Abstract
Gluten-sensitive enteropathy, also known as coeliac disease (CD), is an autoimmune disorder occurring in genetically susceptible individuals that damages the small intestine and interferes with the absorption of other nutrients. As it is triggered by dietary gluten and related prolamins present in wheat, rye and barley, the accepted treatment for CD is a strict gluten-free diet. However, a complete exclusion of gluten-containing cereals from the diet is often difficult, and new therapeutic strategies are urgently needed. A class of proteins that have already emerged as drug targets for other autoimmune diseases are the heat shock proteins (HSPs), which are highly conserved stress-induced chaperones that protect cells against harmful extracellular factors. HSPs are expressed in several tissues, including the gastrointestinal tract, and their levels are significantly increased under stress circumstances. HSPs exert immunomodulatory effects, and also play a crucial role in the maintenance of epithelial cell structure and function, as they are responsible for adequate protein folding, influence the degradation of proteins and cell repair processes after damage, and modulate cell signalling, cell proliferation and apoptosis. The present review discusses the involvement of HSPs in the pathophysiology of CD. Furthermore, HSPs may represent a useful therapeutic target for the treatment of CD due to the cytoprotective, immunomodulatory, and anti-apoptotic effects in the intestinal mucosal barrier.
Keywords: Gluten-sensitive enteropathy, Coeliac disease, Heat shock proteins, Gluten-free diet, Intestinal barrier
Core tip: The only current effective therapy for the treatment of coeliac disease (CD) is a gluten-free diet. However, therapies targeting heat shock proteins (HSPs) for the treatment of various autoimmune disorders and cancers have been developed and have shown promising results. As CD is an autoimmune disorder, these new therapies may prove beneficial as an alternative treatment strategy. This review highlights and discusses recent data concerning the involvement of HSPs in the pathophysiology of CD.
INTRODUCTION
Coeliac disease (CD), or gluten-sensitive enteropathy, is an autoimmune inflammatory disorder characterized by partial or total villous atrophy and crypt hyperplasia of the small intestine in genetically predisposed patients. Ninety-five percent of affected individuals carry one of two specific human leukocyte antigen (HLA) class II alleles, either DQ2 (HLA-DQA1*05-DQB1*02) or DQ8 (HLADQA1*03-DQB1*0302)[1-4]. Since dietary gluten and related prolamins are present in different types of cereals (wheat, barley and rye), medicines, and various other products, including stamp and envelope adhesives, a lifelong exclusion of gluten presents a considerable challenge for patients with CD[5,6]. Although the worldwide incidence of CD has continued to increase over the past decade, most cases remain undiagnosed[7]. The increased incidence suggests that the disease manifestation is similar to that of other immune-mediated diseases, such as inflammatory bowel disease (IBD), allergies or asthma, and results from a combination of genetic predisposition and environmental factors. This hypothesis is supported by the fact that CD is often first detected following physical and emotional stress, such as from surgery, pregnancy, or viral infection[8]. Heat shock proteins (HSPs) are known to exert immunomodulatory effects, and have thus been targeted for the treatment of autoimmune disorders. Recent evidence suggests that the expression of HSPs is altered in CD. This review presents and discusses the role of HSPs and various stress factors in the pathophysiology of CD.
EFFECT OF STRESS ON THE PATHOGENESIS OF CD
Stress represents an acute threat to an organism, which initiates and mediates the physiological adaptations necessary to maintain homeostasis and ensure survival[9]. Stress can be caused by intrinsic factors, such as genes and endoplasmic reticulum stress, or extrinsic factors, such as heat, toxins, radiation, infection, mechanical force and metabolic disturbances. Stress factors affecting the gastrointestinal tract may induce inflammation and reduce its motility[10], resulting in disrupted mucosal integrity and impaired epithelial barrier function[11,12]. Such changes can lead to the development of CD in genetically predisposed individuals[13].
In CD, the transport of incompletely digested wheat gluten peptides, such as gliadin, across a damaged epithelial layer into the lamina propria[14] triggers oxidative stress and the release of pro-inflammatory cytokines[15]. However, gluten can induce adaptive as well as innate immune responses, such as enhancing the production of interleukin (IL)-15 in epithelial cells, which also leads to cell damage through the activation of intraepithelial cytotoxic CD8+ T-cells[16,17]. Activated transglutaminase 2 enzymes in the lamina propria[18] deamidate neutral glutamine residues of gluten, thus creating epitopes with increased immunostimulatory potential[16]. These deamidated peptides are presented to CD4+ T-helper cells by the disease associated HLA-DQ2 and -DQ8 molecules from macrophages, dendritic cells (DCs) and B lymphocytes[19], which promote the differentiation of B-cells producing anti-gliadin and anti-transglutaminase 2 antibodies[20]. T-cells may also produce pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-α and interferon (IFN)-γ, and activate intestinal fibroblasts leading to further damage of the epithelial cell layer, mucosal matrix degradation and tissue remodelling[18]. Moreover, gliadin peptides can directly activate pattern recognition receptors such as Toll-like receptor (TLR) 2 and 4 on macrophages and DCs[21], leading to a further upregulation of proinflammatory cytokines and chemokines[22] (Figure 1). These inflammatory effects of stress lead to additional aggravation of the disease[23].
Figure 1.
