Abstract
AIM: To assessed the clinical significance of protocol liver biopsy (PLB) in pediatric liver transplantation (LT).
METHODS: Between July 2008 and August 2012, 89 and 55 PLBs were performed in pediatric patients at two and five years after LT, respectively. We assessed the histopathological findings using the Metavir scoring system, including activity (A) and fibrosis (F), and we identified factors associated with scores of ≥ A1 and ≥ F1. Our results clarified the timing and effectiveness of PLB.
RESULTS: The incidences of scores of ≥ A1 and ≥ F1 were 24.7% and 24.7%, respectively, at two years after LT and 42.3% and 34.5%, respectively, at five years. Independent risk factors in a multivariate analysis of a score of ≥ A1 at two years included ≥ 2 h of cold ischemic time, no acute cellular rejection and an alanine amino transaminase (ALT) level of ≥ 20 IU/L (P = 0.028, P = 0.033 and P = 0.012, respectively); however, no risk factors were identified for a score of ≥ F1. Furthermore, no independent risk factors associated with scores of ≥ A1 and ≥ F1 at five years were identified using multivariate analysis. A ROC curve analysis of ALT at two years for a score of ≥ A1 demonstrated the recommended cutoff value for diagnosing ≥ A1 histology to be 20 IU/L. The incidence of scores of ≥ A2 or ≥ F2 at two years after LT was 3.4% (three cases), and all patients had an absolute score of ≥ A2. In contrast to that observed for PLBs at five years after LT, the incidence of scores of ≥ A2 or ≥ F2 was 20.0% (11 cases), and all patients had an absolute score of ≥ F2. In all cases, the dose of immunosuppressants was increased after the PLB, and all ten patients who underwent a follow-up liver biopsy improved to scores of ≤ A1 or F1.
CONCLUSION: PLB at two years after LT is an unnecessary examination, because the serum ALT level reflects portal inflammation. In addition, immunosuppressive therapy should be modulated to maintain the ALT concentration at a level less than 20 IU/L. PLB at five years is an excellent examination for the detection of early reversible graft fibrosis because no serum markers reflect this finding.
Keywords: Protocol liver biopsy, Graft fibrosis, Immunosuppression, Liver function test, Pediatric liver transplantation
Core tip: Few studies have investigated the impact of the timing and effectiveness of post-transplant protocol liver biopsy (PLB). We assessed the histopathological findings of these biopsies using the Metavir scoring system, and our results clarified the timing and effectiveness of PLB. PLB at two years after pediatric liver transplantation is an unnecessary examination, because the serum alanine amino transaminase (ALT) level reflects portal inflammation. In addition, immunosuppressive therapy should be modulated to maintain the ALT concentration at a level less than 20 IU/L. PLB at five years is an excellent examination for the detection of early reversible graft fibrosis because no serum markers reflect this finding.
INTRODUCTION
Liver transplantation (LT) is an established curative treatment for pediatric patients with end-stage liver disease or acute liver failure[1-3]. Graft fibrosis and/or chronic rejection can still occasionally lead to graft failure or even death despite improvements and innovations in immunosuppressive therapy, and the histopathological assessments performed after LT remain insufficient. It is therefore necessary to further improve the prognosis by maintaining the function of the liver graft using a minimum degree of immunosuppression to achieve an optimal balance between the effectiveness and side effects of individual immunosuppressants.
The development of liver graft fibrosis after pediatric LT has been reported to occur in 69%-97% of cases, including cases of mild fibrosis[4-8]. Graft dysfunction does not occur unless the fibrosis becomes advanced, and the occurrence of graft fibrosis or portal inflammation cannot be predicted using the standard liver function test (LFT) alone. Therefore, histopathological assessments using protocol liver biopsy (PLB) have recently been reported to be important[4-9]. However, the significance of mild to severe fibrosis is unclear, and the indications for the treatment of abnormal PLB findings are controversial. In addition, few studies have investigated the impact of the timing and effectiveness of PLB. This retrospective study assessed the clinical significance of the timing and effectiveness of PLB after pediatric living donor liver transplantation (LDLT).
