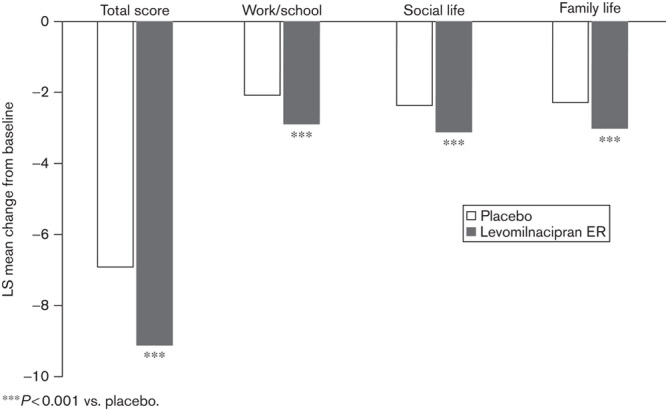
SDS total score and subscales: LS mean change from baseline to end of treatment in the overall patient population (MMRM, ITT population). ***P<0.001 vs. placebo. ITT, intent to treat; LS, least squares; MMRM, mixed-effects model for repeated measures; SDS, Sheehan Disability Scale.
The change in SDS total score from baseline to endpoint was significantly greater for levomilnacipran ER versus placebo in patients categorized by sex and age: women (LSMD=−1.9), men (LSMD=−2.7), younger (<45 years, LSMD=−1.9), middle-aged (≥45 and <60 years, LSMD=−2.3), and older patients (≥60 years, LSMD=−2.8; Fig. 4).
Fig. 4.
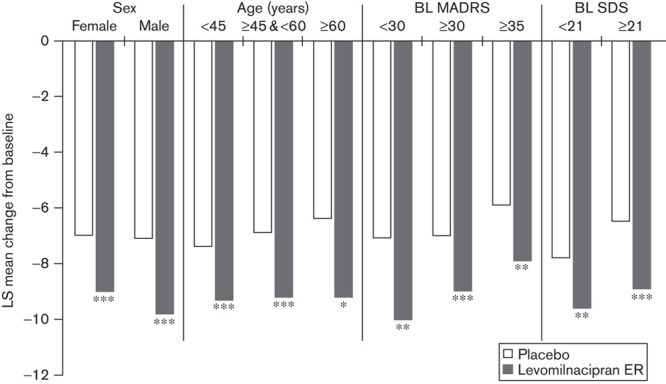
SDS total score: LS mean change from baseline to end of treatment in patient subgroups (MMRM, ITT population). *P<0.05, **P<0.01, ***P<0.001 vs. placebo. BL, baseline; ITT, intent to treat; LS, least squares; MADRS, Montgomery–Åsberg Depression Rating Scale; MMRM, mixed-effects model for repeated measures; SDS, Sheehan Disability Scale.
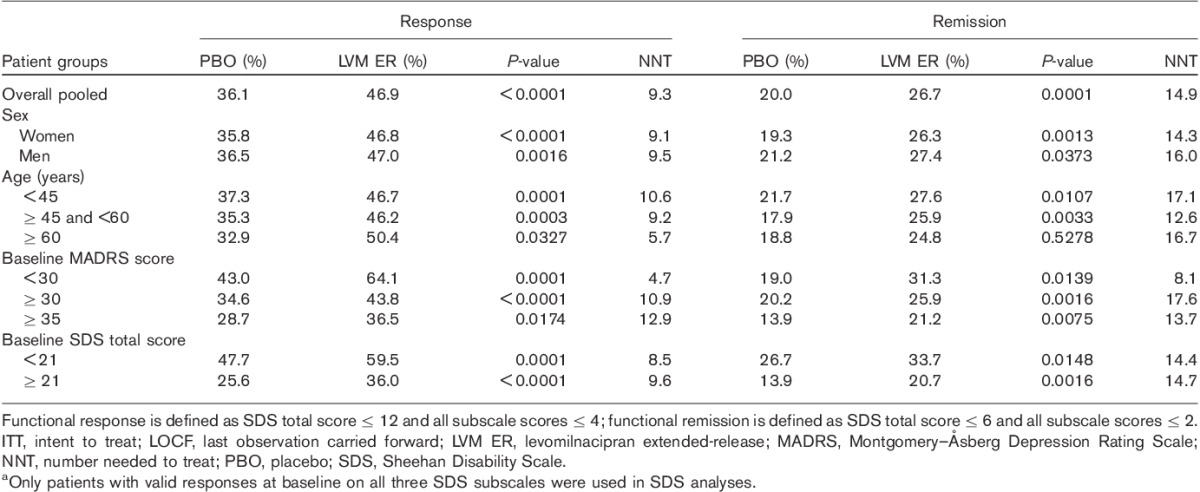
Significantly higher rates of functional response (total score≤12 and all subscale scores≤4) were observed in the pooled population of patients treated with levomilnacipran ER relative to those treated with placebo, as well as among women, men, younger, middle-aged, and older patients (Table 2). Similarly, a significantly greater proportion of levomilnacipran ER-treated patients versus placebo-treated patients achieved functional remission (SDS total score≤6 and subscale scores≤2) at endpoint in the pooled population, among both sexes, and among younger and middle-aged patients (in the relatively smaller older-patient subgroup, the separation between levomilnacipran ER (n=125) and placebo (n=85) did not reach statistical significance; Table 2).