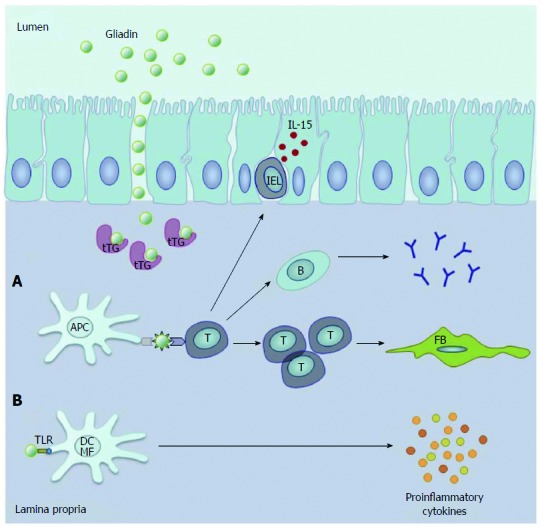
Key processes during the pathogenesis of coeliac disease. In the lamina propria. A: Gluten-derived gliadin peptides deamidated by tissue transglutaminase (tTG) are presented to T-cells by antigen presenting cells (APC). This process leads to the activation of anti-gliadin and anti-tTG antibody producing B-cells and other T-cells promoting the activation of intestinal fibroblasts (FB). Furthermore, gliadin enhances the production of IL-15, which activates intraepithelial T lymphocytes (IEL); B: Gliadin peptides can directly activate Toll-like receptor (TLR) 2 and 4 on macrophages (MF) and dendritic cells (DC), resulting in increased production of proinflammatory cytokines (Reproduced with permission from Sziksz et al[1]).
DEFENSE AGAINST STRESS: ROLE OF HSPs
Stress results in the activation of various proteins such as proteolytic system components, RNA/DNA modifying enzymes, metabolic enzymes, regulatory, transport, detoxifying and membrane-modulating proteins, and molecular chaperones, or HSPs[24]. HSPs were first discovered in Drosophila melanogaster in the early 1960s[25], and have since been observed in all organisms after exposure to cellular stresses[26], such as heat, UV light, cytotoxic agents[27,28], and nutritional (e.g., the absence of glucose and glutamine)[29] and oxidative stress[30]. HSPs are expressed in many tissues, including heart[31], brain[26], muscle[32], lung[33], kidney[34], liver[35], and intestinal and colonic epithelium[36]. These highly conserved molecules are responsible for maintaining adequate protein folding[37] and influencing the degradation of proteins[38] and cell repair processes after damage[39]. Furthermore, HSPs are involved in the modulation of immune responses[40,41], autoimmunity[27], cell signalling[42], cell proliferation[43], apoptosis[44], and tumour cell differentiation and invasion[45]. Based on their molecular weight they can be classified into six major families: small HSPs (molecular weight < 30 kDa), HSP60s, HSP70s, HSP90s, HSP100s[24,46], and other non-ubiquitous HSPs[47] (Table 1).
Table 1.
| Family | Subunit MW (kDa) | Family members | Cellular localization |
| HSP100 | 80-110 | HSP100, HSP104 | Cytoplasm, nucleus, mitochondria, plasma membrane |
| HSP90 | 82-96 | HSP90α, HSP90β | Cytoplasm, nucleus, mitochondria, endoplasmic reticulum |
| HSP70 | 67-76 | HSP70, HSP72, HSP73, HSP80 | Cytoplasm, nucleus, mitochondria, endoplasmic reticulum, lysosomes, extracellular compartments |
| HSP60 | 58-65 | HSP60, HSP65 | Mitochondria |
| Small HSPs | 8-40 | αB-crystallin, HSP25, HSP27, ubiquitin | Cytoplasm, nucleus |
| Others (not ubiquitous) | Various | HSP33 | Various |
MW: Molecular weight; HSP: Heat shock protein.
Oxidative stress and HSPs
Environmental and chemical agents inducing oxidative stress can enhance the generation of reactive oxygen species (ROS)[48,49]. In CD, gluten itself can promote the generation of ROS by stimulating the expression of the inducible form of nitric oxide synthase (iNOS) and increasing nitric oxide levels[50,51]. This process contributes to subsequent mucosal damage and villous atrophy of the small intestine[52]. Interestingly, these same oxygen-free radicals, such as superoxide, also induce the expression of various HSPs which take part in the defence against oxidative stress[53]. The inducible form of HSP70 (HSP70i) reduces iNOS expression by specifically binding to iNOS and its transcription factor Krueppel-like factor 6[54]; moreover, its upregulation was shown to inhibit nuclear factor (NF)-B activation, thereby providing cellular protection against stress[55]. In addition, glutamine-induced HSP72 was shown in vivo to protect against endotoxin-induced shock injury[56], and HSP90 has been shown to exert antioxidative and anti-apoptotic effects against chemical-induced hypoxic injury[57]. HSP60 contributes to the protection of small intestine by enhancing the cytoprotective function of intestinal epithelial cells against H2O2-induced injury[58]. Finally, HSP32, also known as heme oxygenase-1, degrades heme into vasoactive carbon monoxide, free iron and biliverdin, and is also a potent antioxidant[59].
Inflammation and HSPs
HSPs can act as “danger signals” for the immune system at sites of tissue injury[60]. HSPs were shown to contribute to antigen presentation and the proliferation and activation of macrophages and DCs[61], and natural killer cells[62]. HSP70 and HSP90 bind to TLRs on the surface of DCs and macrophages[63] resulting in enhanced expression of pro-inflammatory cytokines[64,65], and HSP60 stimulates the release of TNF-α, IL-12, and IL-1β, via TLR 4 signalling[66]. However, HSP60 can also activate anti-inflammatory processes through TLR 2 signalling, upregulating the suppressive function of regulatory T-cells and shifting the cytokine secretion balance toward a Th2 phenotype[67,68], suggesting that the immunomodulatory effect can be cell and receptor type specific.
