Abstract
Helicobacter pylori (H. pylori) infects more than half of the world’s human population, but only 1% to 3% of infected people consequently develop gastric adenocarcinomas. The clinical outcome of the infection is determined by host genetic predisposition, bacterial virulence factors, and environmental factors. The association between H. pylori infection and chronic active gastritis, peptic ulcer disease, gastric cell carcinoma, and B cell mucosa-associated lymphoid tissue lymphoma has been well established. With the exception of unexplained iron deficiency anemia and idiopathic thrombocytopenic purpura, H. pylori infection has no proven role in extraintestinal diseases. On the other hand, there is data showing that H. pylori infection could be beneficial for some human diseases. The unpredictability of the long-term consequences of H. pylori infection and the economic challenge in eradicating it is why identification of high-risk individuals is crucial.
Keywords: Helicobacter pylori, Virulence factor, Host factors, Gastroduodenal diseases, Extraintestinal disorders
Core tip: Helicobacter pylori (H. pylori) infects more than half of the world’s human population. The association between H. pylori infection and chronic active gastritis, peptic ulcer disease, gastric cell carcinoma, and B cell mucosa-associated lymphoid tissue lymphoma, unexplained iron deficiency anemia and idiopathic thrombocytopenic purpura has been well established. H. pylori screening and treatment is a recommended gastric cancer risk reduction strategy in high-risk populations. The unpredictability of the long-term consequences of H. pylori infection and the economic challenge in eradicating it is why identification of high-risk individuals is crucial.
INTRODUCTION
Helicobacter pylori (H. pylori) is a micro-aerophilic, Gram-negative, slow-growing, spiral-shaped, and flagellated organism which infects more than half of the world’s human population[1]. H. pylori colonization itself does not cause any symptoms, and fewer than 20% of all infected patients will develop symptoms from their infection[2]. Approximately 10% of infected individuals develop peptic ulcer disease, 1% to 3% develop gastric adenocarcinoma, and less 0.1% [mucosa-associated lymphoid tissue (MALT)] develop lymphoma[3].
The outcome of H. pylori infection may involve a combination of bacterial, host, and environmental factors. The association between H. pylori infection and chronic active gastritis, peptic ulcer disease, gastric cell carcinoma, and B cell MALT lymphoma has been well established. On the other hand H. pylori infection could be beneficial for humans[2] (Table 1).
Table 1.
Summary of the pathogenetic and preventive role of Helicobacter pylori
|
Pathogenetic role |
Preventive role |
|
| Proven | Suspected | Suspected |
| Gastro-duodenal diseases | Gastro-intestinal diseases | Gastroesophageal diseases |
| Peptic ulcer | Pancreatic cancer | Gastroesophageal reflux disease |
| Gastric cancer | Colorectal adenoma/carcinoma | Esophageal adenocarcinoma |
| MALT lymphoma | Liver cirrhosis, hepatocellular carcinoma | |
| Extra-intestinal diseases | Extra-intestinal diseases | Extra-esophageal diseases |
| Immune thrombocytopenic purpura | Laryngeal cancer | Bronchial asthma |
| Iron deficiency anemia | Lung cancer | |
| Metabolic syndrome/insulin resistance | ||
| Cardiovascular diseases/ischemic heart disease | ||
| Chronic urticaria | ||
| Henoch-Schönlein purpura | ||
MALT: Mucosa-associated lymphoid tissue.
PATHOGENETIC ASPECTS
Virulence factors of H. pylori
Bacterial virulence factors play a significant role in the outcome and progression of H. pylori infection[4]. The linkages of virulence factors may show how they interact with each other[5].
The cag pathogenicity island (cag PAI) contains 27-31 genes flanked by a 31-p direct repeats. H. pylori exhibits a high degree of genetic heterogeneity due to genomic rearrangements, gene insertions, and/or deletion[6].
At least 18 cag genes encode components of the bacterial type IV secretion system, which functions to export bacterial protein across the bacterial membrane and into host gastric epithelial cells. The presence of cag PAI (cag+) amplifies the risk for severe gastritis, atrophic gastritis, and distal gastric cancer in comparison with cag-deficient (cag-) bacteria[6].
CagA: Cytotoxin-associated gene A product (CagA) is translocated into the host cell by the type IV secretion system. Phosphorylation of CagA at the glutamate-proline-isoleucine-tyrosine-alanine (EPIYA) motifs by the host Abl and Src kinases results in morphological changes to the cell (the so-called “hummingbird phenotype”). Four EPIYA motifs (-A, -B, -C, and -D) are distinguished with different degrees of phosphorylation and geographical distribution[6]. EPIYA-A and EPIYA -B sites are less phosphorylated in comparison with EPIYA-C. EPIYA-C is typically found only in strains from Western countries (Europe, North America, and Australia), and is an indicator of gastric cancer risk. EPIYA-D is found in East Asian strains. EPIYA-D containing strains induce more relief of interleukin-8 (IL-8) from gastric epithelial cells[6] (Figure 1).
Figure 1.
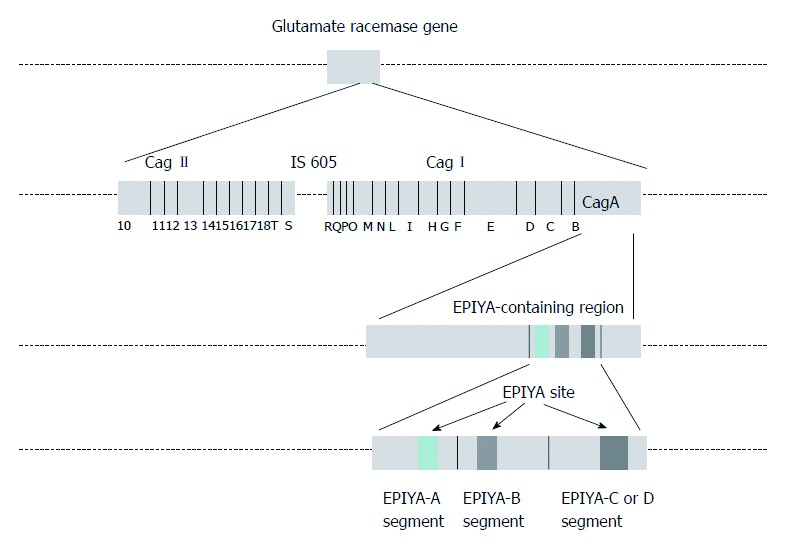
Cytotoxin-associated gene pathogenicity island. CagA: Cytotoxin-associated gene A product; EPIYA: Glutamate-proline-isoleucine-tyrosine-alanine.
Phospho-CagA interacts with numerous intracellular effectors, including eukaryotic tyrosine phosphatase with sustained activation of extracellular signal-regulated kinases 1 and 2 (ERK ½), Crk adaptor, and C-terminal Src kinase[6]. The activation of ERK and focal adhesion kinase with the tyrosine dephosphorylation of the actin binding proteins cortactin, ezrin, and vinculin leads to cell elongation[1,6] (Figure 2).
Figure 2.
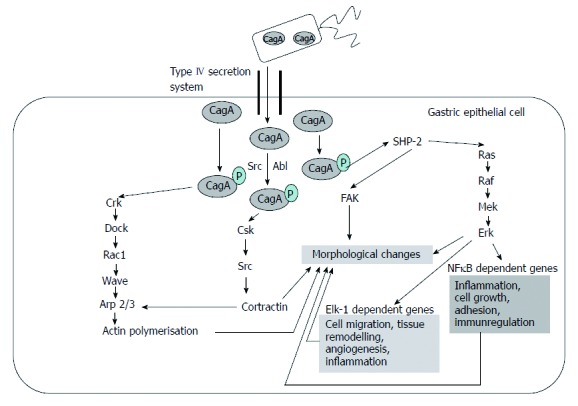
Targets of phosphorylated cytotoxin-associated gene A. Based on the article from Current Opinion in Microbiology, Hatakeyama M, SagA of CagA in Helicobacter pylori pathogenesis, 11, 30-37, Copyright (2008), with permission from Elsevier[7]. CagA: Cytotoxin-associated gene A product; NFκB: Nuclear factor κB; FAK: Focal adhesion kinase; Csk: C-terminal Src kinase.
The targets of non-phosphorylated CagA comprise E-cadherin, β-catenin, hepatocyte growth factor receptor c-Met, phospholipase C gamma, adaptor protein Grb2, kinase partitioning-defective 1b/microtubule affinity-regulating kinase 2, epithelial tight junction scaffolding protein zonula occludens 1, and the transmembrane protein junctional adhesion molecule A. The main effects are pro-inflammatory and mitogenic cell-cell junction disruption and loss of cell polarity that may be important in gastric carcinoma development[1,6] (Figures 3 and 4).
Figure 3.
