Abstract
AIM: To investigate telomerase activity and human telomerase reverse transcriptase (hTERT) expression in normal human gastric mucosal epithelial cells (nhGMECs) and fibroblasts (nhGMFs).
METHODS: nhGMECs and nhGMFs were isolated and cultured from specimens obtained during routine surgery for bleeding peptic ulcer. Telomerase activity in nhGMFs, nhGMECs, and the tumor cell lines BGC-823, SGC-7901 and MKN-28 cells was analyzed using the telomeric repeat amplification protocol assay. hTERT protein was determined in nhGMECs, nhGMFs, BGC-823, SGC-7901 and MKN-28 cells by indirect immunofluorescence.
RESULTS: A similar level of telomerase activity was observed in nhGMECs, nhGMFs and BGC-823, SGC-7901, MKN-28 cell lines. Positive hTERT immunostaining was detected in nhGMECs, nhGMFs, BGC-823, SGC-7901 and MKN-28 cell lines.
CONCLUSION: The use of telomerase or hTERT as diagnostic markers for gastric cancer may require further studies.
Keywords: Gastric cancer, Telomerase, Human telomerase reverse transcriptase, Normal human gastric mucosal epithelial cell, Normal human gastric mucosal fibroblast
Core tip: Telomerase activity and human telomerase reverse transcriptase (hTERT) protein expression were detected in normal human gastric mucosal epithelial cells isolated from human gastric tissues, and were similar to those found in cell lines from human gastric adenocarcinoma. The results of this study suggest that the use of telomerase or hTERT as diagnostic markers for gastric cancer may require further studies.
INTRODUCTION
Despite its decreasing frequency worldwide, gastric cancer remains one of the major causes of cancer-related deaths[1,2]. This is due to the fact that most cases are in the advanced stages of disease when diagnosed. While the 5-year survival of patients with advanced gastric cancer is approximately 20%, early tumor resection can achieve a 5-year survival rate of around 90%[3]. Therefore, early diagnosis is an important measure to improve the prognosis of patients with gastric cancer. Researchers have been looking for early diagnostic markers for gastric cancer for more than ten years[4-8].
Telomerase is a specialized reverse transcriptase that adds telomeric repeats to the ends of eukaryotic chromosomes, and is responsible for continuous cell growth. Human telomerase reverse transcriptase (hTERT) is the major subunit of the telomerase enzyme complex and plays a critical role in the regulation of telomerase activity[1,9]. They are observed in 80%-90% of human tumors including gastric cancer and nearly all cancer-derived cell lines[4,5,10], and are not observed in the majority of normal tissues and somatic cells, therefore could be considered useful markers for the early diagnosis of human gastric cancer. However, hTERT expression was also found in normal gastric tissues; a full-length hTERT mRNA was present in 43% of normal gastric specimens and hTERT protein was expressed at all the proliferation zones in crypts[11]. Therefore, the use of hTERT and subsequently telomerase as gastric cancer markers is unclear.
In the present study, we determined the expression of telomerase and hTERT in primary cultured cells from normal human gastric mucosal epithelium, and evaluated whether they could be used as cytological markers for the diagnosis of gastric cancer.
MATERIALS AND METHODS
Cell culture
After the study protocol was approved by the university and hospital ethical committees and informed consent was obtained from the patients, normal human gastric mucosal epithelial cells (nhGMECs) were isolated from specimens obtained during routine surgery for bleeding peptic ulcer using a method previously developed by us[12]. Cell viability was estimated by methyl thiazolyl tetrazolium assay to examine the general growth process. Periodic acid-Schiff (PAS) staining was used to identify mucinogen granules in epithelial cells and cytokeratin (CK)-18 staining was used to identify epithelial cells. Light microscopy and transmission electron microscopy were used to observe the morphological structures of cells. Toluidine blue (0.5%) staining was used to observe the nucleus of nhGMECs and SGC-7901 cells. Normal human gastric mucosal fibroblasts (nhGMFs) were also isolated from the same specimens. Male or female patients aged 40-71 years provided the gastric samples. BGC-823, SGC-7901 and MKN-28 cell lines maintained in our laboratory were used as controls. All cells were grown in DMEM-F12 medium supplemented with 10% fetal bovine serum without antibiotics.