Altered expression of HSPs has been associated with intestinal inflammation. An increased epithelial expression of HSP70, HSP60 and HSP10 was observed in the colonic mucosa of patients with IBD[69,70]. This upregulation may be protective, as Tanaka et al[71] demonstrated that transgenic mice overexpressing HSP70 showed reduced apoptosis and suppressed expression of pro-inflammatory cytokines after dextran sulfate sodium-induced colitis. HSP47, a collagen-specific molecular chaperone, was also found in mesenchymal and submucosal cells in a murine model of colitis[72].
Apoptosis and HSPs
Apoptosis is essential for the maintenance of intestinal epithelial function, as it regulates the normal turnover of enterocytes[73]. The increased apoptosis of enterocytes in CD contributes to villous atrophy, which is mediated either by the direct toxicity of gliadin domains or by the gliadin-dependent activation of intraepithelial and lamina propria lymphocytes[74]. Gliadin-induced apoptosis can be blocked by Fas cascade inhibitors[75], although the activation of the Fas system can also contribute to cell survival in the gut by inducing the expression of HSP72 and HSP72-driven chemokines[76]. HSP70 can also promote cell survival by inhibiting the mitochondrial translocation of Bax and subsequent release of cytochrome c and activation of caspase-9 and -3[77,78], an intrinsic apoptotic pathway that is initiated by intracellular stress signals[79]. Furthermore, HSP70 is a natural inhibitor of c-Jun N-terminal kinase[80] and is also a modulator of the calcium signalling that play major roles in the regulation of apoptosis[80-83]. Furthermore, HSP60 has been identified as a novel mitochondrial permeability transition regulator. HSP60 is a component of a mitochondrial multi-chaperone complex that includes HSP90 and its related molecule TNF receptor-associated protein 1, which associates with and antagonizes the pro-apoptotic, mitochondrial permeability transition pore modulator, cyclophilin D, thereby contributing to the preservation of organelle integrity and prevention of cell death[84,85].
Intestinal epithelial integrity and HSPs
The intestinal mucosa forms a barrier that is essential for defending the intestine against the harmful effects of different stressors. Oxidative stress, inflammation and increased apoptosis all lead to mucosal damage and increased permeability[86]. The integrity of the epithelial barrier is determined by an apical junctional complex composed of tight and adherent junctions[87]. During heat stress, HSPs play a pivotal role in the preservation of the intestinal barrier by promoting the upregulation of the tight junction protein occludin[88,89]. HSP70s protect intestinal epithelial cells by preserving the integrity of the actin cytoskeleton and cell-cell contact, and HSP72 directly binds and stabilizes other tight junction-associated proteins on colonic epithelial cells, such as zonula occludens[90]. Other HSPs, including members of the HSP110 subfamily, have also been shown to bind to junctional proteins[91]. Tissue integrity is also influenced by matrix metalloproteinases (MMPs)[92], which have been observed as increased in intestinal tissues of patients with CD[93]. Extracellular HSP90α was shown to activate MMP-2, which was enhanced by HSP70 and HSP40, leading to increased cell migration[94]. HSP60 may also induce MMP production in macrophages[95].
HSPs AND CD
HSPs are differentially expressed throughout the gastrointestinal tract, with gastric and colonic epithelial cells showing high expression of HSP25 and HSP72, likely the result of continuously low acidic pH, mechanical stress and/or bacterial fermentation[96]. In contrast, the expression of HSPs in the small intestine is normally negligible[97], but the expression of HSP25 and HSP70 is markedly increased under stress[88]. The predominant localization of HSPs in intestinal epithelial cells suggests their primary role is in maintaining the integrity of the enterocyte layer, as demonstrated by Kojima et al[98] who showed that Bacteriodes fragilis treatment of young adult mouse colonocyte cells increased the expression of HSPs mediated by lipopolysaccharide and other bacteria-derived factors. Using horseradish peroxidase to evaluate human intestinal epithelial permeability, Yang et al[99] found that heat stress increased transport across an epithelial monolayer, which was inhibited by pretreatment with HSP70. Asea[65] and Cario et al[100] provided further supporting evidence by showing that HSP70 can behave as a ligand for TLR 2 and TLR 4[101], the activation of which can contribute to the maintenance of intestinal barrier function by preserving the integrity of tight junction proteins, such as zonula occludens 1, under stressful conditions.
The role of HSPs in the pathophysiology of CD is not well understood, owing in part to the lack of experimental models. However, our lab has shown increased mRNA and protein expression of HSP72 in the duodenal mucosa of children newly diagnosed with CD[102]. The most abundant expression of HSP72 was in villous enterocytes of the epithelium and immune cells of the lamina propria. Clinical symptoms were reduced with a gluten-free diet (GFD), which also reduced the level of intestinal HSP72, though levels were still higher than in control individuals. In contrast, Brottveit et al[103] reported that suspension of a GFD for three days did not alter the mRNA expression of HSP70 or HSP27 in the mucosa of adult CD patients. This apparent discrepancy may be due to the difference in patient age, or in the experimental setting, for example, comparing the effect of dietary gluten elimination in newly diagnosed CD patients vs the return of dietary gluten in patients maintained on a long-term GFD. Iltanen et al[104] found elevated expression of mitochondrial HSP65 in 80% of jejunal biopsies from children diagnosed with CD compared to 24% of specimens from children with a normal biopsy. The levels of HSP65 correlated with the number of + T-cells and serum IgA endomysial autoantibodies, suggesting that HSP65 may be an indicator of disease activity. Yeboah et al[105] examined the duodenal mucosa of CD patients and found a close correlation between the distribution of the small HSP αB-crystallin and the degree of villous atrophy, indicating its involvement in the modulation of mucosal integrity.
