Abstract
The cure rates of Helicobacter pylori (H. pylori) eradication therapy using a proton pump inhibitor (PPI) and antimicrobial agents such as amoxicillin, clarithromycin, and metronidazole are mainly influenced by bacterial susceptibility to antimicrobial agents and the magnitude of the inhibition of acid secretion. Annual cure rates have gradually decreased because of the increased prevalence of H. pylori strains resistant to antimicrobial agents, especially to clarithromycin. Alternative regimens have therefore been developed incorporating different antimicrobial agents. Further, standard PPI therapy (twice-daily dosing) often fails to induce a long-term increase in intragastric pH > 4.0. Increasing the eradication rate requires more frequent and higher doses of PPIs. Therapeutic efficacy related to acid secretion is influenced by genetic factors such as variants of the genes encoding drug-metabolizing enzymes (e.g., cytochrome P450 2C19, CYP2C19), drug transporters (e.g., multidrug resistance protein-1; ABCB1), and inflammatory cytokines (e.g., interleukin-1β). For example, quadruple daily administration of PPI therapy potently inhibits acid secretion within 24 h, irrespective of CYP2C19 genotype. Therefore, tailored H. pylori eradication regimens that address acid secretion and employ optimal antimicrobial agents based on results of antimicrobial agent-susceptibility testing may prove effective in attaining higher eradication rates.
Keywords: Helicobacter pylori, Tailored eradication therapy, Proton pomp inhibitor, Cytochrome P450 2C19, Clarithromycin
Core tip: The eradication for Helicobacter pylori infection is mainly influenced by antibiotic susceptibility and insufficient acid inhibition [e.g., cytochrome P450 2C19 (CYP2C19) genotype, proton pump inhibitor (PPI) dose, and PPI treatment schedule]. When a PPI is administered to CYP2C19 rapid metabolizers and intermediate metabolizers, plasma levels of PPIs cannot be maintained between once-daily doses. The intragastric pH attained with four-times-daily-dosing of PPI is significantly higher than those observed when PPI is administered as once-daily-dosing of four-fold doses or twice-daily-dosing of two-fold doses. We describe a tailored treatment that was designed according to pharmacogenomics and antimicrobial susceptibility to achieve an eradication rate exceeding 95%, irrespective of different CYP2C19 genotypes.
INTRODUCTION
The Maastricht IV/Florence Consensus Report issued by the European Helicobacter Study Group in 2012[1] recommends Helicobacter pylori (H. pylori) eradication therapy as first-line treatment for patients with upper gastrointestinal disorders such as peptic ulcer disease, gastric mucosa associated-lymphoid tissue lymphoma {Evidence level in support of the recommendations formulated in the Maastricht IV/Florence Consensus Report: 1a [Systematic review of randomized controlled trial (RCT)], Grade of recommendation: A}, atrophic gastritis [2a (Systematic review of cohort studies) and B], intestinal metaplasia (2a and B), and functional dyspepsia (1a and A) as well as for patients with extra-gastrointestinal disorders such as idiopathic thrombocytopenic purpura [1b (Individual RCT with narrow CI) and A], vitamin B12 deficiency [3b (Individual case-control study) and B], and iron-deficiency anemia (1a and A)[2-11].
“Test and treat” is a strategy involving a noninvasive test (e.g., serum anti-H. pylori IgG or urea breath test) given to patients with dyspepsia to diagnose H. pylori infection of the gastric mucosa, with treatment initiated upon detection[1]. In Japan, first-line H. pylori eradication therapy is limited to a regimen that employs a proton pump inhibitor (PPI) administered twice-daily (bid) using a standard dose (e.g., omeprazole, rabeprazole, lansoprazole, and esomeprazole), amoxicillin (AMPC) 750 mg bid, and clarithromycin (CAM) 200 mg or 400 mg bid for 1 wk. Unfortunately, the prevalence of CAM-resistant H. pylori strains in Japan is increasing (> 30%)[12,13]. Therefore, alternative regimens are designed to consist of different antimicrobial agents with susceptibility to H. pylori strain, increased dosing dosages of antimicrobial agents and PPIs, increased dosing times of drugs and prolonged treatment periods[14-17].
In this review article, we consider first the factors that influence the cure rate of H. pylori eradication therapy and the importance of inhibiting acid secretion. We then propose optimal treatment strategies based on the most effective antimicrobial agents and the patient’s genotype.
FACTORS CONTRIBUTING TO THE SUCCESS OF H. PYLORI ERADICATION THERAPY
The cure rates of H. pylori infection are influenced by several factors such as antibiotic susceptibility[12,13,18], insufficient inhibition of acid secretion [e.g., cytochrome P450 2C19 (CYP2C19) genotype, PPI dose, and PPI treatment schedule][19], bacterial genotypes that reduce virulence (e.g., cagA-negative strains and the vacA s2 genotype), the environment (e.g., smoking), and compliance (Table 1).
Table 1.
Major factors preventing eradication of Helicobacter pylori infection
| Factor | ||
| Antibiotics | Resistance to antibiotics | Clarithromycin |
| Metronidazole | ||
| Levofloxacin | ||
| Amoxicillin | ||
| Inhibition of acid secretion | CYP2C19 | Rapid metabolizer |
| CYP2C19*17 | *17 carrier | |
| ABCB1 3435 | C/C genotype (Caucasian) | |
| IL-1B-511 | C/C genotype | |
| IL-1B-31 | T/T genotype | |
| Acid inhibitory drug dosing time | Low frequency (oid) | |
| Acid inhibitory drug dosing dose | Insufficient dose | |
| H. pylori phenotype | H. pylori virulence factors | cagA-negative |
| vacA s2 genotype | ||
| Environment | Volume | Much |
| Smoking | Many | |
| Compliance | Poor |
CYP2C19: Cytochrome P450 2C19; IL: Interleukin; ABCB1: Multidrug resistance protein-1; H. pylori: Helicobacter pylori.
Antibiotics targeted to H. pylori strains with known drug sensitivities will likely increase the eradiation rate[20,21]. When patients are treated with antibiotics that are targeted specifically to the infecting H. pylori strain, increased eradication rates are achieved. Therefore, determining the antibiotic susceptibility of H. pylori using either or both culture or genetic testing is of great import, particularly in a population with a high rate of infection with drug-resistant strains. These tests should be conducted before treatment, particularly with second- and third-line treatment[1]. The Maastricht IV Consensus Report[1] recommends that, after a second failure, if culture and susceptibility testing is not possible, molecular genetic tests should be conducted to detect H pylori. Recently, a novel fully-automated rapid genetic analyzer was developed which was capable of determining CAM resistance (e.g., 23S rRNA gene point mutations of A2143G and A2144G) within 60-120 min[20], whereas culture tests required 7-10 d. Note that the treatment strategy should take into account the drug resistance of H. pylori in different patient populations in different localities.