Table 2.
Rates of functional response and remission in the overall population and patient subgroups (LOCF, ITT population)a
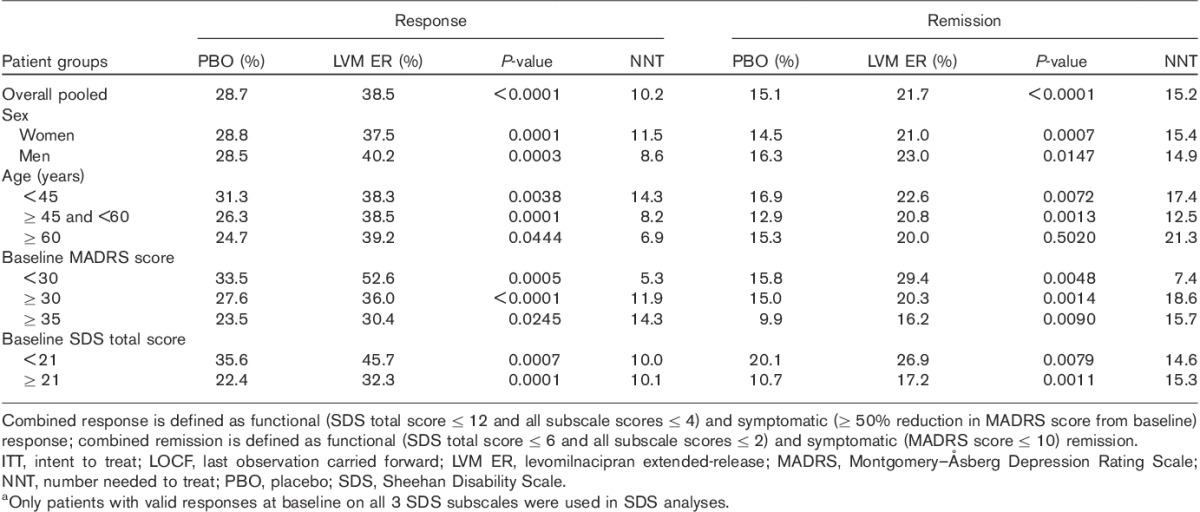
Significantly higher rates of combined response (defined as functional and symptomatic response; SDS total score≤12, all SDS subscale scores ≤4, and ≥50% reduction in MADRS score) were observed in the levomilnacipran ER group versus the placebo group in the pooled population, as well as among women, men, younger, middle-aged, and older patients (Table 3). A significantly greater proportion of levomilnacipran ER-treated patients compared with placebo-treated patients also achieved combined remission (defined as functional and symptomatic remission; SDS total score≤6, all SDS subscale scores ≤2, and MADRS score≤10) at end of treatment in the overall population and in all subgroups, with the exception of the relatively smaller subgroup of older (≥60 years) patients (placebo, n=85; and levomilnacipran ER, n=125), in which the numeric advantage over placebo did not reach statistical significance (Table 3).
Table 3.