Single nucleotide polymorphisms in the 5’ regulatory region of the gene encoding HSP70-1 (HSPA1A) have been linked with CD. Ramos-Arroyo et al[106] found a significantly higher frequency of an HSPA1A allele showing an intermediate electrophoretic mobility in patients with CD. Individuals expressing CD-associated HLA alleles that were homozygous for this intermediate HSPA1A allele were 12-fold more likely to develop CD, indicating that HSPA1A polymorphisms are an additional predisposing factor for CD as a component of a high-risk haplotype. Partanen et al[107] found significantly deviated gene frequencies of the HSPA1B (HSP70-2) gene cluster in 19 families of patients with CD compared to that of a normal population, indicating that a polymorphism of the HLA-linked HSPA1B gene may be involved in the pathophysiology of CD. The main scientific findings indicating involvement of HSPs in CD are summarized in Table 2.
Table 2.
Involvement of heat shock proteins in coeliac disease
| Samples | Investigation | Localization/major findings | Ref. |
| Duodenal biopsies from 16 children with newly diagnosed CD, 9 maintained on GFD, 10 controls | HSP72 mRNA expression, protein level and localization | HSP72 mRNA and protein are increased in CD, and decreased by GFD. HSP72 was localized in villous enterocytes of the epithelium and lamina propria immune cells | [102] |
| Duodenal biopsy specimens from 30 HLA-DQ2 (+) NCGS and 15 CD patients maintained on GFD | HSP27 or HSP70 mRNA expression, before and after challenge with gluten-containing bread daily for 3 d | mRNA expression of HSP27 and HSP70 in the duodenal mucosa was not different in any of the groups | [103] |
| Jejunal biopsies from 78 children with clinical suspicion of CD | Epithelial HSP65 expression | Increased mitochondrial HSP65 expression in the jejunal mucosa in 80% (16/20) of children with CD and in 24% (14/58) of non-CD patients. Strong correlation between HSP65, γδ + T-cells and serum IgA endomysial autoantibodies. HSP65 is a potential mucosal integrity modulator | [104] |
| Duodenal biopsies from 12 patients with CD and 10 controls | Small HSP αB-crystallin expression and distribution | Increased αB-crystallin in CD, localized in the supra-nuclear region of enterocytes in the duodenal mucosa | [105] |
| Blood samples from 128 patients with CD and 94 healthy individuals | HSPA1A gene (HSP70-1) polymorphism | Altered frequency of an intermediate HSPA1A allele in CD (64.5%) vs normal (37.2%). HSP70-1 gene is part of a high-risk haplotype for CD | [106] |
| Blood samples from 19 families with CD patients and 95 healthy individuals | HLA-linked HSPA1B gene (HSP70-2) polymorphism | Altered HSPA1B allele frequencies in CD vs normal and non-affected MHC haplotypes | [107] |
CD: Coeliac disease; GFD: Gluten-free diet; HSP: Heat shock protein; NCGS: Non-coeliac gluten sensitivity; Ig: Immunoglobulin; HLA: Human leukocyte antigen; MHC: Major histocompatibility complex.
HSPs AND THERAPEUTIC TREATMENTS
Although promising results have been found using HSP-based vaccines for the treatment of cancer patients[108], relatively little is known about the therapeutic potential of HSPs in the treatment of gastrointestinal diseases. There is evidence to suggest, however, that targeting of HSPs would be beneficial. The anti-ulcer drug geranylgeranylacetone (GGA) that reduced colitis in a mouse model was found to induce the intestinal expression of HSP70 and to suppress myeloperoxidase activity and reduce TNF-α and IFN-γ levels[109]. Furthermore, it was demonstrated that the upregulation of HSPs by GGA is protective against intestinal damage from non-steroidal anti-inflammatory drugs such as indomethacin[110]. Indeed, overexpression of HSP70 in mice decreased the number of indomethacin-induced apoptotic cells and the level of proinflammatory cytokines and chemokines (IL-1β, IL-6) in the small intestine, suggesting that HSP70 is protective and can reduce the extent of small intestinal lesions[36]. A strong correlation between the expression of HSPs and the advantageous effects of probiotics in IBD has also been suggested[111], and probiotics containing eight different naturally occurring strains of “beneficial” bacteria may induce the expression of HSP25 and HSP72 in colonic epithelial cells[88]. Moreover, probiotic Lactobacillus GG induces the expression of HSP72 in intestinal epithelial cells, contributing to the beneficial clinical effects through preservation of cytoskeletal integrity[112]. These data suggest that HSP-inducers are promising drugs to treat gastrointestinal diseases, including CD, or ameliorate their symptoms.
CONCLUSION
HSPs are a class of highly conserved, stress-induced chaperones that are responsible for proper protein folding and regulating protein degradation, cell repair, immune responses, cell signalling, cell proliferation, apoptosis, and tumour cell differentiation. The increased expression of various HSPs observed in CD suggests that their antioxidant and anti-apoptotic features are protective. Furthermore, HSPs may be involved in the pathophysiology of CD through their immunomodulatory effects, serving as “danger signals” for the immune system at sites of tissue injury.
Intrinsic apoptotic pathways initiated by intracellular stress signals can be blocked by HSPs, which likely contributes to the maintenance of intestinal homeostasis. HSPs suppress the expression of iNOS and reduce the level of nitric oxide, thereby providing cellular protection against stress. HSPs are also involved in tissue repair and remodelling by regulating the production of matrix metalloproteinases in the intestine, which are increased in patients with CD. In conclusion, HSPs appear to influence the key features of CD through their contribution to the maintenance of mucosal barrier integrity, inhibition of apoptosis, and regulation of inflammatory processes. Therefore, therapies targeting the expression of HSPs in the intestinal mucosa should be pursued for the treatment of inflammatory gastrointestinal diseases.