The optimal intragastric pH condition with potent acid inhibition in the stomach makes selected antibacterial agents more stable and bioavailable[21,22]. Controlling intragastric pH with PPIs depends on dosing schedules, dose, and combination of acid inhibitors[23-27], and polymorphisms in the genes encoding drug-metabolizing enzymes and drug transporter genes such as CYP2C19 and multidrug resistance protein-1 (ABCB1) influence pH during treatment[23-29]. In the gastrointestinal tract, ABCB1 is expressed on the apical surfaces of superficial columnar epithelial cells of distal small bowel and the pharmacokinetics of PPIs are influenced by the activity of ABCB1. Polymorphism of ABCB1 is one of the determinants of successful eradication of H. pylori by the triple therapy with lansoprazole, amoxicillin and clarithromycin, and eradication rates for H. pylori infection are 82%, 81% and 67% in patients with the ABCB1 3435 C/C, C/T and T/T genotype, respectively (P = 0.001)[30]. In particular, patients’ pharmacogenetic characteristics [e.g., CYP2C19 rapid metabolizer (RM), interleukin (IL)-1β-511 C/C, and ABCB1 3435 T/T genotype] require a modified treatment regimen to effectively inhibit acid secretion for 24 h[23-29].
The increased levels of IL-1β induced by H. pylori infection potently inhibit acid production, and IL-1β is one hundred times more potent an inhibitor than PPIs on a molar basis[31]. Polymorphisms in the gene encoding IL-1β are associated with individual differences in IL-1β levels[32], and presence of the IL-1B-511 polymorphism has been shown to be related to eradication rate[33-35]. Indeed, the eradication rate achieved for patients with the low IL-1β-producer genotype IL-1B-511 C/C (77.4%) is lower than that of the C/T and T/T genotypes (87.2%; odds ratio for failure: 1.98, 95%CI: 1.38-2.84, P = 0.0002)[35]. Further, H. pylori virulence factors (e.g., cagA and vacA), which play critical roles in gastric mucosal injury and inflammation, determine cure rates[19]. In a meta-analysis, individuals infected with strains with the cagA-negative 69.9% (95%CI: 65.7%-73.9%) vs 83.1% (80.7%-85.3%) for cagA-positive genotypes and vacA s2 genotype [72.1% (64.8%-78.7%) vs vacA s1 genotype 79.2% (75.1%-83.0%] are at increased risk of failure of eradication therapy[19]. The presence of dupA, which is associated with the development of duodenal ulcers, influences the cure rate of eradication therapy[36,37].
CAM RESISTANCE AND ERADICATION TREATMENT
CAM is a key component of H. pylori eradication therapy, exerting its antimicrobial effects by binding to the subunit 23S of the bacterial ribosome, which inhibits protein synthesis. In general, although the cut-off concentration used to define resistance to CAM is higher than 1.0 mg/mL[38], bacterial susceptibility to CAM in most strains is conferred by a single nucleotide polymorphism at either position 2142 or 2143 of the H. pylori 23S rRNA gene (A2142G or A2143G). The most frequent mutation is A2143G (69.8%), followed by A2142G (11.7%) and A2142C (2.6%)[39,40].
From 1990 to 2000, the eradication rates achieved in Japan using CAM-based triple therapy ranged from approximately 85%-91%[12]; in contrast, eradication rates for patients infected with CAM-resistant strains were markedly low (10%-30%)[41,42]. The frequencies of resistance of H. pylori strains to CAM in Japan and Europe exceed 35% and 20%, respectively, and account for the relatively low eradication rates with the CAM-based regimen[12,13,42,43].
The Maastricht IV consensus report recommends first-line eradication treatment using a CAM-based regimen [PPI-CAM-AMPC or -metronidazole (MNZ)] and an alternative eradication using a bismuth-containing quadruple treatment in areas where prevalence of CAM-resistant strains is low, and a bismuth-containing quadruple treatment in areas of high resistance[1]. Extending the duration of CAM-based triple treatment from 7 to 10-14 d improves the eradication success rate by approximately 5%[44,45]. When CAM-based treatment fails, either bismuth-containing quadruple therapy or levofloxacin-based therapy is recommended. In areas of high CAM resistance, levofloxacin-based treatment is recommended after quadruple therapy fails.
IMPORTANCE OF INHIBITING GASTRIC ACID SECRETION DURING ERADICATION THERAPY
Rapid and potent neutralization of intragastric pH after treatment with drugs that inhibit acid secretion is required to cure acid-related diseases. Thus, intragastric pH during treatment is associated with the cure rates of peptic ulcers[46], gastroesophageal reflux disease[47], and aspirin-induced gastroduodenal mucosal injury[48]. Further, eradication treatment fails if acid secretion is not sufficiently inhibited[49-51].
Whereas H. pylori survives with a periplasmic pH of 4.0-8.0, it only grows between pH 6.0-8.0[52]. When bacterial urease activity elevates the pH to 4.0-6.0, H. pylori survives but does not divide[52]. The consistent and potent action of drugs that inhibit acid secretion increases the stability and bioavailability of acid-sensitive antimicrobial agents such as CAM and AMPC by preventing their degradation in the stomach. Further, these inhibitors increase the concentration of antimicrobial agents in the gastric mucosa[21,52,53]. Raising pH from 3.5 to 5.5 increases the in vitro effectiveness of AMPC more than 10-fold[21]. Ampicillin is bactericidal at pH 4.5-7.4 but not at pH 3.0, as this pH inhibits the expression of genes encoding cell envelope components and proteins required for cell division[54]. The activity of CAM is higher at pH 7.4 than at pH 5.0, and activity is intermediate at pH 6.8[55]. Inhibiting acid secretion also allows H. pylori to grow and become more sensitive to antimicrobial agents[52].
In a previous study, the 24-h intragastric pH level in patients treated successfully with eradication therapy (omeprazole 20 mg bid and AMPC 1 g bid) was found to be higher than in patients that failed 7-d treatment[51]. When 24-h pH exceeds 5.5, H. pylori can be eradicated using dual PPI/AMPC therapy without a second antimicrobial agent such as CAM, MNZ, or both[51]. Eradication using PPI/AMPC therapy is preferred for patients with pH > 4.0 for 75% of total treatment duration and above 5.5 in 4-h pH[49]. Univariate analysis shows that pH values depend on the dose of omeprazole (P = 0.003), CYP2C19 genotype (P = 0.001), and H. pylori density (P = 0.044)[49]. We demonstrate further that the median 24-h pH was 6.4 (range, 5.0-7.6) for successful eradication therapy compared with that for failed therapy [pH 5.2 (2.2-6.2), P = 0.0131] and that median percentage time at pH < 4.0 for successful cures [0.5% (0.0%-31.6%)] is significantly shorter compared with failures [26.7% (6.0%-72.2%), P = 0.0017; Figure 1A and B][50]. Therefore, the degree and duration of acid suppression are related to cure rate, and we may conclude that pH > 4 should be maintained for 24 h and 24-h pH higher than 6.0 (Figure 1C). Unfortunately, the PPI standard-dose bid treatment does not maintain pH values > 4.0 long enough to satisfy the above criteria in all of patients[23].
Figure 1.