MATERIALS AND METHODS
Patients
Between July 2008 and August 2012, 144 PLBs were performed in pediatric patients at two and five years after LDLT at the Department of Transplant Surgery, Jichi Medical University, Japan (Table 1). The observation period was between six and 55 mo.
Table 1.
Demographic characteristics of recipients and grafts undergoing protocol liver biopsy at two and five years after living donor liver transplantation
| PLB at two years after LDLT (n = 89) | PLB at five years after LDLT (n = 55) | |
| Recipient characteristics at LDLT | ||
| Gender | Male 37, female 52 | Male 20, female 35 |
| Age (mo) | 22 (0-234) | 19 (7-198) |
| Body weight (kg) | 10.7 (2.6-58.5) | 9.7 (5.9-64.9) |
| Original disease | BA 63, OTCD 9, AS 4, FHF 4, CEPS 3, graft failure 2, WD 1, PSC 1, CPS1D 1, LC 1 | BA 43, OTCD 3, AS 2, WD 2, FHF 1, HB 1, CF 1, CEPS 1, graft failure 1 |
| PELD or MELD | 7.4 (-9.7-39.4) | 8.6 (-8.9-39.4) |
| Operation time | 13 h 25 min (7 h 33 min-30 h 28 min) | 17h 19 min (11 h 11 min-30 h 28 min) |
| Cold ischemic time | 2 h 17 min (36 min-8 h 6 min) | 2 h 06 min (25 min-16 h 19 min) |
| Warm ischemic time | 45 min (30 min-2 h 2 min) | 1 h 00 min (30 min-4 h 27 min) |
| Blood loss volume (mL/kg) | 77.0 (3.1-585.1) | 45.5 (6.7-776.2) |
| Transfusion volume (mL/kg) | 91.3 (0.0-597.7) | 68.1 (0.0-670.7) |
| Donor and graft characteristics at LDLT | ||
| Gender | Father; 45, mother; 44 | Father; 30, mother; 25 |
| Age (yr) | 33 (23-57) | 33 (23-53) |
| ABO compatibility | Identical; 55, compatible; 20, incompatible 14 | Identical; 40, compatible; 8, incompatible 7 |
| GV/SLV (%) | 68.0 (33.0-120.9) | 75.8 (35.7-121.2) |
| Graft type | Lateral segment; 57, left lobe; 23, | Lateral segment; 43, left lobe; 10, |
| S2 monosegment; 5, left lobe + caudate; 4 | left lobe + caudate; 2 | |
| Recipient and graft characteristics at PLB | ||
| Age (mo) | 48 (24-259) | 81 (68-257) |
| Body weight (kg) | 15.6 (7.3-64.6) | 21.4 (14.4-71.6) |
| Total bilirubin (mg/dL) | 0.63 (0.25-3.25) | 0.68 (0.26-2.55) |
| AST (IU/L) | 30 (14-61) | 27 (10-251) |
| ALT (IU/L) | 17 (9-54) | 17 (8-260) |
| γ-GTP (IU/L) | 17 (6-440) | 16 (9-510) |
| Hyaluronic acid (ng/mL) | 21 (9-239) | 17 (9-216) |
| IgG (mg/dL) | 927 (440-2063) | 1148 (475-2961) |
| GV/SLV (%) | 90.6 (70.2-126.9) | 93.0 (58.8-157.0) |
| Spleen volume (mL) | 125 (0-892) | 145 (0-692) |
| Trough of tacrolimus (ng/mL) | 3.4 (0-10.1) | 2.3 (0-15.5) |
PLB: Protocol liver biopsy; LDLT: Living donor liver transplantation; BA: Biliary atresia; OTCD: Ornithine transcarbamylase deficiency; AD: Alagille syndrome; FHF: Fulminant hepatic failure; CEPS: Congenital extrahepatic portsystemic shunt; WD: Wilson disease; PSC: Primary sclerosing cholangitis; CPS1D: Carbamoyl-phosphate synthase 1 deficiency; LC: Liver cirrhosis; HB: Hepatoblastoma; CF: Cystic fibrosis; PELD: Pediatric end-stage liver disease; MELD: Model for end-stage liver disease; GV/SLV: Ratio of graft volume to standard liver volume; AST: Aspartate amino transferase; ALT: Alanine amino transferase; IgG: Immunoglobulin G.