Footnotes
Supported by OTKA-84087/2010, -K81117, -K105530, -PD83431, -PD105361, “Lendulet” Research Grant LP2011-008, 2011 and KMR_12-1-2012-0074; Vannay Á and Veres G are holders of the János Bolyai Research Grant by János Bolyai Research Scholarship of the Hungarian Academy of Sciences
P- Reviewers: Barish CF, Crenn PP, Rhoads JM S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Sziksz E, Himer L, Veres G, Szebeni B, Arató A, Tulassay T, Vannay A. Heat shock proteins (HSPs) in coeliac disease. In: Kruzliak P, editor. Celiac Disease -From Pathophysiology to Advanced Therapies. Rijeka: InTech; 2012. pp. 37–68. [Google Scholar]
- 2.Schuppan D, Dennis MD, Kelly CP. Celiac disease: epidemiology, pathogenesis, diagnosis, and nutritional management. Nutr Clin Care. 2005;8:54–69. [PubMed] [Google Scholar]
- 3.Silano M, Agostoni C, Guandalini S. Effect of the timing of gluten introduction on the development of celiac disease. World J Gastroenterol. 2010;16:1939–1942. doi: 10.3748/wjg.v16.i16.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catassi C, Fasano A. Celiac disease. Curr Opin Gastroenterol. 2008;24:687–691. doi: 10.1097/MOG.0b013e32830edc1e. [DOI] [PubMed] [Google Scholar]
- 5.Fric P, Gabrovska D, Nevoral J. Celiac disease, gluten-free diet, and oats. Nutr Rev. 2011;69:107–115. doi: 10.1111/j.1753-4887.2010.00368.x. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A. Novel therapeutic/integrative approaches for celiac disease and dermatitis herpetiformis. Clin Dev Immunol. 2012;2012:959061. doi: 10.1155/2012/959061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol. 2010;26:116–122. doi: 10.1097/MOG.0b013e3283365263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldassarre M, Laneve AM, Grosso R, Laforgia N. Celiac disease: pathogenesis and novel therapeutic strategies. Endocr Metab Immune Disord Drug Targets. 2008;8:152–158. doi: 10.2174/187153008785700109. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia V, Tandon RK. Stress and the gastrointestinal tract. J Gastroenterol Hepatol. 2005;20:332–339. doi: 10.1111/j.1440-1746.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- 10.Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- 11.Lewis K, McKay DM. Metabolic stress evokes decreases in epithelial barrier function. Ann N Y Acad Sci. 2009;1165:327–337. doi: 10.1111/j.1749-6632.2009.04036.x. [DOI] [PubMed] [Google Scholar]
- 12.John LJ, Fromm M, Schulzke JD. Epithelial barriers in intestinal inflammation. Antioxid Redox Signal. 2011;15:1255–1270. doi: 10.1089/ars.2011.3892. [DOI] [PubMed] [Google Scholar]
- 13.Szaflarska-Poplawska A, Siomek A, Czerwionka-Szaflarska M, Gackowski D, Rózalski R, Guz J, Szpila A, Zarakowska E, Olinski R. Oxidatively damaged DNA/oxidative stress in children with celiac disease. Cancer Epidemiol Biomarkers Prev. 2010;19:1960–1965. doi: 10.1158/1055-9965.EPI-10-0295. [DOI] [PubMed] [Google Scholar]
- 14.Alaedini A, Green PH. Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med. 2005;142:289–298. doi: 10.7326/0003-4819-142-4-200502150-00011. [DOI] [PubMed] [Google Scholar]
- 15.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516–525. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 16.Briani C, Samaroo D, Alaedini A. Celiac disease: from gluten to autoimmunity. Autoimmun Rev. 2008;7:644–650. doi: 10.1016/j.autrev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–231. doi: 10.1111/j.0105-2896.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 18.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–1933. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Qiao SW, Sollid LM, Blumberg RS. Antigen presentation in celiac disease. Curr Opin Immunol. 2009;21:111–117. doi: 10.1016/j.coi.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Szebeni B, Veres G, Dezsofi A, Rusai K, Vannay A, Bokodi G, Vásárhelyi B, Korponay-Szabó IR, Tulassay T, Arató A. Increased mucosal expression of Toll-like receptor (TLR)2 and TLR4 in coeliac disease. J Pediatr Gastroenterol Nutr. 2007;45:187–193. doi: 10.1097/MPG.0b013e318064514a. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 23.Sollid LM, Jabri B. Is celiac disease an autoimmune disorder? Curr Opin Immunol. 2005;17:595–600. doi: 10.1016/j.coi.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Ritossa FM, Vonborstel RC. Chromosome puffs in drosophila induced by ribonuclease. Science. 1964;145:513–514. doi: 10.1126/science.145.3631.513. [DOI] [PubMed] [Google Scholar]
- 26.Stetler RA, Gan Y, Zhang W, Liou AK, Gao Y, Cao G, Chen J. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog Neurobiol. 2010;92:184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev. 2009;8:388–393. doi: 10.1016/j.autrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aufricht C. HSP: helper, suppressor, protector. Kidney Int. 2004;65:739–740. doi: 10.1111/j.1523-1755.2004.00442.x. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui F, Avery PR, Li CY, Zhang X, LaRue SM, Dewhirst MW, Ullrich RL. Induction of the human heat shock promoter HSP70B by nutritional stress: implications for cancer gene therapy. Cancer Invest. 2008;26:553–561. doi: 10.1080/07357900701788015. [DOI] [PubMed] [Google Scholar]