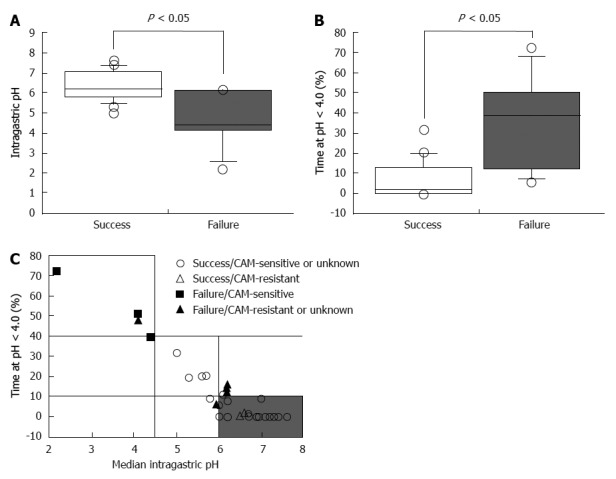
Success of Helicobacter pylori eradication treatment as a function of pH. A, B: Median 24-h pH values (A) and the percentage of the times when pH < 4.0 during eradication therapy according to successful and failed treatment (B); C: Variation of pH and the percentage of time at pH < 4.0[51]. The median pH of successfully treated patients was significantly higher than compared with patients that failed treatment (A). The median percentage of the time when pH < 4.0 in successfully treated patients was significantly shorter compared with unsuccessfully treated patients (B). The majority of patients were cured using triple therapy when the percentage of time at pH < 4.0 during the 24-h post-dose period was < 10% and the 24-h pH was > 6.0 (shaded area) (C). CAM: Clarithromycin.
CYP2C19 POLYMORPHISMS AND H. PYLORI ERADICATION THERAPY
Pharmacokinetics and pharmacodynamics of PPIs according to CYP2C19 genotype
PPIs delivered through the circulatory system are absorbed in the small intestine and reach gastric parietal cells where they bind irreversibly to and alter the function of H+/K+-ATPase, which potently inhibits acid secretion[56,57]. Therefore, PPIs are currently used as the first-line treatment of acid-related diseases[33,50,58,59].
PPIs undergo extensive hepatic metabolism by the CYP system, which includes CYP2C19 and CYP3A4 (Figure 2)[60]. CYP2C19 polymorphisms therefore influence both pharmacokinetics [i.e., peak plasma concentration (Cmax) and area under the curve (AUC) of the plasma concentration] and pharmacodynamics (i.e., intragastric pH) of PPIs (Figure 3A and B). At least 20 CYP2C19 variants (Figure 4) have been identified, with the majority classified into three genotypes: RMs, intermediate metabolizers (IMs), and poor metabolizers (PMs)[60-63]. The inhibition of acid secretion achieved using PPIs (e.g., omeprazole and lansoprazole) to treat PMs is enhanced compared with IMs or RMs because of the different pharmacokinetics among the three CYP2C19 genotypes[26,27,29,64]. In contrast, rabeprazole reduces acid secretion mainly via a non-enzymatic reaction, with minor involvement of CYP2C19 (Figure 2)[60,65], and its acid inhibitory effect is less influenced by CYP2C19 genotypes than that of omeprazole or lansoprazole. Esomeprazole, which is the S-isomer of omeprazole, more effectively inhibits acid secretion than omeprazole[66,67], and this relatively high activity can be attributed to its less extensive metabolism during first-pass hepatic metabolism compared with omeprazole. The increased systemic bioavailability of esomeprazole offers the prospect of improved clinical efficacy and more effective management of acid-related diseases[68]. A 2006 study shows that the CYP2C19*17 variant is an ultrarapid metabolizer genotype[69]. Interethnic differences have been reported in the frequencies of genotypes[63,70,71], with frequency of the *17 allele approximately 20% among Caucasian, African-American, Swedish, and Ethiopian populations but only 4% among Asians.
Figure 2.
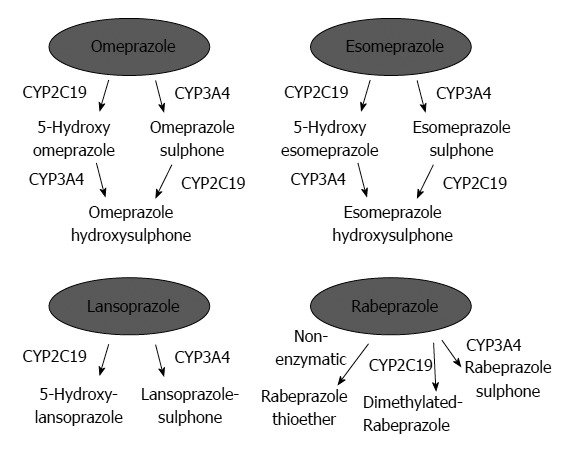
Metabolism of omeprazole, lansoprazole, rabeprazole, and esomeprazole by cytochrome P450 isoenzymes. Reproduced from Chang et al[61].
Figure 3.
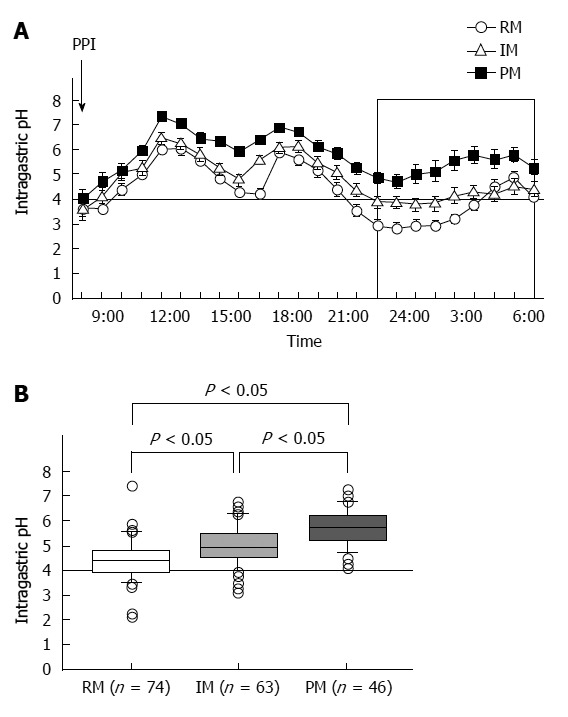
Median 24-h intragastric pH profiles (A) and median 24-h pH values after administering a standard dose of a proton pump inhibitor to patients with the three CYP2C19 genotypes (B). Proton pump inhibitor (PPI) treatment of poor metabolizers (PMs) inhibited gastric acid secretion more effectively than that of rapid metabolizers (RMs) and intermediate metabolizers (IMs).
Figure 4.
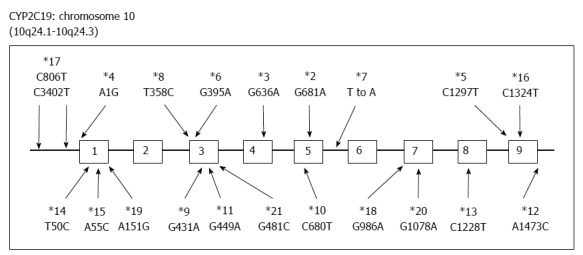
Genetic polymorphisms of CYP2C19. More than 20 variants have been discovered.
Several studies have compared the level of acid inhibition attained among CYP2C19 genotypes on PPI bid administration[23,33,68,72,73]. Effects of lansoprazole (30 mg, bid) have been found to vary markedly with CYP2C19 genotype[33,68], whereas little or no differences in efficacy were observed in patients treated with rabeprazole (10 mg) or esomeprazole (20 mg) bid, regardless of CYP2C19 genotype[68,72,73]. Further, CYP2C19 genotype-dependent differences in intragastric pH attained using esomeprazole and rabeprazole are smaller than those noted with lansoprazole and omeprazole[68]. As such, twice-daily dosing with a second-generation PPI may attenuate the influence of CYP2C19 genotype, but not completely remove it.