Telomerase activity assay
Telomerase activity was determined using the telomeric repeat amplification protocol (TRAP) assay and a telomerase detection kit (Dingguo, Beijing, China). nhGMFs, nhGMECs, BGC-823, SGC-7901 and MKN-28 cells were analyzed according to the manufacturer’s protocol. Protein was extracted from 3 × 106 cells in each group. After 35 polymerase chain reaction cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s, the products were electrophoresed on 12.5% polyacrylamide gels.
hTERT protein detection
hTERT protein was determined in nhGMECs, nhGMFs, BGC-823, SGC-7901 and MKN-28 cells by indirect immunofluorescence. Cells were grown on slides coated with polylysine, fixed in 4% paraformaldehyde for 10 min and then permeabilized with 0.5% Triton X-100 for 10 min at room temperature. An hTERT antibody (Santa Cruz Biotechnology, CA, United States) was added to the slides and incubated for 2 h at 37 °C. After washing with phosphate-buffered saline, the cells were further incubated with TrITC conjugated secondary antibodies for 50 min at 37 °C. Finally, the cell nuclei were stained with diamidino-phenyl-indole (DAPI) (Vector Laboratories, United States) and observed under a Leica TCS SP5 laser scanning confocal microscope. Phosphate-buffered saline replaced the primary antibody in the controls.
RESULTS
Primary culture of nhGMECs
nhGMECs were dissociated and cultured. The viability of these cells showed a maximal increase between the 2nd and 3rd day, reached a peak on the 4th day, and then declined gradually. As shown in Figure 1, cultured cells were PAS-positive and CK-18 positive. On the 2nd day of inoculation, the cells grew in clumps and proliferated rapidly, then gradually ceased to grow after the 4th day and began to detach and die on the 5th day. Transmission electron microscopy revealed microvilli and secretary granules in gastric mucosal epithelial cells. Toluidine blue staining was weakly positive in nhGMECs and strongly positive in SGC-7901 cells. nhGMFs were also isolated and cultured.
Figure 1.
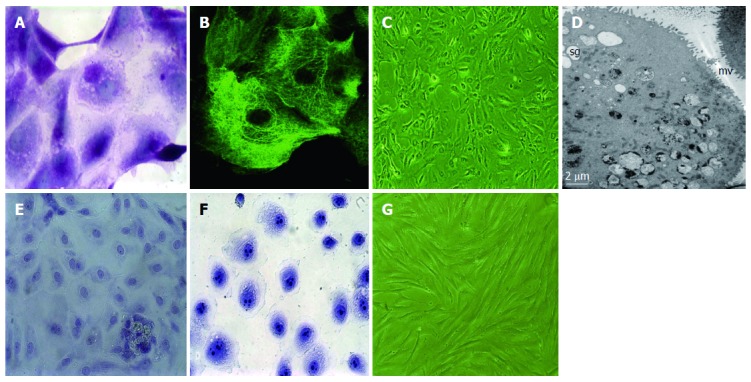
Periodic acid-Schiff, cytokeratin-18 and toluidine blue staining and cell morphology. A: Cytoplasm of normal human gastric mucosal epithelial cells (nhGMECs) was stained purple with periodic acid-Schiff (PAS), and contained neutral mucin granules, magnification × 1000; B: nhGMEC network-structure staining with an antibody against cytokeratin (CK)-18 demonstrates the presence of CK-18; C: Phase-contrast micrograph of nhGMECs after 4 d of culture, magnification × 100; D: Transmission electron microscopy revealed the presence of microvilli (mv) and secretary granules (sg) in nhGMECs, magnification × 1000; E: nhGMECs were detected by toluidine blue staining, and nuclei (light color) were observed, magnification × 400; F: SGC-7901 cells were detected by toluidine blue staining, and nuclei with multiple nucleoli (deep color) were observed, magnification × 400; G: Phase-contrast micrograph of nhGMFs after 13 d of culture, magnification × 100.