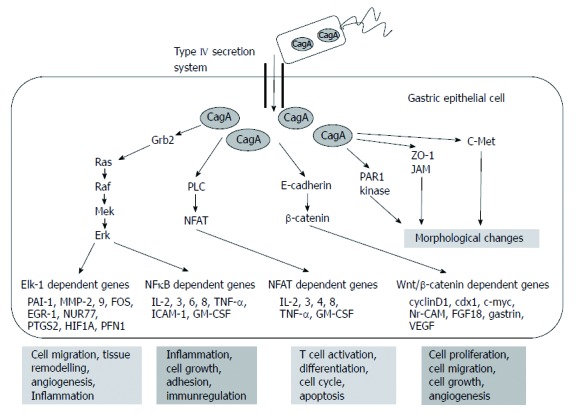
Targets of non-phosphorylated cytotoxin-associated gene a product. Based on the article from Current Opinion in Microbiology, Hatakeyama M, SagA of CagA in Helicobacter pylori pathogenesis, 11, 30-37, Copyright (2008), with permission from Elsevier[7]. CagA: Cytotoxin-associated gene A product; PLC: Phospholipase C gamma; PAR1: Kinase partitioning-defective 1b; ZO-1: Zonula occludens 1; JAM: Junctional adhesion molecule A; NFκB: Nuclear factor κB; TNF-α: Tumor necrosis factor-α; IL: Interleukin.
Figure 4.
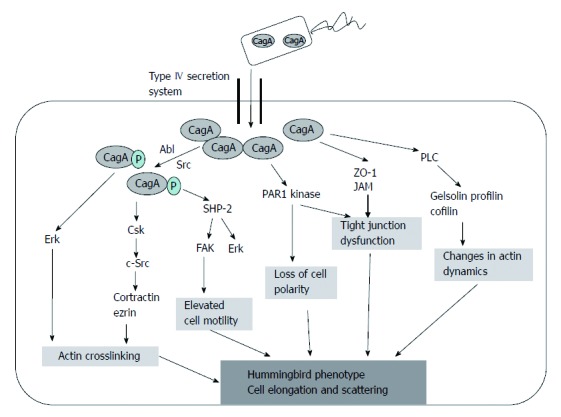
Development of “hummingbird phenotype”. CagA: Cytotoxin-associated gene A product; PLC: Phospholipase C gamma; PAR1: Kinase partitioning-defective 1b; ZO-1: Zonula occludens 1.
Activity of CagA on tumor-suppressor pathways has also been investigated. CagA is able to modulate the H. pylori induced apoptotic signal, but the exact mechanism remains to be elucidated. The initial host response upregulates p53 expression followed by the proteasomal degradation of p53[8].
Almost all cagA+ strains are classified as vacA s1 genotypes (either m1 or m2), whereas almost all cagA- strains are classified as the vacA s2/m2 strain (see below)[5]. Specific vacA genotypes of H. pylori strains are associated with a level of in vitro cytotoxin activity with clinical consequences[9].
Peptidoglycans: Peptidoglycans translocated by the cag secretion system interact with the nucleotide-binding oligomerization domain 1 (Nod1) molecule which leads to the activation of nuclear factor κB (NF-κB), pro-inflammatory secretion of interleukin-8 (IL-8), and β-defensin-2[6,10]. H. pylori enhances the phosphoinositide 3-kinase Akt signaling pathway, leading to decreased apoptosis and increased cell migration. NOD1 ligand binding can activate the interferon (IFN)-stimulated gene factor 3 signaling cascade, resulting in type I IFN production usually associated with protection against viral infection and possibly other mucosal infections[11].
VacA toxin: The cytotoxin gene vacA is present in all strains. The VacA cytotoxin induces the vacuolation, gastric epithelial barrier function disruption, disturbance of late endosomal compartments, and modulation of the inflammatory response. VacA reduces the mitochondrial transmembrane potential, releases cytochrome c from mitochondria, activates caspase 8 and 9, and induces apoptosis[6,12].
Binding of VacA to receptor-type protein tyrosine phosphatase (RPTPβ) regulates cell proliferation, differentiation, and adhesion, which all play a role in ulcerogenesis[13].
Variations in vacA gene structure (in the signal s: s1, s2, or in the middle regions m: m1, m2) make differences in vacuolating activity and specificity. The intermediate (i) region also plays role in the vacuolating activity of H. pylori. All s1, m1 strains were classified as i1 (vacuolating) type, and all s2, m2 strains were classified as i2 (non-vacuolating) type, while s1, m2 alleles could be i1 or i2. A novel intermediate variant (i3) has been identified. The fourth pathogenic region is d, a 69-81 bp-region between the m and i regions[1,5].
The variants in s and m regions seem to be a good indicator of clinical outcomes. However the roles of i and d regions should be further investigated[5]. The s1, m1 strains can induce greater vacuolation, and are associated with peptic ulcer disease and gastric cancer in Western countries, but have no pathogenic role in East Asian countries[1,6]. vacA i1 strains were associated with gastric cancer in Iranian patients[14], but not in the East Asian or Southeast Asian populations[14]. i1 genotype appeared to be a better predictor of carcinoma-associated H. pylori strains than the s or m genotype[15]. In Western countries, d1 strains without the deletion of the d region are predictors of histological inflammation, atrophy, and an increased risk of peptic ulceration and gastric cancer, compared with the presence of the vacA s-, m-, and i-region strains[16].
Adhesins and outer membrane proteins: 4% of the H. pylori genome encodes for outer membrane proteins (BabA, BabB, SabA, and OipA) which function as adhesins and porins, and are implicated in complement resistance and immune regulation[17].
The blood group antigen binding adhesin BabA is thought to mediate host-bacterial interactions and maintain colonization of the H. pylori targeting human Lewis-b surface epitopes[18,19]. The babA2 gene is associated with duodenal ulcer and gastric cancer. When in conjunction with cagA and vacA s1 alleles (“triple-positive strains”), it is associated with a greater risk of the more severe duodenal ulcer and gastric adenocarcinoma in Western populations[1,6,19].
Sialic acid-binding adhesin (SabA) binds to the carbohydrate structure sialyl-Lewis antigen expressed on the gastric epithelium. SabA can mediate the binding of H. pylori to neutrophils and erythrocytes, but the pathophysiological importance of these findings is uncertain[1]. SabA positive status was associated with increased gastric cancer risk and a negative status associated with duodenal ulceration[12].
The outer inflammatory protein (OipA) has a role in the increased expression of mucosal IL-1, -8, -17, tumor necrosis factor-α (TNF-α), and in gastric mucosal inflammation. Upregulation of matrix metalloproteinase 1, inhibition of glycogen synthase kinase 3β, and nuclear accumulation of β-catenin can influence carcinogenesis[6]. OipA positive status was significantly associated with duodenal ulcer and gastric cancer[12].
Others: Duodenal ulcer promoting gene (dupA) product induces the production of IL-8 and -12[5]. DupA may enhance duodenal ulceration and /or decrease gastric cancer development in some populations[1,5,6].
Variants of gene encoding flagellar proteins (flaA) of H. pylori may affect motility and colonization, and, therefore, the carcinogenic effect[6].
Annexin family members (AnxA1 and AnxA4) are involved in epithelial cell membrane repair response induced by H. pylori-generated VacA and CagA-independent plasma membrane disruption. Plasma membrane disruption and AnxA4 can promote cell proliferation[19].
TNF-α-inducing protein (Tipα) binds to cell-surface nucleolin and then enters the gastric cancer cells where TNF-α and chemokine gene expressions are induced by NF-κB activation in a cag PAI independent manner[20].
Bacterial factors like urease, AmiE, AmiF, hydrogenase, and arginase are essential for H. pylori survival in the acidic gastric environment[4].
Immune response to H. pylori: The host’s innate and adaptive immune system plays a crucial role in the initiation and progression of H. pylori infection[21].
Innate immunity effectors and a complex mixture of T helper (Th) 1, Th17, and regulatory T cells (Treg) adaptive immunity effectors are involved in H. pylori infection[22].
H. pylori initially targets gastric epithelial cells which form part of the innate immune response via signaling through pattern recognition receptors, such as Toll-like receptors (mainly TLR2)[21].
The neutrophil-activating protein of H. pylori polarizes Th1 cells, stimulating IL-12 and IL-23 secretion from neutrophils and macrophages. Th1 cytokines, such as gamma interferon (IFN-γ) and TNF-α, can increase the release of pro-inflammatory cytokines and augment apoptosis induced by H. pylori[22,23].
IL-17 expressing Th17 cells are important in the pro-inflammatory immune response to H. pylori. Th17 cells produce Il-17, IL-21, and IL-22 cytokines[6]. H. pylori infected macrophages produce IL-6, IL-23, and transforming growth factor (TGF)-β, which are required for Th17 cell development and maintenance[6,21]. The literature on Th1 and Th17 H. pylori-associated gastric pathology is confusing and requires intensive investigation[6].