We reported that the median 24-h pH [5.4 (3.5–6.8)] of H. pylori-negative CYP2C19 RMs administered esomeprazole 20 mg bid was significantly higher than that achieved with omeprazole [5.0 (2.4-5.9), P = 0.018], rabeprazole [4.8 (2.5-6.4), P = 0.002], or (check) lansoprazole [4.7 (3.7-5.5), P = 0.017][68]. However, these findings suggest that although esomeprazole inhibits acid secretion in CYP2C19 RMs, twice-daily dosing of omeprazole, lansoprazole, and rabeprazole as well as with esomeprazole does not sustain high pH for 24 h for all patients.
Influence of CYP2C19 polymorphisms on PPI-based eradication of H. pylori
The rates for eradicating H. pylori infection using triple therapy (omeprazole 20 mg bid or lansoprazole 30 mg bid, AMPC 250 mg tid, and CAM 200 mg tid for 1 wk) vary with CYP2C19 genotype as follows: 72.7%, RMs; 92.1% IMs; and 97.8%, PMs[18]. The frequency of CYP2C19 RMs is relatively high among patients that experience eradication failure (58.6%, vs 2.9% for PMs). Schwab et al[74] also noted significant differences in eradication rates among RMs (80.2%), IMs (97.8%), and PMs (100%) (P < 0.01), which were associated with corresponding changes in median serum lansoprazole levels (753 ng/mL, PMs; 59 ng/mL, IMs; and 21 ng/mL, RMs; P < 0.001). A meta-analysis performed by those authors[74] further revealed that failure to eradicate H. pylori infection using PPI-based regimens is strongly influenced by the CYP2C19 genotype.
A more recent meta-analysis performed by Tang et al[75] on the effects of CYP2C19 loss-of-function variants revealed significant differences in rates between RMs and IMs (OR = 0.72; 95%CI: 0.59-0.88), between RMs and PMs (0.51; 0.38-0.68), and between IMs and PMs (0.69; 0.52-0.92) regardless of the PPI administered. Although other studies have reported significant differences in eradication rates using other PPI-based eradication therapies (e.g., rabeprazole, esomeprazole, or pantoprazole) among the different CYP2C19 genotypes[76,77], Tang et al[75] found no significant differences in efficacy between rabeprazole or esomeprazole for loss-of-function CYP2C19 variants. Therefore, CYP2C19 loss-of-function variants such as CYP2C19 PMs are associated with increased H. pylori eradication rates in patients taking PPI-based triple therapies with either omeprazole or lansoprazole[75].
EFFICACY OF DIVIDED DOSING WITH PPIS
When PPIs are administered to CYP2C19 RMs and IMs, plasma levels of PPIs cannot be maintained between once-daily doses[23,26,29,78]. RMs rapidly eliminate PPIs from the systemic circulation, and plasma levels of PPIs before dosing and 3 h later are often below detectable levels (plasma half-life, 2-3 h). Following rapid elimination of PPIs, activated or newly synthesized H+, K+-ATPase expressed in gastric parietal cells secretes gastric acid[23,26,29,78]. Although the Cmax for multiple-dosing PPI regimens (e.g., bid or qid) does not increase compared with oid administration, plasma levels of PPIs reached using multiple doses can be sustained throughout the 24-h period and continue to inactivate H+, K+-ATPase consistently for 24 h, achieving sufficient inhibition of acid secretion.
We reported that when rabeprazole (40 mg oid or 20 mg bid) is administered to CYP2C19 RMs, the levels of plasma rabeprazole are often below the limit of detection. However, when rabeprazole (20 mg bid or 10 mg qid) is administered to IMs and RMs, respectively, plasma levels are sustained above 10 ng/mL throughout the 24-h period, and sufficient suppression of acid secretion is achieved[23]. When rabeprazole is administered once, twice, or four times to achieve a daily dose of 40 mg, the median pH values are 4.8 (3.6-6.4), 5.7 (4.1-7.4), and 6.6 (4.9-8.4), respectively (Figure 5A)[25]. Administering 10 mg rabeprazole, 30 mg lansoprazole, and 10 mg esomeprazole qid to RMs achieves sufficient inhibition of acid secretion[23,64,73].
Figure 5.
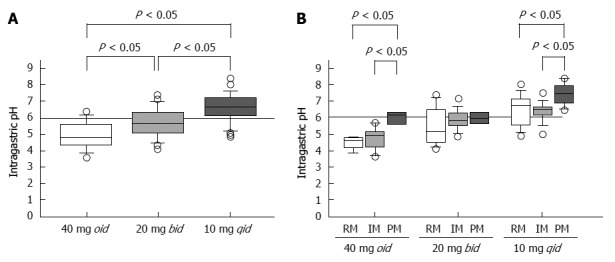
Median 24-h pH values as a function of dosing frequency using 40 mg of rabeprazole (A), and the pH attained using three different dosing regimens as a function of CYP2C19 genotype (B). Reproduced from Sugimoto et al[25]. PMs: Poor metabolizers; RMs: Rapid metabolizers; IMs: Intermediate metabolizers.
However, when patients with the same CYP2C19 genotype were treated with different doses of rabeprazole on different dosing schedules, the median pH attained with 10 mg qid was significantly higher than those observed when the drug was administered as 40 mg oid or 20 mg bid (Figure 5A). Further, multiple doses of a PPI decreased the influence of CYP2C19 genotype on pH (Figure 5B)[25]. Fischbach et al[41] reported that inhibition of acid secretion attained using esomeprazole 20 mg bid or 10 mg qid was similar among CYP2C19 genotypes but differed markedly from that achieved with 40 mg oid. We may therefore reasonably assume that, in order to maintain plasma PPI levels above a certain threshold level throughout the 24 h period, a multiple-dosing regimen would be more effective in inhibiting acid secretion by increasing the dose than by increasing either or both the Cmax or the AUC value.
Previously, sufficient eradication rates were achieved for RM patients treated with either lansoprazole 30 mg qid or rabeprazole 10 mg qid plus AMPC 500 mg qid for 2 wk. These findings suggest that potent inhibition of acid secretion using more frequent dosing intervals than at present bid may help to improve the eradication rate[18,43,79-81]. In addition, interestingly, rabeprazole 20 mg bid plus AMPC 1000 mg bid attains a 59.6% cure rate irrespective of administration of high daily doses of a PPI and AMPC[82]. This observation may be explained by findings that inhibition of acid secretion is insufficient when RMs are treated with rabeprazole 20 mg bid.
EFFICACY OF DIVIDED DOSING WITH AMPC
Similarly, AMPC should have been administered at 500 mg qid, not 1000 mg bid We therefore believe that administering AMPC using a regimen of 750-1000 mg bid is theoretically inappropriate according to pharmacological considerations, as antibiotics with a beta-lactam ring, such as AMPC, exert little post-antibiotic effects on gram-negative rods[83]. Their antibacterial effect depends largely on the duration for which their concentration is maintained at levels above the MIC and not the AUC or Cmax. The regimens reported to achieve high re-eradication rates (96.8%-100%) using dual therapy use a PPI qid plus AMPC qid[18,43,79,84].