Telomerase activity
Telomerase activity was detected in all cultured cells using TRAP assay. Amplified telomeric repeats (160 bp) in nhGMECs and nhGMFs were equal to those in BGC-823, SGC-7901 and MKN-28 tumor cell lines (Figure 2). These results suggested that a similar level of telomerase expression was seen in nhGMECs, nhGMFs and the tumor cell lines.
Figure 2.
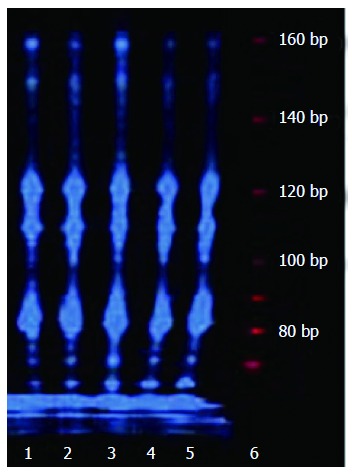
Telomerase activity in normal human gastric mucosal epithelial cells, normal human gastric mucosal fibroblasts and tumor cell lines. Lane 1: Normal human gastric mucosal fibroblasts; lane 2: Normal human gastric mucosal epithelial cells; lane 3: BGC-823 cells; lane 4: SGC-7901 cells; lane 5: MKN-28 cells; lane 6: Marker.
hTERT protein expression
In situ detection of hTERT showed that positive hTERT immunostaining was detected in nhGMECs, nhGMFs, BGC-823, SGC-7901 and MKN-28 cells (Figure 3). Both cellular cytoplasm and nuclear compartments were stained with the hTERT antibody. There was little difference in hTERT expression among nhGMECs, nhGMFs and the tumor cell lines.
Figure 3.
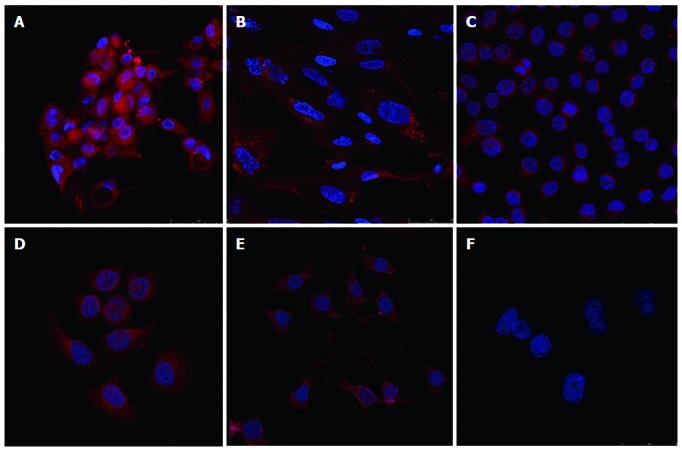
Expression of human telomerase reverse transcriptase in cultured cells, stained with diamidino-phenyl-indole, human telomerase reverse transcriptase antibody, and rhodamine labeled human telomerase reverse transcriptase second antibody. A: Normal human gastric mucosal epithelial cells; B: Normal human gastric mucosal fibroblasts; C: BGC-823 cells; D: SGC-7901 cells; E: MKN-28 cells; F: Negative control.
DISCUSSION
Increased telomerase activation and hTERT expression are generally considered early events in carcinogenesis[10,13]. Their assessment as diagnostic markers in various types of cancers has been carried out for more than ten years. However, some researchers have recently found similar expression of hTERT in both normal and cancerous gastric specimens[11], which challenges the widespread concept that hTERT and telomerase are repressed in normal tissues. Many highly proliferative normal human cells such as lymphocytes, hematopoietic progenitor cells and basal epidermal cells have been shown to express telomerase and hTERT[14,15]. Consistent with this, telomerase activity and hTERT protein were detected in primary cultured nhGMECs in our study. Telomerase activity and hTERT protein expression in nhGMECs were very similar to those in the three human gastric adenocarcinoma cell lines, BGC-823, SGC-7901 and MKN-28. In general, primary cultured nhGMECs are from the proliferation zones of crypts in gastric glands and have good proliferative ability. The presence of telomerase and hTERT in these cells could affect the feasibility of the diagnostic markers in the neoplastic process.