Immunosuppressive therapy
Tacrolimus (Tac) and methylprednisolone (MP) were used as the standard postoperative immunosuppressive regimen. The target trough levels of Tac were 15-20 ng/mL during the first week, 8-12 ng/mL during the first month, 5-8 ng/mL during the first six months, 3-5 ng/mL during the first year and 2-4 ng/mL thereafter. MP was administered at an initial dose of 20 mg/kg intravenously on the morning of the operation and before graft reperfusion. The MP dose was thereafter decreased gradually to 3 mg/kg per day on postoperative day (POD) 1, 0.5 mg/kg per day on POD 7 and 0.25 mg/kg per day at one month after LDLT and was then discontinued within one year except in patients in whom immunosuppression could not be maintained at the lowest dose. Mycophenolate mofetil (MMF) was used when more potent immunosuppression was required, such as in ABO-incompatible recipients older than five years, patients with steroid-resistant acute rejection episodes and patients with liver dysfunction following the cessation of MP therapy.
Diagnosis of acute cellular rejection
All episodes of acute cellular rejection were diagnosed based on the histopathological findings of a liver biopsy. In all specimens, the diagnosis of acute cellular rejection was evaluated by highly experienced pathologists and graded into four classes according to the Banff scheme[10]. The degrees of portal infiltration by lymphocytes (P0-3), bile duct inflammation or damage (B0-3) and venous endothelial inflammation (V0-3) in the Banff scheme were evaluated. A liver biopsy was indicated when all liver function data (aspartate amino transferase, alanine amino transferase (ALT), gamma-glutamyl transpeptidase, and total bilirubin) were elevated compared with the previous data.
PLB procedure and timing
We began to perform PLBs in pediatric patients at two and five years after LT in July 2008 because we experienced cases of normal LFTs coexisting with histopathological portal inflammation and fibrosis, including cases 4 and 5, which are discussed later. In those cases in which the dose of immunosuppressants was increased after the PLB, we generally performed a follow-up liver biopsy between six months and one year after the PLB. In addition to a PLB, an episode biopsy was performed when a recipient with a high serum level of ALT or hyaluronic acid was refractory to an increase in immunosuppressants.
The PLB necessitated an overnight stay at our hospital. A percutaneous transhepatic liver biopsy was performed under analgesia and sedation using ultrasonographically-guided 14 G Monopty (C.R.Bard, Inc. United States). Manual compressive hemostasis was conducted for 20 min, after which compressive bandage hemostasis was performed until the following day. Preventive cefoperazone and sulbactam were also administered on that day.
Assessment of the PLB findings
We assessed the histopathological features of the PLB samples using the Metavir scoring system[11], which grades the activity (A), i.e., the amount of inflammation (specifically, the intensity of necro-inflammatory lesions), on a four-point scale from A0 to A3. Fibrosis (F) was graded on a five-point scale from F0 to F4.
Strategy of increasing the dose of immunosuppressants after LDLT
When the serum level of ALT or hyaluronic acid was found to be high in outpatients, we increased the dose of immunosuppressants if the suspected causes of the elevation of these levels was an immune response. When the serum level of ALT or hyaluronic acid was maintained at a normal level for a few months in the early period or for six months in the late period after LDLT, we gradually decreased the dose of immunosuppressants.
When the PLB score was ≥ A2 or ≥ F2, we increased the dose of immunosuppressants to provide the early treatment of portal inflammation or fibrosis. When the PLB grade was A0 and F0, we gradually decreased the dose of immunosuppressants.