EFFICACY OF TAILORED ERADICATION TREATMENT FOR H. PYLORI INFECTIONS BASED ON SUSCEPTIBILITY TO ANTIBIOTICS AND CYP2C19 GENOTYPE
Tailored eradication therapy shows promise for delivering significantly more successful outcomes than the standard therapies described above. For example, in their preliminary trial, Kawai et al[85] determined the efficacy of a regimen based on bacterial drug susceptibility to CAM that included 70 H. pylori-positive patients administered the following drugs: PPI/AMPC/CAM for patients with CAM-sensitive strains and PPI/AMPC/MNZ for CAM-resistant strains. The tailored treatment regimen achieved a 94.3% eradication rate, which is significantly higher than that achieved with standard treatment (71.4%, PPI/AMPC/CAM)[85], suggesting that treatment based on susceptibility to CAM will be effective, particularly in areas such as Japan with a high prevalence of CAM-resistant strains.
A second example of the increased efficacy of a tailored therapy comes from our own studies in which we administered PPIs according to CYP2C19 genotype and sequence analysis of the gene encoding H. pylori 23S rRNA[86]. Patients infected with a CAM-sensitive strain were treated with CAM 200 mg tid, AMPC 500 mg tid, and personalized doses of lansoprazole (e.g., RMs, 30 mg tid; IMs, 15 mg tid; and PMs, 15 mg bid) for 1 wk. Patients infected with a resistant strain were treated with AMPC 500 mg qid and a personalized dose of lansoprazole (e.g., RMs, 30 mg qid; IMs, 15 mg qid; and PMs, 15 mg bid for 2 wk) [86]. The ITT analyses of eradication rates for standard triple regimen (PPI/AMPC/CAM) are 70.0% compared with 96.0% (P < 0.0001) for tailored treatment (graded A, excellent)[86].
Although this tailored treatment may be optimal with high eradication rate, a setting of drug selection and dosing doses of PPI and antimicrobial agents may be complicated. We therefore propose that a regimen based on administering rabeprazole qid for all patients, irrespective of their CYP2C19 genotype, may achieve higher eradication rates than using a regimen employing a PPI bid, in particular in RMs. We assessed the efficacy of the tailored eradication regimens that control acid secretion using a PPI qid and selected antimicrobial agents based on the CAM-susceptibility of the patient’s H. pylori strain (Figure 6A). Patients infected with CAM-sensitive strains were treated with a tailored regimen of rabeprazole (RPZ) qid, AMPC 500 mg qid, and CAM 200 mg bid for 1 wk, while those infected with resistant strains were given RPZ qid, AMPC 500 mg qid, and MNZ 250 mg bid for 1 wk, irrespective of CYP2C19 genotype. The overall eradication rate achieved using the standard regimen was 77.8% (95%CI: 72.0%-85.5%) according to ITT analysis and that of the tailored regimen was 98.0% (94.3%-99.6%) (Figure 6B). The eradication rates using CAM-based and MNZ-based treatment were similar (96.5% and 98.4%, respectively, P = 0.469), and the eradication rates using the tailored regimen were similar among different CYP2C19 genotypes (RM, 94.3%; IM, 98.3%; and PM, 100%). In contrast, the outcomes achieved using the standard regimen were as follows: RM, 75.7%; IM, 81.7%; and PM, 87.0%) (Figure 6C). A tailored H. pylori eradication regimen based on CAM susceptibility that inhibits acid secretion for 24 h using RPZ 10 mg qid is more effective than the standard therapy used in Japan, as its eradication rate exceeds 95% irrespective of CYP2C19 genotype. Benefits of this tailored treatment are to save a cost of genotyping test and to prevent increased CAM-resistant H. pylori strain. We added limitation of this treatment in revised version. However, because not all patients are CYP2C19 RM and more frequent dosing with the PPI for IMs and PMs is more costly, this tailored treatment may be applicable third-line treatment. To identify efficacy of this tailored treatment for first- and second-line treatment (i.e., eradication rate and cost benefit), further study will be required.
Figure 6.
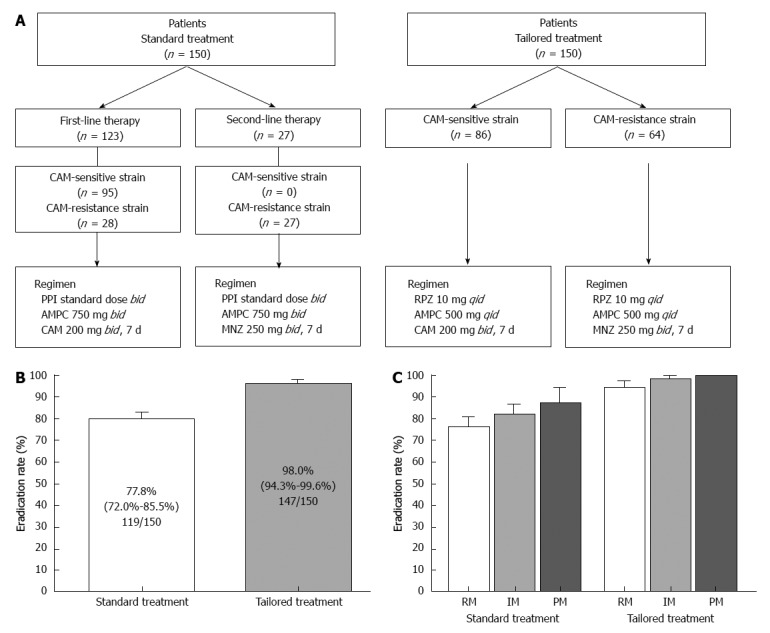
Study design and outcomes. A: Patients were classified into two treatment regimens: standard treatment group (first- or second-line standard Japanese regimen) and tailored treatment group (based on clarithromycin-susceptibility); B: Eradication rates for the standard and tailored regimens for eradication of Helicobacter pylori; C: Eradication rates for the standard and tailored regimens among different CYP2C19 genotypes. CAM: Clarithromycin; PPI: Proton pump inhibitor; AMPC: Amoxicillin; MNZ: Metronidazole; RPZ: Rabeprazole.
CONCLUSION
This review focuses on H. pylori eradication therapy in relation to pharmacogenomics and susceptibility to antimicrobial agents. We highlight the many genetic factors that influence therapeutic outcomes of H. pylori eradication therapy using a PPI and antimicrobial agents. We describe a tailored treatment that was designed according to pharmacogenomics and antimicrobial susceptibility to achieve an eradication rate exceeding 95%, irrespective of eradication history, that overcomes differences among CYP2C19 genotypes. Although a tailored regimen based on an individual’s CYP2C19 genotype is a valid therapeutic consideration, our strategy saves the cost of CYP2C19 genotyping. However, using increased doses of PPIs may not be universally welcomed and may not be tolerated by some patients. In addition, because there are other genetic factors to influence in eradication rate as listed in Table 1, it may be better to consider effects of genetic factors for optimal tailored treatment.