Fibroblasts are widely found in various tissues, both in benign and malignant tissues. They were previously believed to lack telomerase activity and hTERT expression[16]. However, several studies have confirmed the presence of telomerase and hTERT in human fibroblasts in recent years[15,17]. In the present study, telomerase activity and hTERT expression were similarly detected in primary cultured nhGMFs isolated from human gastric tissues, which will interfere with their use as gastric cancer markers.
Novel molecular biology techniques have generated some new ideas for therapeutics such as gene therapy in gastric cancer. Telomerase is one of therapeutic targets[17,18]. Its presence in nhGMECs and nhGMFs isolated from gastric tissues suggests that anti-telomerase treatment may trigger undesired toxicity in normal gastric cells, which will have an influence on the therapeutic outcome.
In conclusion, the present study demonstrated the expression of telomerase and hTERT in primary cultured nhGMECs and nhGMFs isolated from gastric tissues. Combined with the observation that hTERT expression occurs in normal human gastric tissues[6], from a cytological and histological point of view, the use of telomerase and hTERT as diagnostic markers for gastric cancer may require further investigation.
COMMENTS
Background
Gastric cancer remains one of the major causes of cancer-related deaths. This is due to the fact that most cases are in the advanced stages of disease when diagnosed. Therefore, early diagnosis is important in improving the prognosis of patients with gastric cancer. Researchers have been looking for early diagnostic markers for gastric cancer for more than ten years, including telomerase and human telomerase reverse transcriptase (hTERT).
Research frontiers
The assessment of telomerase and hTERT as diagnostic markers in various types of cancers has been carried out for more than ten years. However, the use of hTERT and subsequently telomerase as gastric cancer markers is still unclear. In this study, the authors detected telomerase activity and hTERT expression in primary cultured normal human gastric mucosal epithelial cells (nhGMECs) and normal human gastric mucosal fibroblasts (nhGMFs), which challenges the widespread concept that hTERT and telomerase are repressed in normal tissues.
Innovations and breakthroughs
Telomerase and hTERT are observed in 80%-90% of human tumors including gastric cancer and nearly all cancer-derived cell lines, and are not observed in the majority of normal tissues and somatic cells. However, some researchers have recently found that hTERT mRNA and protein were expressed in normal gastric tissues. In this study, the authors observed that both telomerase and hTERT were expressed in primary cultured normal human gastric mucosal cells including nhGMECs and nhGMFs.
Applications
The present study demonstrated the expression of telomerase and hTERT in primary cultured nhGMECs and nhGMFs isolated from gastric tissues, which suggested from the cytological point of view that using telomerase and hTERT as useful markers for early diagnosis and promising targets for gastric cancer treatment may need further investigation.
Terminology
Telomerase is a specialized reverse transcriptase that adds telomeric repeats to the ends of eukaryotic chromosomes, and is responsible for continuous cell growth. hTERT is the major subunit of the telomerase enzyme complex and plays a critical role in the regulation of telomerase activity. Increased telomerase activation and hTERT expression are generally considered the early events in carcinogenesis.
Peer review
The authors observed telomerase activity and hTERT expression in normal human gastric mucosal epithelial cells and fibroblasts. The results told us again that the use of hTERT and subsequently telomerase as gastric cancer markers is unclear. At the same time, if telomerase or hTERT are used as gastric cancer therapeutic targets, the adverse effects on normal somatic cells should be noted.