Tregs (formerly suppressor T cells) are also implicated in the pathogenesis of H. pylori infection. TGF-β and IL-18 are responsible for Treg development[21]. H. pylori-specific Tregs suppress memory T cell responses that prolong the infection[6]. Tregs suppress the inflammatory reaction driven by IL-17, thereby also favoring bacterial persistence[24].
Antimicrobial defense of macrophages is nitric oxide (NO) dependent. H. pylori’s arginase enzyme can compete with macrophages for the inducible nitric oxide synthase (iNOS) substrate L-arginine so that host NO production is impaired; this leads to enhanced bacterial survival. H. pylori can evade macrophage phagocytosis. VacA protein prevents the fusion of phagosomes with lysosomes needed for phagocytosis. Fused phagosomes contain large numbers of live bacteria[6].
The role of B cells in the host response to H. pylori has been suggested[21]. Immunoglobulin (Ig) G and IgA antibody release from B cells in response to H. pylori may be involved in protective immunity, however it was suggested this antibody-mediated response may be counterproductive. B cells can also produce autoreactive antibodies that may be pathogenic[6]. B cell activation and survival may have implications for MALT lymphoma development[6].
CLINICAL ASPECTS
Gastroduodenal diseases
Peptic ulcer: Some H. pylori colonized individuals may develop corpus gastritis associated with gastric hypochlorhydria, gastric atrophy, gastric ulcer, and an increased risk of gastric cancer. Conversely, others may develop antral-predominant gastritis, which is associated with gastric hyperchlorhydria and an increased risk of duodenal ulcer[8,25].
Since the discovery of H. pylori in the 1980s, the availability of effective eradication therapy has led to a decline in recurrent peptic ulcer disease and its complications. The pathogenetic role of H. pylori in 90% of duodenal ulcers and 80% of gastric ulcers is proven[26,27]. Effective eradication decreased the yearly recurrence rate of duodenal and gastric ulcers from 80% and 60%, respectively, to less than 5%[28].
Gastric cancer: H. pylori is a class I carcinogen in humans[1]. It is considered to be the most common aetiological factor of infection-related cancers (followed by human papilloma, hepatitis B and C, Epstein-Barr, human immunodeficiency, and human herpesvirus-8)[1,29]. H. pylori infection-related cancer represents 5.5% of the global cancer burden[6].
Gastric cancer develops in 2.9% of H. pylori infected patients[30]. H. pylori infection is responsible for about 75% of all non-cardia gastric cancers and 63.4% of all stomach cancers worldwide[1]. H. pylori infection also plays a fundamental role in non-cardia gastric carcinogenesis, but its association with cardia cancer is still uncertain[31].
The prevalence of infection is statistically significantly much higher in patients with intestinal-type gastric cancer (89.2%) compared to the diffuse-type (31.8%)[32]. H. pylori infection is regarded as the trigger of intestinal-type gastric adenocarcinoma[33]. According to Correa and Piazuelo, intestinal-type gastric carcinogenesis progresses as follows: normal gastric mucosa - no atrophic gastritis - multifocal atrophic gastritis without intestinal metaplasia - intestinal metaplasia of complete (small intestine) type - low-grade dysplasia - high-grade dysplasia - invasive adenocarcinoma[34]. Altered cell proliferation, apoptosis, epigenetic modifications to the tumor suppressor genes, oncogene activation, and dysregulation of DNA repair may occur and eventually lead to inflammation-associated carcinogenesis[35].
Eradication of H. pylori infection decreases the risk of premalignant lesions and gastric cancer in infected individuals[36-38]. Follow-up endoscopy and histology is crucial, even in patients with apparently non-malignant gastric ulcers, in improving the malignancy detection rate in populations with a high prevalence of gastric cancer[39].
H. pylori plays a role in the development and progression of gastric (MALT) lymphoma[40]. The average prevalence of H. pylori infection in MALT lymphoma was 79%; it was higher in low-grade (79%) than in high-grade (60%) cases[41]. Treatment for localized stage I gastric MALT lymphoma with H. pylori infection is eradication[40]. Eradication of H. pylori resulted in a complete remission in 60%-80% of patients with MALT lymphoma[42,43], and a 10-year sustained remission in up to 64% of cases[44].
The carcinogenic effect of H. pylori can be modified by dietary and environmental factors. H. pylori infection is more frequent in less developed Asian countries (e.g., India, Bangladesh, Pakistan, and Thailand) in comparison with the more developed Asian countries (e.g., Japan and China). However, the frequency of gastric cancer is paradoxically very low in these less developed regions than in Japan and China (the so-called “Asian enigma”)[33,45]. Several other large populations with high infection prevalence show a very low rate of gastric cancer. The so-called “African enigma” remains unexplained as well, but it does verify that not all H. pylori infected patients have an increased risk of gastric cancer[32,33,46].
Host and environmental factors also affect the development of gastroduodenal diseases in H. pylori infected individuals[6,47]. Individuals with a high-expression of IL-1β polymorphisms (C-T or T-C transitions, at positions -511, -31, and +3954 base pairs from the transcriptional start site) have an increased risk for hypochlorhydria, gastric atrophy, and distal gastric adenocarcinoma in comparison with low-expression polymorphisms; they have no effect on cancers associated with high acid exposure such as esophageal adenocarcinomas and some cardia cancers[47,48]. The combined effects of pro-inflammatory IL-1 genotypes and H. pylori bacterial virulence factors have been reported[48].
Gene polymorphisms (-308 G > A) of the pro-inflammatory cytokine TNF-α that increase the expression of the cytokine and polymorphisms (promoter polymorphisms at positions -592, -819, and -1082) that reduce the production of the anti-inflammatory cytokine (IL-10) have been associated with an increased risk of distal gastric cancer[48-50].
The effects of pro-inflammatory genotypes (IL-1β, TNF-α and IL-10) are additive[6,50].
High dietary salt intake increases the risk of gastric cancer by directly damaging gastric mucus and mucosa, improving temporary epithelial proliferation, increasing the incidence of endogenous mutations, upregulating cytokine production, and H. pylori gene expression modulation, especially that of virulence factors[51-53].
Co-infection with helminths (Ascaris lumbricoides) and Toxoplasma gondii reduces the severity of H. pylori-induced gastritis via a reduced Th1 response with higher levels of Th2 cytokines[54].
Fruit and vegetables are rich sources of carotenoids, vitamin C, folate, and phytochemicals, which may modulate xenobiotic-metabolizing enzymes and have antioxidant activity, thereby playing a preventive role in carcinogenesis[6,55-57].
Smoking is an established risk factor for gastric cancer. Swallowed carcinogenic substances (nitrosamine and other nitroso compounds), greater concentrations of smoking-related DNA adducts in the gastric mucosa, lower levels of free radical scavengers (ascorbic acid and β-carotene), and increased mRNA expression of chemokines in the gastric mucosa are in the background[58].
Pancreatic cancer
Epidemiological studies have suggested that H. pylori might be involved in the pathogenesis of pancreatic cancer (OR = 1.87, 2.1), however results are inconsistent[59,60]. A meta-analysis showed significant association between H. pylori seropositivity and development of pancreatic cancer (pooled adjusted OR = 1.38), but further research is needed to confirm this result[61,62].
Despite good scientific reasoning for the involvement of H. pylori in pancreatic diseases, direct pancreatic infection seems unlikely[63] (Table 2).
Table 2.
Putative pathomechanisms of Helicobacter pylori
| Disease | Putative pathomechanisms |
| Pathogenetic role | |
| Pancreatic cancer | Inflammatory cytokine ↑[61] |
| Angiogenic factors ↑[61] | |
| Reactive oxygen species ↑[61] | |
| Somatostatin synthesis ↓[64,65] | |
| Secretin release ↑[64,65] | |
| Basal pancreatic bicarbonate output ↑[64,65] | |
| Bacterial overgrowth, production of N-nitroso compounds ↑[66] | |
| Absorption of antioxidants ↓[67] | |
| Colorectal adenoma/ carcinoma | Direct damage[69] |
| Inflammation ↑[69] | |
| Bacterial overgrowth, bacterial fermentation (ammonia)↑[69-71,74,75] | |
| NO release ↑[76] | |
| Hypergastrinemia[68,69] | |
| Hepatobiliary disease | Ammonia ↑[90] |
| Endotoxemia[90] | |
| Inflammation ↑[90] | |
| Hepatic fibrosis ↑[87] | |
| Hepatoma cell adhesion and invasion ↑[91] | |
| Laryngeal cancer | Sensitivity to smoke and dust ↑[92] |
| Cell proliferation ↑[92] | |
| Apoptosis ↓[92] | |
| Lung cancer | Direct damage[97] |
| Sensitivity to smoke and dust ↑[98] | |
| Inhalation of gastrin and urea[95] | |
| Hypergastrinemia[94] | |
| Activation of docking protein p130cas[95] | |
| Inflammation ↑[94] | |
| Insulin resistance/ metabolic syndrome | Inflammation ↑[103,105] |
| Vasoconstrictor factors ↑[103,105] | |
| Adiponectin ↓[104] | |
| Atherogenesis | Inflammation ↑[108] |
| Autoimmunity[108] | |
| Fibrinogen ↑[112] | |
| Platelet aggregation ↑[114] | |
| Chronic urticaria | Vascular permeability ↑[83] |
| Complement consumption ↑[83] | |
| Pathogenetic antibodies ↑[83] | |
| Henoch-Schönlein purpura | IgA ↑[82] |
| Cryoglobulins ↑[82] | |
| C3 ↓[82] | |
| Possible preventive role | |
| Gastroesophageal reflux disease | Sympathetic tone ↑[128] |
| Cholinergic stimulation[128] | |
| Esophageal adenocarcinoma | Sympathetic tone ↑[128] |
| Cholinergic stimulation[128] | |
| Acid production ↓[129] | |
| Bronchial asthma | Polarization of Th-1 ↓[131] |
| Allergic Th-2 response ↓[131] | |
| Tregs ↓[132,133] | |
| Interleukin-1 receptor associated kinase M (IRAK-M) ↑[133] |
↑: Increase; ↓: Decrease.