Footnotes
Supported by Grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, No. 22790640 and No. 24590912
P- Reviewers: Lin CJ, Senates E S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
References
- 1.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 3.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, Oguma K, Okada H, Shiratori Y. The effect of eradicating helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037–1042. doi: 10.1111/j.1572-0241.2005.41384.x. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244–1252. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- 6.Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639–642. [PubMed] [Google Scholar]
- 7.Wotherspoon AC, Doglioni C, de Boni M, Spencer J, Isaacson PG. Antibiotic treatment for low-grade gastric MALT lymphoma. Lancet. 1994;343:1503. [PubMed] [Google Scholar]
- 8.Sugimoto M, Kajimura M, Shirai N, Furuta T, Kanaoka S, Ikuma M, Sato Y, Hishida A. Outcome of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Intern Med. 2006;45:405–409. doi: 10.2169/internalmedicine.45.1473. [DOI] [PubMed] [Google Scholar]
- 9.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 10.Tebbe B, Geilen CC, Schulzke JD, Bojarski C, Radenhausen M, Orfanos CE. Helicobacter pylori infection and chronic urticaria. J Am Acad Dermatol. 1996;34:685–686. doi: 10.1016/s0190-9622(96)80086-7. [DOI] [PubMed] [Google Scholar]
- 11.Annibale B, Marignani M, Monarca B, Antonelli G, Marcheggiano A, Martino G, Mandelli F, Caprilli R, Delle Fave G. Reversal of iron deficiency anemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Ann Intern Med. 1999;131:668–672. doi: 10.7326/0003-4819-131-9-199911020-00006. [DOI] [PubMed] [Google Scholar]
- 12.Asaka M, Sugiyama T, Kato M, Satoh K, Kuwayama H, Fukuda Y, Fujioka T, Takemoto T, Kimura K, Shimoyama T, et al. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001;6:254–261. doi: 10.1046/j.1523-5378.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 13.Murakami K, Sato R, Okimoto T, Nasu M, Fujioka T, Kodama M, Kagawa J, Sato S, Abe H, Arita T. Eradication rates of clarithromycin-resistant Helicobacter pylori using either rabeprazole or lansoprazole plus amoxicillin and clarithromycin. Aliment Pharmacol Ther. 2002;16:1933–1938. doi: 10.1046/j.1365-2036.2002.01368.x. [DOI] [PubMed] [Google Scholar]
- 14.Cammarota G, Cianci R, Cannizzaro O, Cuoco L, Pirozzi G, Gasbarrini A, Armuzzi A, Zocco MA, Santarelli L, Arancio F, et al. Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:1339–1343. doi: 10.1046/j.1365-2036.2000.00846.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharara AI, Chaar HF, Aoun E, Abdul-Baki H, Araj GF, Kanj SS. Efficacy and safety of rabeprazole, amoxicillin, and gatifloxacin after treatment failure of initial Helicobacter pylori eradication. Helicobacter. 2006;11:231–236. doi: 10.1111/j.1523-5378.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 16.Murakami K, Sato R, Okimoto T, Nasu M, Fujioka T, Kodama M, Kagawa J. Efficacy of triple therapy comprising rabeprazole, amoxicillin and metronidazole for second-line Helicobacter pylori eradication in Japan, and the influence of metronidazole resistance. Aliment Pharmacol Ther. 2003;17:119–123. doi: 10.1046/j.1365-2036.2003.01401.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami E, Machado RS, Ogata SK, Langner M, Fukushima E, Carelli AP, Bonucci VC, Patricio FR. Furazolidone-based triple therapy for H pylori gastritis in children. World J Gastroenterol. 2006;12:5544–5549. doi: 10.3748/wjg.v12.i34.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta T, Shirai N, Takashima M, Xiao F, Hanai H, Sugimura H, Ohashi K, Ishizaki T, Kaneko E. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001;69:158–168. doi: 10.1067/mcp.2001.113959. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto M, Yamaoka Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch Immunol Ther Exp (Warsz) 2009;57:45–56. doi: 10.1007/s00005-009-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuta T, Soya Y, Sugimoto M, Nishino M, Yamade M, Uotani T, Kodaira C, Sahara S, Ichikawa H, Yamada T, et al. Rapid automated genotyping of CYP2C19 and Helicobacter pylori 23S rRNA gene in gastric juice. J Gastroenterol Hepatol Res. 2013;2:506–512. [Google Scholar]
- 21.Grayson ML, Eliopoulos GM, Ferraro MJ, Moellering RC. Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1989;8:888–889. doi: 10.1007/BF01963775. [DOI] [PubMed] [Google Scholar]
- 22.Hunt RH. pH and Hp--gastric acid secretion and Helicobacter pylori: implications for ulcer healing and eradication of the organism. Am J Gastroenterol. 1993;88:481–483. [PubMed] [Google Scholar]
- 23.Sugimoto M, Furuta T, Shirai N, Kajimura M, Hishida A, Sakurai M, Ohashi K, Ishizaki T. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004;76:290–301. doi: 10.1016/j.clpt.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto M, Furuta T, Shirai N, Nakamura A, Kajimura M, Hishida A, Ohashi K, Ishizaki T. Comparison of an increased dosage regimen of rabeprazole versus a concomitant dosage regimen of famotidine with rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotypes. Clin Pharmacol Ther. 2005;77:302–311. doi: 10.1016/j.clpt.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto M, Shirai N, Nishino M, Kodaira C, Uotani T, Yamade M, Sahara S, Ichikawa H, Sugimoto K, Miyajima H, et al. Rabeprazole 10 mg q.d.s. decreases 24-h intragastric acidity significantly more than rabeprazole 20 mg b.d. or 40 mg o.m., overcoming CYP2C19 genotype. Aliment Pharmacol Ther. 2012;36:627–634. doi: 10.1111/apt.12014. [DOI] [PubMed] [Google Scholar]
- 26.Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, Xiao F, Kosuge K, Nakagawa K, Hanai H, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929–1937. doi: 10.1046/j.1365-2036.2001.01108.x. [DOI] [PubMed] [Google Scholar]
- 27.Shirai N, Furuta T, Xiao F, Kajimura M, Hanai H, Ohashi K, Ishizaki T. Comparison of lansoprazole and famotidine for gastric acid inhibition during the daytime and night-time in different CYP2C19 genotype groups. Aliment Pharmacol Ther. 2002;16:837–846. doi: 10.1046/j.1365-2036.2002.01229.x. [DOI] [PubMed] [Google Scholar]
- 28.Kodaira C, Sugimoto M, Nishino M, Yamade M, Shirai N, Uchida S, Ikuma M, Yamada S, Watanabe H, Hishida A, et al. Effect of MDR1 C3435T polymorphism on lansoprazole in healthy Japanese subjects. Eur J Clin Pharmacol. 2009;65:593–600. doi: 10.1007/s00228-009-0625-8. [DOI] [PubMed] [Google Scholar]
- 29.Furuta T, Ohashi K, Kosuge K, Zhao XJ, Takashima M, Kimura M, Nishimoto M, Hanai H, Kaneko E, Ishizaki T. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65:552–561. doi: 10.1016/S0009-9236(99)70075-5. [DOI] [PubMed] [Google Scholar]
- 30.Furuta T, Sugimoto M, Shirai N, Matsushita F, Nakajima H, Kumagai J, Senoo K, Kodaira C, Nishino M, Yamade M, et al. Effect of MDR1 C3435T polymorphism on cure rates of Helicobacter pylori infection by triple therapy with lansoprazole, amoxicillin and clarithromycin in relation to CYP 2C19 genotypes and 23S rRNA genotypes of H. pylori. Aliment Pharmacol Ther. 2007;26:693–703. doi: 10.1111/j.1365-2036.2007.03408.x. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe MM, Nompleggi DJ. Cytokine inhibition of gastric acid secretion--a little goes a long way. Gastroenterology. 1992;102:2177–2178. doi: 10.1016/0016-5085(92)90360-b. [DOI] [PubMed] [Google Scholar]