Statistical analysis
The significance of the differences between two groups was evaluated using the chi-squared test. Associations between the recipient, donor or graft variables and abnormal histopathological findings were evaluated using univariate and backward selection multivariate Cox regression methods. A ROC curve analysis was performed to identify the cutoff value for the correlation between the ALT level and abnormal histopathological findings. All statistical analyses were performed using the StatView software package (SAS Institute, Cary, NC) and EZR (Saitama Medical Center, Jichi Medical University, Japan). Differences of P < 0.05 were considered to be significant.
RESULTS
Results of PLB at two years after LDLT
The incidence of scores of ≥ A1 and ≥ F1 at two years after LDLT was 24.7% and 24.7%, respectively. The activity score was A0 in 67 patients, A1 in 19 patients and A2 in three patients, and the fibrosis score was F0 in 67 patients, F1 in 21 patients and F2 in one patient.
The impact of various recipient and graft variables on scores of ≥ A1 and ≥ F1 was assessed, and the results are summarized in Table 2. A univariate analysis revealed the following variables to be risk factors for a score of ≥ A1 at two years after LDLT: HLA-A mismatch, no acute cellular rejection, ALT level of ≥ 20 IU/L, and hyaluronic acid level of ≥ 20 ng/mL (P = 0.029, P = 0.029, P = 0.021 and P = 0.039, respectively). The only variable with P < 0.1000 was ≥ 2h of cold ischemic time (P = 0.055). A multivariate analysis including these variables identified ≥ 2 h of cold ischemic time, no acute cellular rejection and ALT level of ≥ 20 IU/L to be independent risk factors for a score of ≥ A1 at two years after LDLT (P = 0.028, P = 0.033 and P = 0.012, respectively) (Table 3). The ROC curve analysis of the ALT level at two years after LDLT in the patients with a score of ≥ A1, the recommended cutoff value for diagnosing a score of ≥ A1 was 20 IU/L (sensitivity: 50.0%, specificity: 76.1%, area under the curve: 0.685 and 95%CI: 0.557-0.813) (Figure 1). Univariate analysis identified the risk factor for a score of ≥ F1 at two years after LDLT to be the aspartate amino transferase level (P = 0.016). The variables with P < 0.100 included a recipient age of < 12 mo and an ALT level of ≥ 20 IU/L (P = 0.062 and P = 0.075, respectively). A multivariate analysis of these variables found none to be independent risk factors for a score of ≥ F1 at two years after LDLT (Table 3).
Table 2.
Risk factors for ≥ A1 and ≥ F1 of protocol liver biopsy at two years after living donor liver transplantation: univariate analysis
| Variables | Incidence of ≥ A1 (%) | P value | Incidence of ≥ F1 (%) | P value |
| Recipient age at LDLT | ||||
| < 12 mo (n = 30) vs ≥ 12 mo (n = 59) | 26.7 vs 23.7 | 0.762 | 36.7 vs 18.6 | 0.062 |
| Recipient body weight at LDLT | ||||