Colorectal adenoma/carcinoma
On the basis of the epidemiological results showing high mortality rates from gastric and colorectal cancer in similar areas, it can be speculated that gastric cancer and colorectal cancer have common risk factors like H. pylori infection[68]. Although the role of H. pylori in colorectal carcinogenesis has been widely examined, the association has remained inconclusive[69]. Several studies demonstrated conflicting positive and negative associations[68,69]. A meta-analysis showed that H. pylori infection was associated with an increased risk of colorectal adenoma (OR = 1.66) and colorectal cancer (OR = 1.39), however there was significant heterogeneity among the studies[70]. The inconsistent results might be due to sample bias, small sample size, varying frequencies of cagA+ strains in the study population, incomplete colonoscopies, and evaluation of H. pylori infection with the IgG serum test[69].
H. pylori was detected in colorectal carcinoma tissue in a pilot study[71]. Higher prevalence was proven in adenoma and colorectal cancer compared with control[72,73]. H. pylori was more prevalent in moderate/severe dysplastic adenomas compared with mild dysplasia, and in tubular and tubulovillous adenoma compared with villous type[72].
The pathogenetic mechanisms of H. pylori induced colorectal carcinogenesis are not fully understood[69] (Table 2). However, not every study confirms the correlation between atrophic gastritis, hypergastrinemia, and colorectal cancer[68]. Conversely, atrophic gastritis and hypergastrinemia demonstrated a significant elevation in the odds ratio (3.15) for rectal cancer[68]. Overall, chronic atrophic gastritis did not seem to contribute to an increase in colorectal adenoma risk. Chronic atrophic gastritis and its progression appear to further increase the risk for proximal colorectal adenoma formation[77]. The inconsistent results correlating hypergastrinemia and colorectal carcinogenesis may be explained by the fact that gastrin precursors (progastrin and glycine-extended gastrin) act as important promoters of colorectal carcinogenesis, but cannot be measured by most commercially available assays[77,78].
Concomitant H. pylori infection with metabolic syndrome further increases the possibility of colorectal adenoma formation; however the pathomechanism for this possible association is still unclear[69]. Insulin might exert proliferative effects on colonic tumor cells directly or indirectly via the insulin-like growth factor pathway[79]. Chronic inflammation, increased pro-inflammatory cytokine production, and decreased anti-inflammatory adiponectin production might be associated with carcinogenesis[69,80]. Triglycerides are energy sources for cancer cell growth and are linked with increased synthesis of bile acids, which have a carcinogenesis promoting effect[81].
Extra-intestinal diseases
It has been shown that H. pylori may play a potential pathogenic role in extra-intestinal diseases via multiple mechanisms[82]. Atrophic gastritis caused by infection, an increase in gastric vascular permeability and therefore increased exposure to alimentary antigens, release of inflammatory mediators, and systemic immune responses (auto-immunity, pro-inflammatory substances, and immune complex formation induced by molecular mimicry and cross-reactive antibodies) have been suspected in the background[82,83].
With the exception of unexplained iron deficiency anemia (evidence level 1a) and idiopathic thrombocytopenic purpura (evidence level 1b), H. pylori infection has no proven role in other extra-intestinal diseases[82,84,85].
Hepatobiliary diseases
Helicobacter DNA has been detected in hepatic tissues from patients with various hepatobiliary diseases, hepatitis C virus-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC)[86,87]. The association between H. pylori and Child-Pugh classification is inconsistent[87]. It can be proposed that H. pylori infection may play a role in hepatic carcinogenesis as well[88]. The odds ratio for the association between H. pylori infection and the risk of HCC was 13.63[89] (Table 2).
Respiratory tract disorders
Laryngeal cancer: Colonization of bacteria in the upper aerodigestive tract was confirmed, however the relationship between H. pylori infection and laryngeal cancer risk have produced conflicting results. Meta-analysis showed a 2.03-fold increased risk[92].
H. pylori was detected in larynx cancerous tissue. The presence of the cagA gene in larynx cancer tissues significantly decreased survival rate and increased the possibility of disease recurrence[93] (Table 2).
Lung cancer: The results of previous studies of H. pylori seropositivity and lung cancer are inconclusive[94], with an odds ratio between 1.24 and 17.78 on the basis of the epidemiological studies[95]. The NHANES study observed an inverse association between H. pylori and lung cancer in older participants, with a significant inverse association for cagA+ strains; this was without histological examination[96]. A case-control study found no evidence of an association between H. pylori and lung cancer in Finish male smokers. Neither overall H. pylori seropositivity nor CagA-specific H. pylori seropositivity were associated with lung cancer[94]. Causal relationships must be confirmed with exact determination of smoking status[95] (Table 2).
Insulin resistance and metabolic syndrome
Epidemiological studies showed significant associations with metabolic syndrome (OR = 1.39)[99,100]. Furthermore, multiple linear regression analysis showed that H. pylori seropositivity was significantly associated with higher systolic blood pressure, lower high-density lipoprotein (HDL)-cholesterol level, and higher low-density lipoprotein (LDL)-cholesterol level[99]. It has been suggested that H. pylori eradication could lead to an improvement of atherogenic blood lipid profile, insulin resistance, and low-grade inflammation, which were deduced from a decreased C-reactive protein level[101]. Other studies did not find an association between H. pylori infection and insulin resistance[102,103].
The relationship between H. pylori infection and metabolic syndrome is both poorly understood[104] (Table 2) and controversial[106-108].
Cardiovascular diseases
Studies investigating the pathogenetic role of H. pylori in cardiovascular diseases have produced conflicting results[108-111]. A meta-analysis of 18 epidemiological studies involving 10,000 patients did not find any positive association between H. pylori and cardiovascular risk factors and coronary heart diseases[112]. A higher prevalence of more virulent cagA+ H. pylori was reported in patients with ischemic heart disease, unstable angina, acute myocardial infarction, and restenosis after percutaneous transluminal coronary angioplasty and essential hypertension[108,111,113].
Evidence on the relationship between H. pylori infection and ischemic heart disease is weak, with some inconclusive, albeit plausible, mechanisms (Table 2). There are also no adequate interventional studies done to demonstrate that H. pylori eradication is associated with a lower incidence of ischemic heart disease[108].
Dermatological disorders
Chronic urticaria: A correlation between H. pylori infection and chronic urticaria has been suggested (Table 2). H. pylori eradication in patients with chronic urticaria leads to symptomatic improvement in some patients, while others showed no improvement[83].
Hematological disorders
Immune thrombocytopenic purpura: The prevalence of H. pylori infection in patients with immune thrombocytopenic purpura (ITP) is significantly higher than that in age- and gender-matched controls[108,115,116]. The most plausible mechanism is cross-mimicry involving H. pylori, platelet antigens, and infected host factors (antibody production cross-reacts with platelet glycoprotein antigens)[108,117].
Eradication of H. pylori results in an increasing platelet count in nearly half of infected ITP patients, although geographical differences in the efficacy of eradication were also presumed[83,115,116]. The European Helicobacter Study Group consensus in 2012 and the Second Asia-Pacific Consensus Guidelines have recommended H. pylori infection eradication in patients with chronic idiopathic thrombocytopenic purpura[84,85]. However, larger randomized controlled trials with long-term follow-up are still required before a firm conclusion can be drawn[108].
Henoch-Schönlein purpura: A study in China found increasing evidence suggesting that Henoch-Schönlein purpura (HSP), especially abdominal HSP, might be associated with H. pylori infection (OR = 4.62); this underlines the necessity of screening H. pylori infection in children with HSP with gastrointestinal manifestations[82].