- 32.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 33.Furuta T, Sagehashi Y, Shirai N, Sugimoto M, Nakamura A, Kodaira M, Kenmotsu K, Nagano M, Egashira T, Ueda K, et al. Influence of CYP2C19 polymorphism and Helicobacter pylori genotype determined from gastric tissue samples on response to triple therapy for H pylori infection. Clin Gastroenterol Hepatol. 2005;3:564–573. doi: 10.1016/s1542-3565(04)00779-7. [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto M, Furuta T, Shirai N, Ikuma M, Hishida A, Ishizaki T. Influences of proinflammatory and anti-inflammatory cytokine polymorphisms on eradication rates of clarithromycin-sensitive strains of Helicobacter pylori by triple therapy. Clin Pharmacol Ther. 2006;80:41–50. doi: 10.1016/j.clpt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Sugimoto M, Furuta T, Yamaoka Y. Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori. J Gastroenterol Hepatol. 2009;24:1725–1732. doi: 10.1111/j.1440-1746.2009.06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128:833–848. doi: 10.1053/j.gastro.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiota S, Nguyen LT, Murakami K, Kuroda A, Mizukami K, Okimoto T, Kodama M, Fujioka T, Yamaoka Y. Association of helicobacter pylori dupA with the failure of primary eradication. J Clin Gastroenterol. 2012;46:297–301. doi: 10.1097/MCG.0b013e318243201c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamek RJ, Suerbaum S, Pfaffenbach B, Opferkuch W. Primary and acquired Helicobacter pylori resistance to clarithromycin, metronidazole, and amoxicillin--influence on treatment outcome. Am J Gastroenterol. 1998;93:386–389. doi: 10.1111/j.1572-0241.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 39.Ménard A, Santos A, Mégraud F, Oleastro M. PCR-restriction fragment length polymorphism can also detect point mutation A2142C in the 23S rRNA gene, associated with Helicobacter pylori resistance to clarithromycin. Antimicrob Agents Chemother. 2002;46:1156–1157. doi: 10.1128/AAC.46.4.1156-1157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone GG, Shortridge D, Versalovic J, Beyer J, Flamm RK, Graham DY, Ghoneim AT, Tanaka SK. A PCR-oligonucleotide ligation assay to determine the prevalence of 23S rRNA gene mutations in clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1997;41:712–714. doi: 10.1128/aac.41.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 42.Megraud F. Helicobacter pylori and antibiotic resistance. Gut. 2007;56:1502. doi: 10.1136/gut.2007.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furuta T, Shirai N, Takashima M, Xiao F, Hanai H, Nakagawa K, Sugimura H, Ohashi K, Ishizaki T. Effects of genotypic differences in CYP2C19 status on cure rates for Helicobacter pylori infection by dual therapy with rabeprazole plus amoxicillin. Pharmacogenetics. 2001;11:341–348. doi: 10.1097/00008571-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Calvet X, García N, López T, Gisbert JP, Gené E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:603–609. doi: 10.1046/j.1365-2036.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 45.Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–562. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 46.Barer D, Ogilvie A, Henry D, Dronfield M, Coggon D, French S, Ellis S, Atkinson M, Langman M. Cimetidine and tranexamic acid in the treatment of acute upper-gastrointestinal-tract bleeding. N Engl J Med. 1983;308:1571–1575. doi: 10.1056/NEJM198306303082606. [DOI] [PubMed] [Google Scholar]
- 47.Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51 Suppl 1:59–67. doi: 10.1159/000200917. [DOI] [PubMed] [Google Scholar]
- 48.Sugimoto M, Nishino M, Kodaira C, Yamade M, Uotani T, Ikuma M, Furuta T. Impact of acid inhibition on esophageal mucosal injury induced by low-dose aspirin. Digestion. 2012;85:9–17. doi: 10.1159/000329295. [DOI] [PubMed] [Google Scholar]
- 49.Yang JC, Wang HL, Chern HD, Shun CT, Lin BR, Lin CJ, Wang TH. Role of omeprazole dosage and cytochrome P450 2C19 genotype in patients receiving omeprazole-amoxicillin dual therapy for Helicobacter pylori eradication. Pharmacotherapy. 2011;31:227–238. doi: 10.1592/phco.31.3.227. [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, Ishizaki T, Hishida A. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12:317–323. doi: 10.1111/j.1523-5378.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 51.Labenz J, Stolte M, Blum AL, Jorias I, Leverkus F, Sollböhmer M, Bertrams J, Börsch G. Intragastric acidity as a predictor of the success of Helicobacter pylori eradication: a study in peptic ulcer patients with omeprazole and amoxicillin. Gut. 1995;37:39–43. doi: 10.1136/gut.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott D, Weeks D, Melchers K, Sachs G. The life and death of Helicobacter pylori. Gut. 1998;43 Suppl 1:S56–S60. doi: 10.1136/gut.43.2008.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idström JP, Cederberg C, Spiller RC. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111:358–367. doi: 10.1053/gast.1996.v111.pm8690200. [DOI] [PubMed] [Google Scholar]
- 54.Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther. 2012;36:972–979. doi: 10.1111/apt.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heifets LB, Lindholm-Levy PJ, Comstock RD. Clarithromycin minimal inhibitory and bactericidal concentrations against Mycobacterium avium. Am Rev Respir Dis. 1992;145:856–858. doi: 10.1164/ajrccm/145.4_Pt_1.856. [DOI] [PubMed] [Google Scholar]
- 56.Hixson LJ, Kelley CL, Jones WN, Tuohy CD. Current trends in the pharmacotherapy for peptic ulcer disease. Arch Intern Med. 1992;152:726–732. [PubMed] [Google Scholar]
- 57.Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+,K+ ATPase. Annu Rev Pharmacol Toxicol. 1995;35:277–305. doi: 10.1146/annurev.pa.35.040195.001425. [DOI] [PubMed] [Google Scholar]
- 58.Walsh JH, Peterson WL. The treatment of Helicobacter pylori infection in the management of peptic ulcer disease. N Engl J Med. 1995;333:984–991. doi: 10.1056/NEJM199510123331508. [DOI] [PubMed] [Google Scholar]
- 59.Furuta T, Shirai N, Watanabe F, Honda S, Takeuchi K, Iida T, Sato Y, Kajimura M, Futami H, Takayanagi S, et al. Effect of cytochrome P4502C19 genotypic differences on cure rates for gastroesophageal reflux disease by lansoprazole. Clin Pharmacol Ther. 2002;72:453–460. doi: 10.1067/mcp.2002.127637. [DOI] [PubMed] [Google Scholar]