Footnotes
Supported by National Natural Science Foundation of China, No. 30270609
P- Reviewer: Tanyi M S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Gigek CO, Leal MF, Silva PN, Lisboa LC, Lima EM, Calcagno DQ, Assumpção PP, Burbano RR, Smith Mde A. hTERT methylation and expression in gastric cancer. Biomarkers. 2009;14:630–636. doi: 10.3109/13547500903225912. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Li PF, Geng M, Cao YC, Yin YC. Correlation between chemosensitivity to anticancer drugs and telomerase reverse transcriptase mRNA expression in gastric cancer. Diagn Pathol. 2013;8:33. doi: 10.1186/1746-1596-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stock M, Otto F. Gene deregulation in gastric cancer. Gene. 2005;360:1–19. doi: 10.1016/j.gene.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Hiyama E, Hiyama K. Telomerase as tumor marker. Cancer Lett. 2003;194:221–233. doi: 10.1016/s0304-3835(02)00709-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Chen RJ. Prevalence of telomerase activity in human cancer. J Formos Med Assoc. 2011;110:275–289. doi: 10.1016/S0929-6646(11)60043-0. [DOI] [PubMed] [Google Scholar]
- 6.Hu LH, Chen FH, Li YR, Wang L. Real-time determination of human telomerase reverse transcriptase mRNA in gastric cancer. World J Gastroenterol. 2004;10:3514–3517. doi: 10.3748/wjg.v10.i23.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang Y, Zhang J, Sun P, Shang J. Circulating cell-free human telomerase reverse transcriptase mRNA in plasma and its potential diagnostic and prognostic value for gastric cancer. Int J Clin Oncol. 2013;18:478–486. doi: 10.1007/s10147-012-0405-9. [DOI] [PubMed] [Google Scholar]
- 8.Tani N, Ichikawa D, Ikoma D, Tomita H, Sai S, Ikoma H, Okamoto K, Ochiai T, Ueda Y, Otsuji E, et al. Circulating cell-free mRNA in plasma as a tumor marker for patients with primary and recurrent gastric cancer. Anticancer Res. 2007;27:1207–1212. [PubMed] [Google Scholar]
- 9.Liu JL, Ge LY, Zhang GN. Telomerase activity and human telomerase reverse transcriptase expression in colorectal carcinoma. World J Gastroenterol. 2006;12:465–467. doi: 10.3748/wjg.v12.i3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulmann C, Lantuejoul S, Grace A, Leader M, Patchett S, Kay E. Telomerase activity in proximal and distal gastric neoplastic and preneoplastic lesions using immunohistochemical detection of hTERT. Dig Liver Dis. 2005;37:439–445. doi: 10.1016/j.dld.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Li L, Liu Z, Liu C, Liu Z, Strååt K, Björkholm M, Jia J, Xu D. Expression of the full-length telomerase reverse transcriptase (hTERT) transcript in both malignant and normal gastric tissues. Cancer Lett. 2008;260:28–36. doi: 10.1016/j.canlet.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Cheng YB, Fang DC, Guo LP, Wang ZQ, Luo YH, Zhou JC. A convenient primary culture of human gastric epithelial cells. Chin J Gastroenterol; 2007;12:31–35. [Google Scholar]
- 13.Wang W, Luo HS, Yu BP. Expression of NF-kappaB and human telomerase reverse transcriptase in gastric cancer and precancerous lesions. World J Gastroenterol. 2004;10:177–181. doi: 10.3748/wjg.v10.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA, et al. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 15.Ge Z, Liu C, Björkholm M, Gruber A, Xu D. Mitogen-activated protein kinase cascade-mediated histone H3 phosphorylation is critical for telomerase reverse transcriptase expression/telomerase activation induced by proliferation. Mol Cell Biol. 2006;26:230–237. doi: 10.1128/MCB.26.1.230-237.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Tahara E, Ide T, Ishikawa F. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet. 1998;18:65–68. doi: 10.1038/ng0198-65. [DOI] [PubMed] [Google Scholar]
- 17.Philippi C, Loretz B, Schaefer UF, Lehr CM. Telomerase as an emerging target to fight cancer--opportunities and challenges for nanomedicine. J Control Release. 2010;146:228–240. doi: 10.1016/j.jconrel.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Buseman CM, Wright WE, Shay JW. Is telomerase a viable target in cancer? Mutat Res. 2012;730:90–97. doi: 10.1016/j.mrfmmm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