It was found that eradication of H. pylori infection resulted in prompt resolution of the HSP, or at least prevented its recurrence[118].
More investigations are needed to confirm the pathogenetic role of H. pylori in HSP (Table 2). HSP children with serious gastrointestinal symptoms must be screened and treated for H. pylori infection[82].
Iron deficiency anemia: Several epidemiological studies have shown lower ferritin levels among patients with H. pylori infection, although there were studies that produced a negative association[108]. Meta-analyses showed an association between H. pylori infection and iron deficiency anemia (IDA)[119,120]. H. pylori eradication improves iron absorption[121].
Possible pathomechanisms are: increased iron loss due to active hemorrhage secondary to gastritis, peptic ulcer, gastric cancer, reduced iron absorption caused by achlorhydria induced by chronic pangastritis, reduced secretion of ascorbic acid to the gastric mucosa, and iron utilization for protein synthesis by the bacterium for colonization in the host environment[122]. Elevated serum prohepcidin might also indicate the role of inflammation in its aetiology[123].
Testing and eradication of H. pylori for unexplained IDA are supported by the current evidence and approved by the Maastricht IV Consensus and the Second Asia-Pacific Consensus Guidelines[84,85]. However, larger sample randomized controlled trials are necessary to clarify the reason why only a small proportion of H. pylori-positive patients develop IDA[108].
Possible beneficial clinical consequences of H. pylori infection
Gastroesophageal reflux disease/esophageal adenocarcinoma: A meta-analysis showed that H. pylori infection displays a negative association with the development of endoscopic gastroesophageal reflux disease (GORD). Eradication of the infection may be a risk factor for development of de novo GORD[124].
H. pylori infection protects against gastroesophageal reflux[2]. H. pylori-induced corpus gastritis and profound suppression of gastric acid secretion have also been shown to prevent patients from developing GORD[125]. cagA+ H. pylori strains have a more protective effect against GORD[126], and it was found that H. pylori infection was inversely associated with Barrett’s esophagus[127].
The Maastricht consensus IV confirmed a negative association between the prevalence of H. pylori and the severity of GORD. The consensus stated that H. pylori status exerts no effect on symptom severity, recurrence, or treatment efficacy in GORD. H. pylori eradication does not exacerbate pre-existing GORD or affect treatment efficacy[85].
Esophageal adenocarcinoma risk due to H. pylori infection was 0.58-fold, and squamous cell carcinoma risk was 0.80-fold compared with that of controls. Compared with cagA- H. pylori, cagA+ H. pylori markedly decreased esophageal cancer risk[129].
The underlying mechanism in the background of the protective effect of H. pylori against GORD is not fully understood (Table 2).
H. pylori infection acts as neither a preventive factor nor a risk factor for squamous cell carcinoma. This discrepancy might be due to the relatively small number and heterogeneity of the included studies[129].
There is further need to assess the benefits of H. pylori in connection with GORD and its complications.
Bronchial asthma: An infection in the early phase of life is essential for the normal maturation of the immune system, achieving a balance between T-helper type 1 (protective immunity) and T-helper type 2 (allergic diseases) cytokine responses, which can reduce the risk of atopy later[129].
H. pylori infection might play a role in the development of chronic bronchitis, bronchiectasis, tuberculosis, and lung cancer[130]. Moreover, H. pylori might have an influence on the developing immune system, which might reduce the risk of asthma in later life[131].
The associations between H. pylori and asthma were contradictory. Inverse associations were reported, but other studies demonstrated different results[131]. A meta-analysis found weak evidence (OR = 0.81, 0.84) for an inverse association between H. pylori infection and asthma in children and adults, respectively[131,132]. Another meta-analysis failed to prove a significant association between H. pylori infection and asthma risk[130].
The mechanism of the preventive effect of H. pylori on asthma has been unambiguous (Table 2).
It seemed that H. pylori infection (especially cagA+ strains) may prevent children from developing asthma, but must be studied in the future[131] due to the inconsistent result[134].
CONCLUSION
The clinical outcome of H. pylori infection is determined by host genetic predisposition, bacterial strain factors, and environmental factors[1]. Bacterial virulence factors (VacA, CagA) can modulate the immune response involved in the initiation of the carcinogenesis in the stomach. Host genetic factors including IL-1β, IL-10, and TNF-α influence the inflammatory response and the exasperation of mucosal damage. Environmental factors, including salt intake and smoking tobacco, are well-known harmful aetiological factors. The ingestion of fruit and vegetables has some protective effect[135].
The mechanisms of H. pylori-associated gastric carcinogenesis are still poorly defined; further recognition may provide possibilities to develop effective strategies for gastric cancer prevention and treatment[1].
Indications for H. pylori therapy have been extended and now include idiopathic thrombocytopenic purpura, iron deficiency anemia, and vitamin B12 deficiency. New data are presented on the role of H. pylori in neurodegenerative disorders and in metabolic syndrome. H. pylori is associated with a small increase in the risk for colorectal adenoma and colon cancer[80] (Table 3).
Table 3.
| Renal diseases |
| Renal resistive index, proteinuria |
| Hepatobiliary diseases |
| Alcoholic damages of the liver, cholestatic autoimmune liver diseases (primary biliary diseases, primary sclerosing cholangitis), cholelithiasis, cholangiocellular carcinoma |
| Pancreatic disorders |
| Autoimmune pancreatitis |
| Intestinal diseases |
| Enteric diseases, inflammatory bowel diseases |
| Neurological diseases |
| Alzheimer-disease, idiopathic parkinsonism |
| Dermatological diseases |
| Alopecia areata, atopic dermatitis, lichen planus, chronic prurigo multiformis, nodular prurigo, pruritus, psoriasis, recurrent aphthous stomatitis, rosacea, Sweet’s syndrome |
| Ophthalmological diseases |
| Glaucoma, central serous chorioretinopathy, uveitis, blepharitis |
| Autoimmune disorders |
| Autoimmune thyroiditis, Behçet’s disease, Sjögren’s syndrome, progressive systemic sclerosis |
| Others |
| Impaired bioavailability of medication such as thyroxin and l-dopa, pre-eclampsia, chronic prostatitis, growth retardation |
H. pylori screening and treatment is a recommended gastric cancer risk reduction strategy in high-risk populations. In low-risk populations for gastric cancer, H. pylori screening is not recommended[84]. The removal of H. pylori from a large section of the population may be economically difficult, and the long-term consequences are still unpredictable. Identification of high-risk individuals is thus very important[40].