- 60.Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13 Suppl 3:27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 61.Chang M, Dahl ML, Tybring G, Götharson E, Bertilsson L. Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics. 1995;5:358–363. doi: 10.1097/00008571-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Chang M, Tybring G, Dahl ML, Götharson E, Sagar M, Seensalu R, Bertilsson L. Interphenotype differences in disposition and effect on gastrin levels of omeprazole--suitability of omeprazole as a probe for CYP2C19. Br J Clin Pharmacol. 1995;39:511–518. doi: 10.1111/j.1365-2125.1995.tb04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubota T, Chiba K, Ishizaki T. Genotyping of S-mephenytoin 4’-hydroxylation in an extended Japanese population. Clin Pharmacol Ther. 1996;60:661–666. doi: 10.1016/S0009-9236(96)90214-3. [DOI] [PubMed] [Google Scholar]
- 64.Furuta T, Shirai N, Xiao F, Ohashi K, Ishizaki T. Effect of high-dose lansoprazole on intragastic pH in subjects who are homozygous extensive metabolizers of cytochrome P4502C19. Clin Pharmacol Ther. 2001;70:484–492. doi: 10.1067/mcp.2001.119721. [DOI] [PubMed] [Google Scholar]
- 65.Yasuda S, Horai Y, Tomono Y, Nakai H, Yamato C, Manabe K, Kobayashi K, Chiba K, Ishizaki T. Comparison of the kinetic disposition and metabolism of E3810, a new proton pump inhibitor, and omeprazole in relation to S-mephenytoin 4’-hydroxylation status. Clin Pharmacol Ther. 1995;58:143–154. doi: 10.1016/0009-9236(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 66.Abelö A, Andersson TB, Antonsson M, Naudot AK, Skånberg I, Weidolf L. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 2000;28:966–972. [PubMed] [Google Scholar]
- 67.Hassan-Alin M, Andersson T, Niazi M, Röhss K. A pharmacokinetic study comparing single and repeated oral doses of 20 mg and 40 mg omeprazole and its two optical isomers, S-omeprazole (esomeprazole) and R-omeprazole, in healthy subjects. Eur J Clin Pharmacol. 2005;60:779–784. doi: 10.1007/s00228-004-0841-1. [DOI] [PubMed] [Google Scholar]
- 68.Sahara S, Sugimoto M, Uotani T, Ichikawa H, Yamade M, Iwaizumi M, Yamada T, Osawa S, Sugimoto K, Umemura K, et al. Twice-daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice-daily omeprazole, rabeprazole or lansoprazole. Aliment Pharmacol Ther. 2013;38:1129–1137. doi: 10.1111/apt.12492. [DOI] [PubMed] [Google Scholar]
- 69.Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103–113. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Ishizaki T, Sohn DR, Kobayashi K, Chiba K, Lee KH, Shin SG, Andersson T, Regårdh CG, Lou YC, Zhang Y. Interethnic differences in omeprazole metabolism in the two S-mephenytoin hydroxylation phenotypes studied in Caucasians and Orientals. Ther Drug Monit. 1994;16:214–215. doi: 10.1097/00007691-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 71.de Morais SM, Goldstein JA, Xie HG, Huang SL, Lu YQ, Xia H, Xiao ZS, Ile N, Zhou HH. Genetic analysis of the S-mephenytoin polymorphism in a Chinese population. Clin Pharmacol Ther. 1995;58:404–411. doi: 10.1016/0009-9236(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 72.Shimatani T, Inoue M, Kuroiwa T, Horikawa Y. Rabeprazole 10 mg twice daily is superior to 20 mg once daily for night-time gastric acid suppression. Aliment Pharmacol Ther. 2004;19:113–122. doi: 10.1046/j.1365-2036.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- 73.Lou HY, Chang CC, Sheu MT, Chen YC, Ho HO. Optimal dose regimens of esomeprazole for gastric acid suppression with minimal influence of the CYP2C19 polymorphism. Eur J Clin Pharmacol. 2009;65:55–64. doi: 10.1007/s00228-008-0552-0. [DOI] [PubMed] [Google Scholar]
- 74.Schwab M, Schaeffeler E, Klotz U, Treiber G. CYP2C19 polymorphism is a major predictor of treatment failure in white patients by use of lansoprazole-based quadruple therapy for eradication of Helicobacter pylori. Clin Pharmacol Ther. 2004;76:201–209. doi: 10.1016/j.clpt.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Tang HL, Li Y, Hu YF, Xie HG, Zhai SD. Effects of CYP2C19 loss-of-function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a meta-analysis of randomized clinical trials. PLoS One. 2013;8:e62162. doi: 10.1371/journal.pone.0062162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurzawski M, Gawrońska-Szklarz B, Wrześniewska J, Siuda A, Starzyńska T, Droździk M. Effect of CYP2C19*17 gene variant on Helicobacter pylori eradication in peptic ulcer patients. Eur J Clin Pharmacol. 2006;62:877–880. doi: 10.1007/s00228-006-0183-2. [DOI] [PubMed] [Google Scholar]
- 77.Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. Am J Gastroenterol. 2006;101:1467–1475. doi: 10.1111/j.1572-0241.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 78.Horai Y, Kimura M, Furuie H, Matsuguma K, Irie S, Koga Y, Nagahama T, Murakami M, Matsui T, Yao T, et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther. 2001;15:793–803. doi: 10.1046/j.1365-2036.2001.00980.x. [DOI] [PubMed] [Google Scholar]
- 79.Furuta T, Shirai N, Xiao F, Takashita M, Sugimoto M, Kajimura M, Ohashi K, Ishizaki T. High-dose rabeprazole/amoxicillin therapy as the second-line regimen after failure to eradicate H. pylori by triple therapy with the usual doses of a proton pump inhibitor, clarithromycin and amoxicillin. Hepatogastroenterology. 2003;50:2274–2278. [PubMed] [Google Scholar]
- 80.Bayerdörffer E, Miehlke S, Mannes GA, Sommer A, Höchter W, Weingart J, Heldwein W, Klann H, Simon T, Schmitt W. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology. 1995;108:1412–1417. doi: 10.1016/0016-5085(95)90689-4. [DOI] [PubMed] [Google Scholar]
- 81.Shirai N, Sugimoto M, Kodaira C, Nishino M, Ikuma M, Kajimura M, Ohashi K, Ishizaki T, Hishida A, Furuta T. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007;63:743–749. doi: 10.1007/s00228-007-0302-8. [DOI] [PubMed] [Google Scholar]
- 82.Isomoto H, Inoue K, Furusu H, Enjoji A, Fujimoto C, Yamakawa M, Hirakata Y, Omagari K, Mizuta Y, Murase K, et al. High-dose rabeprazole-amoxicillin versus rabeprazole-amoxicillin-metronidazole as second-line treatment after failure of the Japanese standard regimen for Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;18:101–107. doi: 10.1046/j.1365-2036.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 83.Athamna A, Athamna M, Medlej B, Bast DJ, Rubinstein E. In vitro post-antibiotic effect of fluoroquinolones, macrolides, beta-lactams, tetracyclines, vancomycin, clindamycin, linezolid, chloramphenicol, quinupristin/dalfopristin and rifampicin on Bacillus anthracis. J Antimicrob Chemother. 2004;53:609–615. doi: 10.1093/jac/dkh130. [DOI] [PubMed] [Google Scholar]
- 84.Miehlke S, Kirsch C, Schneider-Brachert W, Haferland C, Neumeyer M, Bästlein E, Papke J, Jacobs E, Vieth M, Stolte M, et al. A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter. 2003;8:310–319. doi: 10.1046/j.1523-5378.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 85.Kawai T, Yamagishi T, Yagi K, Kataoka M, Kawakami K, Sofuni A, Itoi T, Sakai Y, Moriyasu F, Osaka Y, et al. Tailored eradication therapy based on fecal Helicobacter pylori clarithromycin sensitivities. J Gastroenterol Hepatol. 2008;23 Suppl 2:S171–S174. doi: 10.1111/j.1440-1746.2008.05408.x. [DOI] [PubMed] [Google Scholar]
- 86.Furuta T, Shirai N, Kodaira M, Sugimoto M, Nogaki A, Kuriyama S, Iwaizumi M, Yamade M, Terakawa I, Ohashi K, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007;81:521–528. doi: 10.1038/sj.clpt.6100043. [DOI] [PubMed] [Google Scholar]