- 30.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghayour-Mobarhan M, Rahsepar AA, Tavallaie S, Rahsepar S, Ferns GA. The potential role of heat shock proteins in cardiovascular disease: evidence from in vitro and in vivo studies. Adv Clin Chem. 2009;48:27–72. [PubMed] [Google Scholar]
- 32.Geiger PC, Gupte AA. Heat shock proteins are important mediators of skeletal muscle insulin sensitivity. Exerc Sport Sci Rev. 2011;39:34–42. doi: 10.1097/JES.0b013e318201f236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong HR, Wispé JR. The stress response and the lung. Am J Physiol. 1997;273:L1–L9. doi: 10.1152/ajplung.1997.273.1.L1. [DOI] [PubMed] [Google Scholar]
- 34.Kelly KJ. Heat shock (stress response) proteins and renal ischemia/reperfusion injury. Contrib Nephrol. 2005;148:86–106. doi: 10.1159/000086054. [DOI] [PubMed] [Google Scholar]
- 35.Tashiro S. Mechanism of liver regeneration after liver resection and portal vein embolization (ligation) is different? J Hepatobiliary Pancreat Surg. 2009;16:292–299. doi: 10.1007/s00534-009-0058-x. [DOI] [PubMed] [Google Scholar]
- 36.Asano T, Tanaka K, Yamakawa N, Adachi H, Sobue G, Goto H, Takeuchi K, Mizushima T. HSP70 confers protection against indomethacin-induced lesions of the small intestine. J Pharmacol Exp Ther. 2009;330:458–467. doi: 10.1124/jpet.109.152181. [DOI] [PubMed] [Google Scholar]
- 37.Papp E, Nardai G, Söti C, Csermely P. Molecular chaperones, stress proteins and redox homeostasis. Biofactors. 2003;17:249–257. doi: 10.1002/biof.5520170124. [DOI] [PubMed] [Google Scholar]
- 38.Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med. 2011;51:5–16. doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratt WB, Galigniana MD, Morishima Y, Murphy PJ. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004;40:41–58. doi: 10.1042/bse0400041. [DOI] [PubMed] [Google Scholar]
- 40.Hauet-Broere F, Wieten L, Guichelaar T, Berlo S, van der Zee R, Van Eden W. Heat shock proteins induce T cell regulation of chronic inflammation. Ann Rheum Dis. 2006;65 Suppl 3:iii65–iii68. doi: 10.1136/ard.2006.058495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- 42.Csermely P, Söti C, Blatch GL. Chaperones as parts of cellular networks. Adv Exp Med Biol. 2007;594:55–63. doi: 10.1007/978-0-387-39975-1_6. [DOI] [PubMed] [Google Scholar]
- 43.Pechan PM. Heat shock proteins and cell proliferation. FEBS Lett. 1991;280:1–4. doi: 10.1016/0014-5793(91)80190-e. [DOI] [PubMed] [Google Scholar]
- 44.Padmini E, Lavanya S. HSP70-mediated control of endothelial cell apoptosis during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2011;156:158–164. doi: 10.1016/j.ejogrb.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis. 2010;33:789–801. doi: 10.1111/j.1365-2761.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- 47.Graf PC, Jakob U. Redox-regulated molecular chaperones. Cell Mol Life Sci. 2002;59:1624–1631. doi: 10.1007/PL00012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivabene R, Mancini E, De Vincenzi M. In vitro cytotoxic effect of wheat gliadin-derived peptides on the Caco-2 intestinal cell line is associated with intracellular oxidative imbalance: implications for coeliac disease. Biochim Biophys Acta. 1999;1453:152–160. doi: 10.1016/s0925-4439(98)00095-7. [DOI] [PubMed] [Google Scholar]
- 49.Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res. 2009;674:137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 50.van Straaten EA, Koster-Kamphuis L, Bovee-Oudenhoven IM, van der Meer R, Forget PP. Increased urinary nitric oxide oxidation products in children with active coeliac disease. Acta Paediatr. 1999;88:528–531. doi: 10.1080/08035259950169530. [DOI] [PubMed] [Google Scholar]
- 51.Diosdado B, van Oort E, Wijmenga C. “Coelionomics”: towards understanding the molecular pathology of coeliac disease. Clin Chem Lab Med. 2005;43:685–695. doi: 10.1515/CCLM.2005.117. [DOI] [PubMed] [Google Scholar]
- 52.Murray IA, Daniels I, Coupland K, Smith JA, Long RG. Increased activity and expression of iNOS in human duodenal enterocytes from patients with celiac disease. Am J Physiol Gastrointest Liver Physiol. 2002;283:G319–G326. doi: 10.1152/ajpgi.00324.2001. [DOI] [PubMed] [Google Scholar]
- 53.Omar R, Pappolla M. Oxygen free radicals as inducers of heat shock protein synthesis in cultured human neuroblastoma cells: relevance to neurodegenerative disease. Eur Arch Psychiatry Clin Neurosci. 1993;242:262–267. doi: 10.1007/BF02190384. [DOI] [PubMed] [Google Scholar]
- 54.Kiang JG. Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury. Cell Res. 2004;14:450–459. doi: 10.1038/sj.cr.7290247. [DOI] [PubMed] [Google Scholar]
- 55.Zlatković J, Bernardi RE, Filipović D. Protective effect of Hsp70i against chronic social isolation stress in the rat hippocampus. J Neural Transm. 2014;121:3–14. doi: 10.1007/s00702-013-1066-1. [DOI] [PubMed] [Google Scholar]
- 56.Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol (1985) 2001;90:2403–2410. doi: 10.1152/jappl.2001.90.6.2403. [DOI] [PubMed] [Google Scholar]
- 57.Chen SL, Yang CT, Yang ZL, Guo RX, Meng JL, Cui Y, Lan AP, Chen PX, Feng JQ. Hydrogen sulphide protects H9c2 cells against chemical hypoxia-induced injury. Clin Exp Pharmacol Physiol. 2010;37:316–321. doi: 10.1111/j.1440-1681.2009.05289.x. [DOI] [PubMed] [Google Scholar]