Rates of combined response and remission in the overall population and patient subgroups (LOCF, ITT population)a
Sheehan Disability Scale outcomes in patients categorized by baseline of severity of depression
When the patient population was categorized by baseline severity of depressive symptoms into three groups (MADRS<30, MADRS≥30, and MADRS≥35), levomilnacipran ER-treated patients had significantly greater improvement than placebo-treated patients in SDS total scores at treatment end in all three groups (Fig. 4). There was also a significantly higher rate of response and remission in levomilnacipran ER-treated patients compared with placebo-treated in patients in all three severity subgroups (Table 2). Similarly, levomilnacipran ER-treated patients had significantly higher rates of combined (symptomatic and functional) response and remission compared with placebo-treated patients regardless of the depression severity at baseline (Table 3).
Sheehan Disability Scale outcomes in patients categorized by baseline level of functional impairment
The pooled population was stratified into two functional impairment groups. Patients with SDS scores lower than the median SDS score (<21) were defined as having lower functional impairment and patients with an SDS total score of 21 or higher were defined as having higher functional impairment. In both impairment subgroups, levomilnacipran ER-treated patients relative to placebo-treated patients showed significantly greater improvement in functional impairment by end of treatment, as measured by a change in the SDS total score (lower impairment, LSMD=−1.8; higher impairment, LSMD=−2.5; Fig. 4). Levomilnacipran ER treatment compared with placebo treatment was also associated with significantly greater rates of functional response and functional remission (Table 2). In addition, combined (functional and symptomatic) response and remission rates were significantly higher for levomilnacipran ER versus placebo in patients with lower and higher functional impairment (Table 3).
Discussion
In this post-hoc analysis of a large pooled dataset from five phase II/III studies, the SDS total score change was significantly greater for levomilnacipran ER versus placebo, indicating that levomilnacipran ER-treated patients showed significantly greater improvement in functional impairment over the course of treatment compared with placebo-treated patients. Significantly greater improvement in SDS total scores at end of treatment with levomilnacipran ER compared with placebo was also observed when patients were categorized by sex and age, suggesting improvement in functional impairment across a wide age range and in both sexes.
Many studies have attempted to identify patient characteristics and/or baseline demographics that are predictors of symptomatic improvement during antidepressant treatment; results have been generally conflicting because of variability in patient populations, response definitions, and antidepressant treatments (Esposito and Goodnick, 2003; Nierenberg, 2003; Trivedi et al., 2006). In one large study, a greater likelihood of symptomatic remission was associated with being female, white, and having higher levels of education and income; lower likelihood of remission was associated with more concurrent psychiatric or medical disorders, lower function, and lower quality of life at baseline (Trivedi et al., 2006). Another study examining possible predictors of time to symptomatic remission in older patients found that those with poor health, lower income, previous depressive episodes, lack of social support, or older age at depressive onset were more likely to take longer to recover; in that analysis, sex, level of education, and level of functional disability were not important factors in determining time to remission (Bosworth et al., 2002). One characteristic that has consistently been associated with lower response rates is severity of depressive symptoms (Kocsis, 1990; Vallejo et al., 1991; Keller et al., 1992; Trivedi et al., 2006). However, in a recent post-hoc analysis, desvenlafaxine was found to improve depressive symptoms significantly more than placebo in a large pool of patients, regardless of baseline depression severity (Guico-Pabia et al., 2011).
Despite recent focus on assessing functional impairment as an important ‘real-world’ outcome of antidepressant treatment (Zimmerman et al., 2006; Sheehan and Sheehan, 2008; Soares et al., 2009; Langlieb and Guico-Pabia, 2010; Sheehan et al., 2011; Mancini et al., 2012), there have been relatively few studies examining whether there are baseline characteristics that predict the likelihood of improved functional impairment during treatment. One recent post-hoc analysis of a large pool of data from studies on the SNRI duloxetine versus placebo reported that female sex, a shorter time since the first depressive episode, absence of previous antidepressant use, and mild versus more severe pain were all prognostic factors for improved functioning following antidepressant treatment (Mancini et al., 2012). Guico-Pabia et al. (2011), found that baseline depression severity was not a predictor of functional response in patients treated with desvenlafaxine, which reduced functional impairment (and improved depressive symptoms) regardless of baseline depression severity.