Footnotes
P- Reviewers: Ali IKM, Bach H, Liu ZJ, Luo JC, Matar G, Nagahara H S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Wang CH
References
- 1.Wen S, Moss SF. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009;282:1–8. doi: 10.1016/j.canlet.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra S. Is Helicobacter pylori good or bad? Eur J Clin Microbiol Infect Dis. 2013;32:301–304. doi: 10.1007/s10096-012-1773-9. [DOI] [PubMed] [Google Scholar]
- 3.Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 4.Molnar B, Galamb O, Sipos F, Leiszter K, Tulassay Z. Molecular pathogenesis of Helicobacter pylori infection: the role of bacterial virulence factors. Dig Dis. 2010;28:604–608. doi: 10.1159/000320060. [DOI] [PubMed] [Google Scholar]
- 5.Yamaoka Y. Pathogenesis of Helicobacter pylori-Related Gastroduodenal Diseases from Molecular Epidemiological Studies. Gastroenterol Res Pract. 2012;2012:371503. doi: 10.1155/2012/371503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatakeyama M. SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol. 2008;11:30–37. doi: 10.1016/j.mib.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Ruggiero P. Helicobacter pylori infection: what’s new. Curr Opin Infect Dis. 2012;25:337–344. doi: 10.1097/QCO.0b013e3283531f7c. [DOI] [PubMed] [Google Scholar]
- 9.Atherton JC, Cao P, Peek RM, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 10.Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18:479–486. doi: 10.1016/j.tim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, Chiba T, Fuss IJ, Kitani A, Strober W. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest. 2010;120:1645–1662. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaoka Y, Ojo O, Fujimoto S, Odenbreit S, Haas R, Gutierrez O, El-Zimaity HM, Reddy R, Arnqvist A, Graham DY. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006;55:775–781. doi: 10.1136/gut.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujikawa A, Shirasaka D, Yamamoto S, Ota H, Yahiro K, Fukada M, Shintani T, Wada A, Aoyama N, Hirayama T, et al. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33:375–381. doi: 10.1038/ng1112. [DOI] [PubMed] [Google Scholar]
- 14.Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 15.Ogiwara H, Graham DY, Yamaoka Y. vacA i-region subtyping. Gastroenterology. 2008;134:1267; author reply 1268. doi: 10.1053/j.gastro.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 16.Ogiwara H, Sugimoto M, Ohno T, Vilaichone RK, Mahachai V, Graham DY, Yamaoka Y. Role of deletion located between the intermediate and middle regions of the Helicobacter pylori vacA gene in cases of gastroduodenal diseases. J Clin Microbiol. 2009;47:3493–3500. doi: 10.1128/JCM.00887-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dossumbekova A, Prinz C, Gerhard M, Brenner L, Backert S, Kusters JG, Schmid RM, Rad R. Helicobacter pylori outer membrane proteins and gastric inflammation. Gut. 2006;55:1360–1361; author reply 1361. [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin LL, Huang HC, Ogihara S, Wang JT, Wu MC, McNeil PL, Chen CN, Juan HF. Helicobacter pylori Disrupts Host Cell Membranes, Initiating a Repair Response and Cell Proliferation. Int J Mol Sci. 2012;13:10176–10192. doi: 10.3390/ijms130810176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suganuma M, Watanabe T, Yamaguchi K, Takahashi A, Fujiki H. Human gastric cancer development with TNF-α-inducing protein secreted from Helicobacter pylori. Cancer Lett. 2012;322:133–138. doi: 10.1016/j.canlet.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Ihan A, Pinchuk IV, Beswick EJ. Inflammation, immunity, and vaccines for Helicobacter pylori infection. Helicobacter. 2012;17 Suppl 1:16–21. doi: 10.1111/j.1523-5378.2012.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller A, Solnick JV. Inflammation, immunity, and vaccine development for Helicobacter pylori. Helicobacter. 2011;16 Suppl 1:26–32. doi: 10.1111/j.1523-5378.2011.00877.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsai HF, Hsu PN. Interplay between Helicobacter pylori and immune cells in immune pathogenesis of gastric inflammation and mucosal pathology. Cell Mol Immunol. 2010;7:255–259. doi: 10.1038/cmi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabir S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter. 2011;16:1–8. doi: 10.1111/j.1523-5378.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 25.Malfertheiner P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig Dis. 2011;29:459–464. doi: 10.1159/000332213. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenberg A. Time trends of ulcer mortality in Europe. Gastroenterology. 2007;132:2320–2327. doi: 10.1053/j.gastro.2007.03.108. [DOI] [PubMed] [Google Scholar]
- 27.Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs in peptic ulcer disease. Can J Gastroenterol. 2006;20:277–280. doi: 10.1155/2006/175217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagymási K, Tulassay Z. [Peptic ulcer: facts and questions -- 2010] Orv Hetil. 2010;151:1054–1061. doi: 10.1556/OH.2010.28892. [DOI] [PubMed] [Google Scholar]
- 29.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 30.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 31.Venkateshwari A, Krishnaveni D, Venugopal S, Shashikumar P, Vidyasagar A, Jyothy A. Helicobacter pylori infection in relation to gastric cancer progression. Indian J Cancer. 2011;48:94–98. doi: 10.4103/0019-509X.75840. [DOI] [PubMed] [Google Scholar]
- 32.Pandey R, Misra V, Misra SP, Dwivedi M, Kumar A, Tiwari BK. Helicobacter pylori and gastric cancer. Asian Pac J Cancer Prev. 2010;11:583–588. [PubMed] [Google Scholar]
- 33.Hu Y, Fang JY, Xiao SD. Can the incidence of gastric cancer be reduced in the new century? J Dig Dis. 2013;14:11–15. doi: 10.1111/j.1751-2980.2012.00647.x. [DOI] [PubMed] [Google Scholar]
- 34.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK, Chen JS; China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 37.Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–1540. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farinati F, Cardin R, Della Libera G, Herszenyi L, Marafin C, Molari A, Plebani M, Rugge M, Naccarato R. The role of anti-oxidants in the chemoprevention of gastric cancer. Eur J Cancer Prev. 1994;3 Suppl 2:93–97. doi: 10.1097/00008469-199412002-00017. [DOI] [PubMed] [Google Scholar]
- 39.Tulassay Z, Stolte M, Engstrand L, Butruk E, Malfertheiner P, Dítê P, Tchernev K, Wong BC, Gottlow M, Eklund S, et al. Twelve-month endoscopic and histological analysis following proton-pump inhibitor-based triple therapy in Helicobacter pylori-positive patients with gastric ulcers. Scand J Gastroenterol. 2010;45:1048–1058. doi: 10.3109/00365520903575737. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira AC, Isomoto H, Moriyama M, Fujioka T, Machado JC, Yamaoka Y. Helicobacter and gastric malignancies. Helicobacter. 2008;13 Suppl 1:28–34. doi: 10.1111/j.1523-5378.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asenjo LM, Gisbert JP. Prevalence of Helicobacter pylori infection in gastric MALT lymphoma: a systematic review. Rev Esp Enferm Dig. 2007;99:398–404. doi: 10.4321/s1130-01082007000700006. [DOI] [PubMed] [Google Scholar]
- 42.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105–110. doi: 10.1016/j.cgh.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Wündisch T, Dieckhoff P, Greene B, Thiede C, Wilhelm C, Stolte M, Neubauer A. Second cancers and residual disease in patients treated for gastric mucosa-associated lymphoid tissue lymphoma by Helicobacter pylori eradication and followed for 10 years. Gastroenterology. 2012;143:936–942; quiz e13-14. doi: 10.1053/j.gastro.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Singh K, Ghoshal UC. Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma. World J Gastroenterol. 2006;12:1346–1351. doi: 10.3748/wjg.v12.i9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki R, Shiota S, Yamaoka Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infect Genet Evol. 2012;12:203–213. doi: 10.1016/j.meegid.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, Yamaoka Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793–1803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- 48.Shanks AM, El-Omar EM. Helicobacter pylori infection, host genetics and gastric cancer. J Dig Dis. 2009;10:157–164. doi: 10.1111/j.1751-2980.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- 49.Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro Alves C, Campos ML, Van Doorn LJ, Caldas C, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 50.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 51.Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204–2213. doi: 10.3748/wjg.15.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–4715. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 54.Ek C, Whary MT, Ihrig M, Bravo LE, Correa P, Fox JG. Serologic evidence that ascaris and toxoplasma infections impact inflammatory responses to Helicobacter pylori in Colombians. Helicobacter. 2012;17:107–115. doi: 10.1111/j.1523-5378.2011.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 56.Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488–492. doi: 10.1093/jnci/djs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang ZW, Farthing MJ. The roles of vitamin C in Helicobacter pylori associated gastric carcinogenesis. Chin J Dig Dis. 2005;6:53–58. doi: 10.1111/j.1443-9573.2005.00194.x. [DOI] [PubMed] [Google Scholar]