- 58.Takada M, Otaka M, Takahashi T, Izumi Y, Tamaki K, Shibuya T, Sakamoto N, Osada T, Yamamoto S, Ishida R, et al. Overexpression of a 60-kDa heat shock protein enhances cytoprotective function of small intestinal epithelial cells. Life Sci. 2010;86:499–504. doi: 10.1016/j.lfs.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Aztatzi-Santillán E, Nares-López FE, Márquez-Valadez B, Aguilera P, Chánez-Cárdenas ME. The protective role of heme oxygenase-1 in cerebral ischemia. Cent Nerv Syst Agents Med Chem. 2010;10:310–316. doi: 10.2174/187152410793429764. [DOI] [PubMed] [Google Scholar]
- 60.Osterloh A, Breloer M. Heat shock proteins: linking danger and pathogen recognition. Med Microbiol Immunol. 2008;197:1–8. doi: 10.1007/s00430-007-0055-0. [DOI] [PubMed] [Google Scholar]
- 61.Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. 2002;14:45–51. doi: 10.1016/s0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 62.Multhoff G. Activation of natural killer cells by heat shock protein 70. 2002. Int J Hyperthermia. 2009;25:169–175. doi: 10.1080/02656730902902001. [DOI] [PubMed] [Google Scholar]
- 63.Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–451. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 64.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 65.Asea A. Heat shock proteins and toll-like receptors. Handb Exp Pharmacol. 2008;(183):111–127. doi: 10.1007/978-3-540-72167-3_6. [DOI] [PubMed] [Google Scholar]
- 66.Flohé SB, Brüggemann J, Lendemans S, Nikulina M, Meierhoff G, Flohé S, Kolb H. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J Immunol. 2003;170:2340–2348. doi: 10.4049/jimmunol.170.5.2340. [DOI] [PubMed] [Google Scholar]
- 67.Zanin-Zhorov A, Cohen IR. Signaling via TLR2 and TLR4 Directly Down-Regulates T Cell Effector Functions: The Regulatory Face of Danger Signals. Front Immunol. 2013;4:211. doi: 10.3389/fimmu.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Ludwig D, Stahl M, Ibrahim ET, Wenzel BE, Drabicki D, Wecke A, Fellermann K, Stange EF. Enhanced intestinal expression of heat shock protein 70 in patients with inflammatory bowel diseases. Dig Dis Sci. 1999;44:1440–1447. doi: 10.1023/a:1026616221950. [DOI] [PubMed] [Google Scholar]
- 70.Rodolico V, Tomasello G, Zerilli M, Martorana A, Pitruzzella A, Gammazza AM, David S, Zummo G, Damiani P, Accomando S, et al. Hsp60 and Hsp10 increase in colon mucosa of Crohn’s disease and ulcerative colitis. Cell Stress Chaperones. 2010;15:877–884. doi: 10.1007/s12192-010-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka K, Mizushima T. Protective role of HSF1 and HSP70 against gastrointestinal diseases. Int J Hyperthermia. 2009;25:668–676. doi: 10.3109/02656730903213366. [DOI] [PubMed] [Google Scholar]
- 72.Kitamura H, Yamamoto S, Nakase H, Matsuura M, Honzawa Y, Matsumura K, Takeda Y, Uza N, Nagata K, Chiba T. Role of heat shock protein 47 in intestinal fibrosis of experimental colitis. Biochem Biophys Res Commun. 2011;404:599–604. doi: 10.1016/j.bbrc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Watson AJ. Necrosis and apoptosis in the gastrointestinal tract. Gut. 1995;37:165–167. doi: 10.1136/gut.37.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Sabatino A, Ciccocioppo R, D’Alò S, Parroni R, Millimaggi D, Cifone MG, Corazza GR. Intraepithelial and lamina propria lymphocytes show distinct patterns of apoptosis whereas both populations are active in Fas based cytotoxicity in coeliac disease. Gut. 2001;49:380–386. doi: 10.1136/gut.49.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giovannini C, Matarrese P, Scazzocchio B, Varí R, D’Archivio M, Straface E, Masella R, Malorni W, De Vincenzi M. Wheat gliadin induces apoptosis of intestinal cells via an autocrine mechanism involving Fas-Fas ligand pathway. FEBS Lett. 2003;540:117–124. doi: 10.1016/s0014-5793(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 76.Choi K, Ni L, Jonakait GM. Fas ligation and tumor necrosis factor α activation of murine astrocytes promote heat shock factor-1 activation and heat shock protein expression leading to chemokine induction and cell survival. J Neurochem. 2011;116:438–448. doi: 10.1111/j.1471-4159.2010.07124.x. [DOI] [PubMed] [Google Scholar]
- 77.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 78.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 79.Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, Vickers SM, Saluja AK. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009;136:1772–1782. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001;20:446–456. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gabai VL, Yaglom JA, Volloch V, Meriin AB, Force T, Koutroumanis M, Massie B, Mosser DD, Sherman MY. Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol Cell Biol. 2000;20:6826–6836. doi: 10.1128/mcb.20.18.6826-6836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhães PJ, Di Virgilio F, Pozzan T. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- 83.Creagh EM, Carmody RJ, Cotter TG. Heat shock protein 70 inhibits caspase-dependent and -independent apoptosis in Jurkat T cells. Exp Cell Res. 2000;257:58–66. doi: 10.1006/excr.2000.4856. [DOI] [PubMed] [Google Scholar]