In the current levomilnacipran ER analyses, it is notable that improved functional impairment was observed in levomilnacipran ER-treated patients compared with placebo-treated patients, regardless of sex or age. Therefore in this study neither age nor sex predicted the likelihood of improvement in functional impairment, which is in contrast to the findings of Mancini et al. (2012), who reported that duloxetine-treated female patients were more likely than male patients to show functional improvement (Mancini et al., 2012). The different results may be due to variations in study design and/or to different drug pharmacology. For example, some researchers (Nutt, 2008; Briley and Moret, 2010; Moret and Briley, 2011) have posited that antidepressants with a prominent noradrenergic component may be especially effective in improving function in depressed patients. In contrast to the SNRIs duloxetine, venlafaxine, and desvenlafaxine, which show preference for inhibiting serotonin compared with norepinephrine reuptake, levomilnacipran ER is a more potent inhibitor of norepinephrine compared with serotonin reuptake (Auclair et al., 2013). Additional research may be warranted to better understand the potential association between baseline characteristics and improvement in functional impairment during antidepressant treatment.
A significantly greater proportion of patients in the overall population treated with levomilnacipran ER relative to placebo achieved functional response and remission, as well as combined (functional and symptomatic) response and remission. This was also true for all sex and age subgroups, with the exception of remission in patients who were 60 years or older. In this group, the increased proportion of remitters relative to placebo did not reach statistical significance, most likely because of the smaller sample size. The clinically meaningful improvement in functional impairment observed among levomilnacipran ER-treated patients across age and sex demographics in an acute timeframe is an important outcome, given that in previous studies with other antidepressants residual functional impairment persisted even after depressive symptoms resolved (Sheehan et al., 2011).
In this analysis, levomilnacipran ER-treated patients in all three baseline symptom severity subgroups (MADRS<30, MADRS≥30, and MADRS≥35) showed significantly more improvement in functional impairment than did placebo-treated patients, on the basis of both total score change and functional and combined response and remission rates. Similarly, when patients were categorized into two groups on the basis of baseline functional impairment (lower level of functional impairment vs. higher level of functional impairment), levomilnacipran ER treatment resulted in improved outcomes relative to placebo treatment regardless of the baseline level of functional impairment.
In light of previous studies that found that higher levels of baseline depressive severity were associated with smaller advantages in response rates for antidepressants relative to placebo, the current results are intriguing. Additional research may be warranted to better understand the relationship between symptom severity and functional outcomes, as well as the relationship between baseline level of functional impairment and treatment outcomes.
Limitations of these analyses include their post-hoc, retrospective nature and the lack of corrections for multiple comparisons. The included trials were heterogeneous with regard to design (flexible vs. fixed dosing, inclusion/exclusion criteria) and duration (8 vs. 10 weeks), and the short length of treatment may not have been adequate to fully evaluate improvement in functional impairment. The inclusion and exclusion criteria in the primary studies may limit the ability to generalize the results. Lack of an active comparator limits comparisons to currently available antidepressant treatments.
Pooling the populations from several individual studies provided increased statistical power to better assess the effects of levomilnacipran ER on function; it also provided larger sample sizes for subgroup analyses. As functional impairment is a common and debilitating sequela of depression (Kessler et al., 2003; McKnight and Kashdan, 2009), understanding patient factors that may be associated with functional improvement during treatment is an important but often overlooked component in managing depression and facilitating return to wellness. Assessing functional impairment during the treatment of patients with MDD is an essential step in the evolution of patient management, as is the understanding of how various patient populations are likely to respond to a given antidepressant treatment.
In this post-hoc analysis, meaningful improvement in functional impairment in favor of levomilnacipran ER versus placebo was observed in the overall pooled population, as well as in patients categorized by sex, age, baseline severity of depressive symptoms, and baseline level of functional impairment. These results suggest that levomilnacipran ER treatment improves functional disability in patients with MDD.
Acknowledgements
The authors would like to acknowledge that writing assistance and editorial support for the preparation of this manuscript was provided by Jennifer Kaiser, PhD, and Adam Ruth, PhD, of Prescott Medical Communications Group, Chicago, Illinois, and a contractor for Forest Research Institute.
This study was supported by funding from Forest Research Institute, a subsidiary of Forest Laboratories Inc. (New York, New York, USA) and Pierre Fabre Médicament (Toulouse, France). Forest Laboratories Inc. was involved in the study design, data collection (through contracted clinical investigator sites), analysis and interpretation of data, and the decision to present these results.
Conflicts of interest
Angelo Sambunaris acknowledges a potential conflict of interest as he has received consultant fees from Forest Research Institute Inc. Carl P. Gommoll, Changzheng Chen, and William M. Greenberg acknowledge a potential conflict of interest as being employees of Forest Research Institute Inc. at the time of study.