- 58.Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Kubo M, Tanizaki Y, Matsumoto T, Iida M, Kiyohara Y. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study. Am J Epidemiol. 2008;168:1409–1415. doi: 10.1093/aje/kwn276. [DOI] [PubMed] [Google Scholar]
- 59.Raderer M, Wrba F, Kornek G, Maca T, Koller DY, Weinlaender G, Hejna M, Scheithauer W. Association between Helicobacter pylori infection and pancreatic cancer. Oncology. 1998;55:16–19. doi: 10.1159/000011830. [DOI] [PubMed] [Google Scholar]
- 60.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, Perez-Perez G, Taylor PR, Virtamo J, Albanes D. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93:937–941. doi: 10.1093/jnci/93.12.937. [DOI] [PubMed] [Google Scholar]
- 61.Trikudanathan G, Philip A, Dasanu CA, Baker WL. Association between Helicobacter pylori infection and pancreatic cancer. A cumulative meta-analysis. JOP. 2011;12:26–31. [PubMed] [Google Scholar]
- 62.Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis. 2013;34:2193–2197. doi: 10.1093/carcin/bgt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jesnowski R, Isaksson B, Möhrcke C, Bertsch C, Bulajic M, Schneider-Brachert W, Klöppel G, Lowenfels AB, Maisonneuve P, Löhr JM. Helicobacter pylori in autoimmune pancreatitis and pancreatic carcinoma. Pancreatology. 2010;10:462–466. doi: 10.1159/000264677. [DOI] [PubMed] [Google Scholar]
- 64.Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95:948–960. doi: 10.1093/jnci/95.13.948. [DOI] [PubMed] [Google Scholar]
- 65.Risch HA. Pancreatic cancer: Helicobacter pylori colonization, N-nitrosamine exposures, and ABO blood group. Mol Carcinog. 2012;51:109–118. doi: 10.1002/mc.20826. [DOI] [PubMed] [Google Scholar]
- 66.Howatson AG, Carter DC. Pancreatic carcinogenesis: effect of secretin in the hamster- nitrosamine model. J Natl Cancer Inst. 1987;78:101–105. doi: 10.1093/jnci/78.1.101. [DOI] [PubMed] [Google Scholar]
- 67.Annibale B, Capurso G, Delle Fave G. Consequences of Helicobacter pylori infection on the absorption of micronutrients. Dig Liver Dis. 2002;34 Suppl 2:S72–S77. doi: 10.1016/s1590-8658(02)80170-0. [DOI] [PubMed] [Google Scholar]
- 68.Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Hanaoka T, Tsugane S. Atrophic gastritis, Helicobacter pylori, and colorectal cancer risk: a case-control study. Helicobacter. 2007;12:328–332. doi: 10.1111/j.1523-5378.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 69.Lin YL, Chiang JK, Lin SM, Tseng CE. Helicobacter pylori infection concomitant with metabolic syndrome further increase risk of colorectal adenomas. World J Gastroenterol. 2010;16:3841–3846. doi: 10.3748/wjg.v16.i30.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Q, Yang ZP, Xu P, Gao LC, Fan DM. Association between Helicobacter pylori infection and the risk of colorectal neoplasia: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e352–e364. doi: 10.1111/codi.12284. [DOI] [PubMed] [Google Scholar]
- 71.Jones M, Helliwell P, Pritchard C, Tharakan J, Mathew J. Helicobacter pylori in colorectal neoplasms: is there an aetiological relationship? World J Surg Oncol. 2007;5:51. doi: 10.1186/1477-7819-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kountouras J, Kapetanakis N, Zavos C, Romiopoulos I. Impact of Helicobacter pylori infection on normal colorectal mucosa, adenomatous polyps and adenocarcinoma sequence. Colorectal Dis. 2014;16:390–391. doi: 10.1111/codi.12356. [DOI] [PubMed] [Google Scholar]
- 73.Nam KW, Baeg MK, Kwon JH, Cho SH, Na SJ, Choi MG. Helicobacter pylori seropositivity is positively associated with colorectal neoplasms. Korean J Gastroenterol. 2013;61:259–264. doi: 10.4166/kjg.2013.61.5.259. [DOI] [PubMed] [Google Scholar]
- 74.Visek WJ. Diet and cell growth modulation by ammonia. Am J Clin Nutr. 1978;31:S216–S220. doi: 10.1093/ajcn/31.10.S216. [DOI] [PubMed] [Google Scholar]
- 75.Clinton SK, Bostwick DG, Olson LM, Mangian HJ, Visek WJ. Effects of ammonium acetate and sodium cholate on N-methyl-N’-nitro-N-nitrosoguanidine-induced colon carcinogenesis of rats. Cancer Res. 1988;48:3035–3039. [PubMed] [Google Scholar]
- 76.Cavallo P, Cianciulli A, Mitolo V, Panaro MA. Lipopolysaccharide (LPS) of helicobacter modulates cellular DNA repair systems in intestinal cells. Clin Exp Med. 2011;11:171–179. doi: 10.1007/s10238-010-0118-1. [DOI] [PubMed] [Google Scholar]
- 77.Inoue I, Mukoubayashi C, Yoshimura N, Niwa T, Deguchi H, Watanabe M, Enomoto S, Maekita T, Ueda K, Iguchi M, et al. Elevated risk of colorectal adenoma with Helicobacter pylori-related chronic gastritis: a population-based case-control study. Int J Cancer. 2011;129:2704–2711. doi: 10.1002/ijc.25931. [DOI] [PubMed] [Google Scholar]
- 78.Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta. 2004;1704:1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 80.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 81.Hill MJ. Bile flow and colon cancer. Mutat Res. 1990;238:313–320. doi: 10.1016/0165-1110(90)90023-5. [DOI] [PubMed] [Google Scholar]
- 82.Xiong LJ, Tong Y, Wang ZL, Mao M. Is Helicobacter pylori infection associated with Henoch-Schonlein purpura in Chinese children? a meta-analysis. World J Pediatr. 2012;8:301–308. doi: 10.1007/s12519-012-0373-1. [DOI] [PubMed] [Google Scholar]
- 83.Hernando-Harder AC, Booken N, Goerdt S, Singer MV, Harder H. Helicobacter pylori infection and dermatologic diseases. Eur J Dermatol. 2009;19:431–444. doi: 10.1684/ejd.2009.0739. [DOI] [PubMed] [Google Scholar]
- 84.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 85.Malfertheiner P, Selgrad M, Bornschein J. Helicobacter pylori: clinical management. Curr Opin Gastroenterol. 2012;28:608–614. doi: 10.1097/MOG.0b013e32835918a7. [DOI] [PubMed] [Google Scholar]
- 86.Rabelo-Gonçalves EM, Sgardioli IC, Lopes-Cendes I, Escanhoela CA, Almeida JR, Zeitune JM. Improved detection of Helicobacter pylori DNA in formalin-fixed paraffin-embedded (FFPE) tissue of patients with hepatocellular carcinoma using laser capture microdissection (LCM) Helicobacter. 2013;18:244–245. doi: 10.1111/hel.12040. [DOI] [PubMed] [Google Scholar]
- 87.Esmat G, El-Bendary M, Zakarya S, Ela MA, Zalata K. Role of Helicobacter pylori in patients with HCV-related chronic hepatitis and cirrhosis with or without hepatocellular carcinoma: possible association with disease progression. J Viral Hepat. 2012;19:473–479. doi: 10.1111/j.1365-2893.2011.01567.x. [DOI] [PubMed] [Google Scholar]
- 88.Tu QV, Okoli AS, Kovach Z, Mendz GL. Hepatocellular carcinoma: prevalence and molecular pathogenesis of Helicobacter spp. Future Microbiol. 2009;4:1283–1301. doi: 10.2217/fmb.09.90. [DOI] [PubMed] [Google Scholar]
- 89.Xuan SY, Xin YN, Chen AJ, Dong QJ, Qiang X, Li N, Zheng MH, Guan HS. Association between the presence of H pylori in the liver and hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2008;14:307–312. doi: 10.3748/wjg.14.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abdel-Hady H, Zaki A, Badra G, Lotfy M, Selmi C, Giorgini A, El-Sayed M, Badr R. Helicobacter pylori infection in hepatic encephalopathy: Relationship to plasma endotoxins and blood ammonia. Hepatol Res. 2007;37:1026–1033. doi: 10.1111/j.1872-034X.2007.00146.x. [DOI] [PubMed] [Google Scholar]
- 91.Liu X, Liang J, Li G. Lipopolysaccharide promotes adhesion and invasion of hepatoma cell lines HepG2 and HepG2.2.15. Mol Biol Rep. 2010;37:2235–2239. doi: 10.1007/s11033-009-9710-4. [DOI] [PubMed] [Google Scholar]
- 92.Zhuo XL, Wang Y, Zhuo WL, Zhang XY. Possible association of Helicobacter pylori infection with laryngeal cancer risk: an evidence-based meta-analysis. Arch Med Res. 2008;39:625–628. doi: 10.1016/j.arcmed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 93.Burduk PK. Association between infection of virulence cagA gene Helicobacter pylori and laryngeal squamous cell carcinoma. Med Sci Monit. 2013;19:584–591. doi: 10.12659/MSM.889011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koshiol J, Flores R, Lam TK, Taylor PR, Weinstein SJ, Virtamo J, Albanes D, Perez-Perez G, Caporaso NE, Blaser MJ. Helicobacter pylori seropositivity and risk of lung cancer. PLoS One. 2012;7:e32106. doi: 10.1371/journal.pone.0032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deng B, Li Y, Zhang Y, Bai L, Yang P. Helicobacter pylori infection and lung cancer: a review of an emerging hypothesis. Carcinogenesis. 2013;34:1189–1195. doi: 10.1093/carcin/bgt114. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62:1262–1269. doi: 10.1136/gutjnl-2012-303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moss SF. The carcinogenic effect of H. pylori on the gastric epithelial cell. J Physiol Pharmacol. 1999;50:847–856. [PubMed] [Google Scholar]