- 84.Ghosh JC, Siegelin MD, Dohi T, Altieri DC. Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res. 2010;70:8988–8993. doi: 10.1158/0008-5472.CAN-10-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 86.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 87.Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 88.Petrof EO, Ciancio MJ, Chang EB. Role and regulation of intestinal epithelial heat shock proteins in health and disease. Chin J Dig Dis. 2004;5:45–50. doi: 10.1111/j.1443-9573.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 89.Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290:G204–G212. doi: 10.1152/ajpgi.00401.2005. [DOI] [PubMed] [Google Scholar]
- 90.Musch MW, Sugi K, Straus D, Chang EB. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology. 1999;117:115–122. doi: 10.1016/s0016-5085(99)70557-3. [DOI] [PubMed] [Google Scholar]
- 91.Tsapara A, Matter K, Balda MS. The heat-shock protein Apg-2 binds to the tight junction protein ZO-1 and regulates transcriptional activity of ZONAB. Mol Biol Cell. 2006;17:1322–1330. doi: 10.1091/mbc.E05-06-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zitka O, Kukacka J, Krizkova S, Huska D, Adam V, Masarik M, Prusa R, Kizek R. Matrix metalloproteinases. Curr Med Chem. 2010;17:3751–3768. doi: 10.2174/092986710793213724. [DOI] [PubMed] [Google Scholar]
- 93.Daum S, Bauer U, Foss HD, Schuppan D, Stein H, Riecken EO, Ullrich R. Increased expression of mRNA for matrix metalloproteinases-1 and -3 and tissue inhibitor of metalloproteinases-1 in intestinal biopsy specimens from patients with coeliac disease. Gut. 1999;44:17–25. doi: 10.1136/gut.44.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sims JD, McCready J, Jay DG. Extracellular heat shock protein (Hsp)70 and Hsp90α assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS One. 2011;6:e18848. doi: 10.1371/journal.pone.0018848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 96.Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205. [DOI] [PubMed] [Google Scholar]
- 97.Arvans DL, Vavricka SR, Ren H, Musch MW, Kang L, Rocha FG, Lucioni A, Turner JR, Alverdy J, Chang EB. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol. 2005;288:G696–G704. doi: 10.1152/ajpgi.00206.2004. [DOI] [PubMed] [Google Scholar]
- 98.Kojima K, Musch MW, Ren H, Boone DL, Hendrickson BA, Ma A, Chang EB. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology. 2003;124:1395–1407. doi: 10.1016/s0016-5085(03)00215-4. [DOI] [PubMed] [Google Scholar]
- 99.Yang PC, He SH, Zheng PY. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. J Gastroenterol Hepatol. 2007;22:1823–1831. doi: 10.1111/j.1440-1746.2006.04710.x. [DOI] [PubMed] [Google Scholar]
- 100.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 101.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 102.Sziksz E, Veres G, Vannay A, Prókai A, Gál K, Onody A, Korponay-Szabó IR, Reusz G, Szabó A, Tulassay T, et al. Increased heat shock protein 72 expression in celiac disease. J Pediatr Gastroenterol Nutr. 2010;51:573–578. doi: 10.1097/MPG.0b013e3181ea0092. [DOI] [PubMed] [Google Scholar]
- 103.Brottveit M, Beitnes AC, Tollefsen S, Bratlie JE, Jahnsen FL, Johansen FE, Sollid LM, Lundin KE. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. 2013;108:842–850. doi: 10.1038/ajg.2013.91. [DOI] [PubMed] [Google Scholar]
- 104.Iltanen S, Rantala I, Laippala P, Holm K, Partanen J, Maki M. Expression of HSP-65 in jejunal epithelial cells in patients clinically suspected of coeliac disease. Autoimmunity. 1999;31:125–132. doi: 10.3109/08916939908994056. [DOI] [PubMed] [Google Scholar]
- 105.Yeboah FA, White D. AlphaB-crystallin expression in celiac disease - a preliminary study. Croat Med J. 2001;42:523–526. [PubMed] [Google Scholar]
- 106.Ramos-Arroyo MA, Feijoó E, Sánchez-Valverde F, Aranburu E, Irisarri N, Olivera JE, Valiente A. Heat-shock protein 70-1 and HLA class II gene polymorphisms associated with celiac disease susceptibility in Navarra (Spain) Hum Immunol. 2001;62:821–825. doi: 10.1016/s0198-8859(01)00277-4. [DOI] [PubMed] [Google Scholar]
- 107.Partanen J, Milner C, Campbell RD, Mäki M, Lipsanen V, Koskimies S. HLA-linked heat-shock protein 70 (HSP70-2) gene polymorphism and celiac disease. Tissue Antigens. 1993;41:15–19. doi: 10.1111/j.1399-0039.1993.tb01971.x. [DOI] [PubMed] [Google Scholar]
- 108.Calderwood SK, Stevenson MA, Murshid A. Heat shock proteins, autoimmunity, and cancer treatment. Autoimmune Dis. 2012;2012:486069. doi: 10.1155/2012/486069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ohkawara T, Nishihira J, Takeda H, Miyashita K, Kato K, Kato M, Sugiyama T, Asaka M. Geranylgeranylacetone protects mice from dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2005;40:1049–1057. doi: 10.1080/00365520510023161. [DOI] [PubMed] [Google Scholar]
- 110.Mizushima T. HSP-dependent protection against gastrointestinal diseases. Curr Pharm Des. 2010;16:1190–1196. doi: 10.2174/138161210790945986. [DOI] [PubMed] [Google Scholar]
- 111.Dotan I, Rachmilewitz D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr Opin Gastroenterol. 2005;21:426–430. [PubMed] [Google Scholar]
- 112.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 113.Otaka M, Odashima M, Watanabe S. Role of heat shock proteins (molecular chaperones) in intestinal mucosal protection. Biochem Biophys Res Commun. 2006;348:1–5. doi: 10.1016/j.bbrc.2006.07.028. [DOI] [PubMed] [Google Scholar]