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders 2000:Fourth editionWashington, DC:American Psychiatric Association; Text revision [Google Scholar]
- Asnis G, Bose A, Gommoll C, Chen C, Greenberg WM.The efficacy and safety of levomilnacipran SR 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase III, randomized, double-blind, placebo-controlled study.J Clin Psychiatry 2013;74:242–248 [DOI] [PubMed] [Google Scholar]
- Auclair AL, Martel JC, Assie MB, Bardin L, Heusler P, Cussac D, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety.Neuropharmacology 2013;70:338–347 [DOI] [PubMed] [Google Scholar]
- Bakish D, Bose A, Gommoll C, Chen C, Nunez R, Greenberg WM, et al. Levomilnacipran ER 40 mg and 80 mg in patients with major depressive disorder: a phase III, randomized, double-blind, fixed-dose, placebo-controlled study.J Psychiatry Neurosci 2014;39:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosworth HB, McQuoid DR, George LK, Steffens DC.Time-to-remission from geriatric depression: psychosocial and clinical factors.Am J Geriatr Psychiatry 2002;10:551–559 [PubMed] [Google Scholar]
- Briley M, Moret C.Improvement of social adaptation in depression with serotonin and norepinephrine reuptake inhibitors.Neuropsychiatr Dis Treat 2010;6:647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Endicott J, Keller M.Outcome of patients with chronic affective disorder: a five-year follow-up.Am J Psychiatry 1990;147:1627–1633 [DOI] [PubMed] [Google Scholar]
- Coryell W, Scheftner W, Keller M, Endicott J, Maser J, Klerman GL.The enduring psychosocial consequences of mania and depression.Am J Psychiatry 1993;150:720–727 [DOI] [PubMed] [Google Scholar]
- Esposito K, Goodnick P.Predictors of response in depression.Psychiatr Clin North Am 2003;26:353–365 [DOI] [PubMed] [Google Scholar]
- Gommoll C, Greenberg WM, Changzheng C.A randomized double-blind, placebo-controlled, study of flexible-doses of levomilnacipran ER (40–120 mg/day) in patients with major depressive disorder.J Drug Assess 2014;3:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guico-Pabia CJ, Fayyad RS, Soares CN.Assessing the relationship between functional impairment/recovery and depression severity: a pooled analysis.Int Clin Psychopharmacol 2011;27:1–7 [DOI] [PubMed] [Google Scholar]
- Guy W.The clinician global severity and impression scales. ECDEU assessment manual for psychopharmacology 1976Rockville, MD:National Institute of Mental Health;218–222DHEW Publication No. 76-338 [Google Scholar]
- Hamilton M.A rating scale for depression.J Neurol Neurosurg Psychiatry 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RM, Dunner DL, Keitner G, Klein DN, Koran LM, Kornstein SG, et al. Does psychosocial functioning improve independent of depressive symptoms? A comparison of nefazodone, psychotherapy, and their combination.Biol Psychiatry 2002;51:123–133 [DOI] [PubMed] [Google Scholar]
- Israel JA.Remission in depression: definition and initial treatment approaches.J Psychopharmacol 2006;20:5–10 [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, et al. Psychosocial disability during the long-term course of unipolar major depressive disorder.Arch Gen Psychiatry 2000;57:375–380 [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, Akiskal HS.Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders.J Affect Disord 2008;108:49–58 [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T.Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects.Arch Gen Psychiatry 1992;49:809–816 [DOI] [PubMed] [Google Scholar]
- Kennedy N, Foy K, Sherazi R, McDonough M, McKeon P.Long-term social functioning after depression treated by psychiatrists: a review.Bipolar Disord 2007;9:25–37 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R).JAMA 2003;289:3095–3105 [DOI] [PubMed] [Google Scholar]
- Kocsis JH.New issues in the prediction of antidepressant response.Psychopharmacol Bull 1990;26:49–53 [PubMed] [Google Scholar]
- Langlieb AM, Guico-Pabia CJ.Beyond symptomatic improvement: assessing real-world outcomes in patients with major depressive disorder.Prim Care Companion J Clin Psychiatry 2010;12:e1–e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M, Sheehan DV, Demyttenaere K, Amore M, Deberdt W, Quail D, Sagman D.Evaluation of the effect of duloxetine treatment on functioning as measured by the Sheehan Disability Scale: pooled analysis of data from six randomized, double-blind, placebo-controlled clinical studies.Int Clin Psychopharmacol 2012;27:298–309 [DOI] [PubMed] [Google Scholar]