- 98.Zhuo WL, Zhu B, Xiang ZL, Zhuo XL, Cai L, Chen ZT. Assessment of the relationship between Helicobacter pylori and lung cancer: a meta-analysis. Arch Med Res. 2009;40:406–410. doi: 10.1016/j.arcmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 99.Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol. 2008;103:3005–3010. doi: 10.1111/j.1572-0241.2008.02151.x. [DOI] [PubMed] [Google Scholar]
- 100.Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. 2009;14:144–150. doi: 10.1111/j.1523-5378.2009.00705.x. [DOI] [PubMed] [Google Scholar]
- 101.Gen R, Demir M, Ataseven H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation. South Med J. 2010;103:190–196. doi: 10.1097/SMJ.0b013e3181cf373f. [DOI] [PubMed] [Google Scholar]
- 102.Gillum RF. Infection with Helicobacter pylori, coronary heart disease, cardiovascular risk factors, and systemic inflammation: the Third National Health and Nutrition Examination Survey. J Natl Med Assoc. 2004;96:1470–1476. [PMC free article] [PubMed] [Google Scholar]
- 103.Naja F, Nasreddine L, Hwalla N, Moghames P, Shoaib H, Fatfat M, Sibai A, Gali-Muhtasib H. Association of H. pylori infection with insulin resistance and metabolic syndrome among Lebanese adults. Helicobacter. 2012;17:444–451. doi: 10.1111/j.1523-5378.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 104.Ando T, Ishikawa T, Takagi T, Imamoto E, Kishimoto E, Okajima A, Uchiyama K, Handa O, Yagi N, Kokura S, et al. Impact of Helicobacter pylori eradication on circulating adiponectin in humans. Helicobacter. 2013;18:158–164. doi: 10.1111/hel.12028. [DOI] [PubMed] [Google Scholar]
- 105.Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16:79–88. doi: 10.1111/j.1523-5378.2011.00822.x. [DOI] [PubMed] [Google Scholar]
- 106.Albaker WI. Helicobacter pylori infection and its relationship to metabolic syndrome: is it a myth or fact? Saudi J Gastroenterol. 2011;17:165–169. doi: 10.4103/1319-3767.80377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eshraghian A, Eshraghian H, Ranjbar Omrani G. Insulin resistance and metabolic syndrome: is Helicobacter pylori criminal? Minerva Gastroenterol Dietol. 2011;57:379–385. [PubMed] [Google Scholar]
- 108.Tan HJ, Goh KL. Extragastrointestinal manifestations of Helicobacter pylori infection: facts or myth? A critical review. J Dig Dis. 2012;13:342–349. doi: 10.1111/j.1751-2980.2012.00599.x. [DOI] [PubMed] [Google Scholar]
- 109.Christodoulou DK, Milionis HJ, Pappa P, Katsanos KH, Sigounas D, Florentin M, Elisaf M, Tsianos EV. Association of Helicobacter pylori infection with cardiovascular disease--is it just a myth? Eur J Intern Med. 2011;22:191–194. doi: 10.1016/j.ejim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 110.Schöttker B, Adamu MA, Weck MN, Müller H, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and major cardiovascular events: a population-based cohort study. Atherosclerosis. 2012;220:569–574. doi: 10.1016/j.atherosclerosis.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki H, Franceschi F, Nishizawa T, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2011;16 Suppl 1:65–69. doi: 10.1111/j.1523-5378.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 112.Danesh J, Peto R. Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. BMJ. 1998;316:1130–1132. doi: 10.1136/bmj.316.7138.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vahdat K, Pourbehi MR, Ostovar A, Hadavand F, Bolkheir A, Assadi M, Farrokhnia M, Nabipour I. Association of pathogen burden and hypertension: the Persian Gulf Healthy Heart Study. Am J Hypertens. 2013;26:1140–1147. doi: 10.1093/ajh/hpt083. [DOI] [PubMed] [Google Scholar]
- 114.Fagoonee S, De Angelis C, Elia C, Silvano S, Oliaro E, Rizzetto M, Pellicano R. Potential link between Helicobacter pylori and ischemic heart disease: does the bacterium elicit thrombosis? Minerva Med. 2010;101:121–125. [PubMed] [Google Scholar]
- 115.Yu T, Wu D, Zhao XY. Infection and eradication of Helicobacter Pylorus affecting etiology and curative effect of idiopathic thrombocytopenic purpura: a META analysis. Zhongguo Shiyanxue Yexue Zazhi. 2011;19:1255–1259. [PubMed] [Google Scholar]
- 116.Franchini M, Veneri D. Helicobacter pylori-associated immune thrombocytopenia. Platelets. 2006;17:71–77. doi: 10.1080/09537100500438057. [DOI] [PubMed] [Google Scholar]
- 117.Hasni SA. Role of Helicobacter pylori infection in autoimmune diseases. Curr Opin Rheumatol. 2012;24:429–434. doi: 10.1097/BOR.0b013e3283542d0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Q, Lin X, Wu Z, He L, Wang W, Cao Q, Zhang J. Immuno-histochemistry analysis of Helicobacter pylori antigen in renal biopsy specimens from patients with glomerulonephritis. Saudi J Kidney Dis Transpl. 2013;24:751–758. doi: 10.4103/1319-2442.113871. [DOI] [PubMed] [Google Scholar]
- 119.Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter. 2008;13:323–340. doi: 10.1111/j.1523-5378.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 120.Qu XH, Huang XL, Xiong P, Zhu CY, Huang YL, Lu LG, Sun X, Rong L, Zhong L, Sun DY, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol. 2010;16:886–896. doi: 10.3748/wjg.v16.i7.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang ZF, Yang N, Zhao G, Zhu L, Zhu Y, Wang LX. Effect of Helicobacter pylori eradication on iron deficiency. Chin Med J (Engl) 2010;123:1924–1930. [PubMed] [Google Scholar]
- 122.Realdi G, Dore MP, Fastame L. Extradigestive manifestations of Helicobacter pylori infection: fact and fiction. Dig Dis Sci. 1999;44:229–236. doi: 10.1023/a:1026677728175. [DOI] [PubMed] [Google Scholar]
- 123.Ozkasap S, Yarali N, Isik P, Bay A, Kara A, Tunc B. The role of prohepcidin in anemia due to Helicobacter pylori infection. Pediatr Hematol Oncol. 2013;30:425–431. doi: 10.3109/08880018.2013.783144. [DOI] [PubMed] [Google Scholar]
- 124.Xie T, Cui X, Zheng H, Chen D, He L, Jiang B. Meta-analysis: eradication of Helicobacter pylori infection is associated with the development of endoscopic gastroesophageal reflux disease. Eur J Gastroenterol Hepatol. 2013;25:1195–1205. doi: 10.1097/MEG.0b013e328363e2c7. [DOI] [PubMed] [Google Scholar]
- 125.El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997;113:15–24. doi: 10.1016/s0016-5085(97)70075-1. [DOI] [PubMed] [Google Scholar]
- 126.Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, Perez-Perez GI, Schoenberg JB, Stanford JL, Rotterdam H, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–590. [PubMed] [Google Scholar]
- 127.Rubenstein JH, Inadomi JM, Scheiman J, Schoenfeld P, Appelman H, Zhang M, Metko V, Kao JY. Association between Helicobacter pylori and Barrett’s esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol. 2014;12:239–245. doi: 10.1016/j.cgh.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shahabi S, Rasmi Y, Jazani NH, Hassan ZM. Protective effects of Helicobacter pylori against gastroesophageal reflux disease may be due to a neuroimmunological anti-inflammatory mechanism. Immunol Cell Biol. 2008;86:175–178. doi: 10.1038/sj.icb.7100119. [DOI] [PubMed] [Google Scholar]
- 129.Zhuo X, Zhang Y, Wang Y, Zhuo W, Zhu Y, Zhang X. Helicobacter pylori infection and oesophageal cancer risk: association studies via evidence-based meta-analyses. Clin Oncol (R Coll Radiol) 2008;20:757–762. doi: 10.1016/j.clon.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y, Bi Y, Zhang L, Wang C. Is Helicobacter pylori infection associated with asthma risk? A meta-analysis based on 770 cases and 785 controls. Int J Med Sci. 2012;9:603–610. doi: 10.7150/ijms.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Q, Yu C, Sun Y. The association between asthma and Helicobacter pylori: a meta-analysis. Helicobacter. 2013;18:41–53. doi: 10.1111/hel.12012. [DOI] [PubMed] [Google Scholar]
- 132.Zhou X, Wu J, Zhang G. Association between Helicobacter pylori and asthma: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:460–468. doi: 10.1097/MEG.0b013e32835c280a. [DOI] [PubMed] [Google Scholar]
- 133.Shiu J, Czinn SJ, Kobayashi KS, Sun Y, Blanchard TG. IRAK-M expression limits dendritic cell activation and proinflammatory cytokine production in response to Helicobacter pylori. PLoS One. 2013;8:e66914. doi: 10.1371/journal.pone.0066914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karimi A, Fakhimi-Derakhshan K, Imanzadeh F, Rezaei M, Cavoshzadeh Z, Maham S. Helicobacter pylori infection and pediatric asthma. Iran J Microbiol. 2013;5:132–135. [PMC free article] [PubMed] [Google Scholar]
- 135.Bornschein J, Malfertheiner P. Gastric carcinogenesis. Langenbecks Arch Surg. 2011;396:729–742. doi: 10.1007/s00423-011-0810-y. [DOI] [PubMed] [Google Scholar]
- 136.Gonciarz M, Włoch M, Gonciarz Z. Helicobacter pylori in liver diseases. J Physiol Pharmacol. 2006;57 Suppl 3:155–161. [PubMed] [Google Scholar]
- 137.Bohr UR, Annibale B, Franceschi F, Roccarina D, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection -- other Helicobacters. Helicobacter. 2007;12 Suppl 1:45–53. doi: 10.1111/j.1523-5378.2007.00533.x. [DOI] [PubMed] [Google Scholar]