- McKnight PE, Kashdan TB.The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research.Clin Psychol Rev 2009;29:243–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M.A new depression scale designed to be sensitive to change.Br J Psychiatry 1979;134:382–389 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Mansuy L, Ruth A, Bose A, Li H, Li D.Efficacy and safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study.J Clin Psychiatry 2013;74:363–369 [DOI] [PubMed] [Google Scholar]
- Moret C, Briley M.The importance of norepinephrine in depression.Neuropsychiatr Dis Treat 2011;7:9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B.Depression, chronic diseases, and decrements in health: results from the World Health Surveys.Lancet 2007;370:851–858 [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD.Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study.Lancet 1997;349:1436–1442 [DOI] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010.Lancet 2012;380:2197–2223 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB.The burden of severe depression: a review of diagnostic challenges and treatment alternatives.J Psychiatr Res 2007;41:189–206 [DOI] [PubMed] [Google Scholar]
- Nierenberg AA.Predictors of response to antidepressants general principles and clinical implications.Psychiatr Clin North Am 2003;26:345–352viii [DOI] [PubMed] [Google Scholar]
- Nutt DJ.Relationship of neurotransmitters to the symptoms of major depressive disorder.J Clin Psychiatry 2008;69Suppl E14–7 [PubMed] [Google Scholar]
- Sambunaris A, Bose A, Gommoll C, Chen C, Greenberg WM, Sheehan DV.A Phase III double-blind, placebo-controlled, flexible-dose study of levomilnacipran extended-release in patients with major depressive disorder.J Clin Psychopharmacol 2014;34:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Raj BA.The measurement of disability.Int Clin Psychopharmacol 1996;11Suppl 389–95 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Spann ME, Thompson HF, Prakash A.Assessing remission in major depressive disorder and generalized anxiety disorder clinical trials with the discan metric of the Sheehan disability scale.Int Clin Psychopharmacol 2011;26:75–83 [DOI] [PubMed] [Google Scholar]
- Sheehan KH, Sheehan DV.Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale.Int Clin Psychopharmacol 2008;23:70–83 [DOI] [PubMed] [Google Scholar]
- Soares CN, Kornstein SG, Thase ME, Jiang Q, Guico-Pabia CJ.Assessing the efficacy of desvenlafaxine for improving functioning and well-being outcome measures in patients with major depressive disorder: a pooled analysis of 9 double-blind, placebo-controlled, 8-week clinical trials.J Clin Psychiatry 2009;70:1365–1371 [DOI] [PubMed] [Google Scholar]
- Solomon DA, Leon AC, Endicott J, Mueller TI, Coryell W, Shea MT, Keller MB.Psychosocial impairment and recurrence of major depression.Compr Psychiatry 2004;45:423–430 [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice.Am J Psychiatry 2006;163:28–40 [DOI] [PubMed] [Google Scholar]
- Trivedi MH.Treating depression to full remission.J Clin Psychiatry 2009;70:e01. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Dunner DL, Kornstein SG, Thase ME, Zajecka JM, Rothschild AJ, et al. Psychosocial outcomes in patients with recurrent major depressive disorder during 2 years of maintenance treatment with venlafaxine extended release.J Affect Disord 2010;126:420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo J, Gasto C, Catalan R, Bulbena A, Menchon JM.Predictors of antidepressant treatment outcome in melancholia: psychosocial, clinical and biological indicators.J Affect Disord 1991;21:151–162 [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB.Deterioration in psychosocial functioning predicts relapse/recurrence after cognitive therapy for depression.J Affect Disord 2009;112:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PS, Simon G, Kessler RC.The economic burden of depression and the cost-effectiveness of treatment.Int J Methods Psychiatr Res 2003;12:22–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study.JAMA 1989;262:914–919 [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Posternak MA, Friedman M, Attiullah N, Boerescu D.How should remission from depression be defined? The depressed patient’s perspective.Am J Psychiatry 2006;163:148–150 [DOI] [PubMed] [Google Scholar]