| < 10 kg (n = 43) vs ≥ 10 kg (n = 46) | 23.3 vs 26.1 | 0.757 | 27.9 vs 21.7 | 0.500 |
| Original disease | ||||
| Cholestatic diseases (n = 69) vs others (n = 20) | 33.3 vs 38.1 | 0.637 | 33.3 vs 38.1 | 0.637 |
| PELD or MELD | ||||
| ≥ 20 (n = 22) vs < 20 (n = 67) | 22.7 vs 25.4 | 0.803 | 31.8 vs 22.4 | 0.374 |
| Donor age | ||||
| ≥ 35 yr (n = 39) vs < 35 yr (n = 50) | 23.1 vs 26.0 | 0.751 | 25.6 vs 24.0 | 0.858 |
| Gender combinations between donor and recipient | ||||
| Mismatch (n = 50) vs match (n = 39) | 24.0 vs 25.6 | 0.858 | 22.0 vs 28.2 | 0.501 |
| ABO compatibility | ||||
| Incompatible (n = 14) vs others (n = 75) | 21.4 vs 25.3 | 0.755 | 14.3 vs 26.7 | 0.324 |
| HLA-A | ||||
| Mismatch (n = 65) vs match (n = 24) | 30.8 vs 8.3 | 0.029 | 27.7 vs 16.7 | 0.285 |
| HLA-B | ||||
| Mismatch (n = 84) vs match (n = 5) | 26.2 vs 0.0 | 0.187 | 25.0 vs 20.0 | 0.802 |
| HLA-DRB1 | ||||
| Mismatch (n = 76) vs match (n = 13) | 26.3 vs 15.4 | 0.398 | 26.3 vs 15.4 | 0.398 |
| Lymphocyte cross-matching | ||||
| ≥ 4 × (n = 7) vs negative (n = 82) | 0.0 vs 26.8 | 0.114 | 28.6 vs 24.4 | 0.805 |
| GV/SLV | ||||
| < 40 % (n = 6) vs ≥ 40 % (n = 83) | 33.3 vs 24.1 | 0.612 | 16.7 vs 25.3 | 0.636 |
| Graft type | ||||
| Lateral segment graft (n = 57) vs others (n = 32) | 21.1 vs 31.3 | 0.285 | 29.8 vs 15.6 | 0.136 |
| Operation time | ||||
| ≥ 20 h (n = 12) vs < 20 h (n = 77) | 16.7 vs 26.0 | 0.113 | 25.0 vs 24.7 | 0.975 |
| Cold ischemic time | ||||
| ≥ 2 h (n = 49) vs < 2 h (n = 40) | 32.7 vs 15.0 | 0.055 | 28.6 vs 20.0 | 0.351 |
| Warm ischemic time | ||||
| ≥ 45 min (n = 45) vs < 45 min (n = 44) | 20.0 vs 29.5 | 0.297 | 26.7 vs 22.7 | 0.666 |
| Blood loss volume | ||||
| ≥ 100 mL/kg (n = 30) vs < 100 mL/kg (n = 59) | 16.7 vs 28.8 | 0.209 | 26.7 vs 23.7 | 0.762 |
| Transfusion volume | ||||
| ≥ 100 mL/kg (n = 41) vs < 100 mL/kg (n = 48) | 22.0 vs 27.1 | 0.576 | 22.0 vs 27.1 | 0.576 |
| Splenectomy | ||||
| Yes (n = 7) vs No (n = 82) | 42.9 vs 23.2 | 0.247 | 28.6 vs 24.4 | 0.805 |
| Portal vein complications | ||||
| Yes (n = 11) vs No (n = 78) | 9.1 vs 26.9 | 0.199 | 27.3 vs 24.4 | 0.834 |
| Hepatic arterial complications | ||||
| Yes (n = 6) vs No (n = 83) | 16.7 vs 25.3 | 0.636 | 33.3 vs 24.1 | 0.509 |
| Hepaticojejunostomic anastomotic stricture | ||||
| Yes (n = 14) vs No (n = 75) | 21.4 vs 25.3 | 0.755 | 28.6 vs 24.0 | 0.716 |
| Cytomegalovirus infection | ||||
| Yes (n = 29) vs No (n = 60) | 31.0 vs 21.7 | 0.337 | 27.6 vs 23.3 | 0.663 |
| Acute cellular rejection | ||||
| Yes (n = 29) vs No (n = 60) | 10.3 vs 31.7 | 0.029 | 17.2 vs 28.3 | 0.255 |
| Total bilirubin at PLB | ||||
| ≥ 0.7 mg/dL (n = 29) vs < 0.7 mg/dL (n = 60) | 17.2 vs 28.3 | 0.255 | 24.1 vs 25.0 | 0.929 |
| AST at PLB | ||||
| ≥ 30 IU/L (n = 49) vs < 30 IU/L (n = 40) | 24.5 vs 25.0 | 0.956 | 34.7 vs 12.5 | 0.016 |
| ALT at PLB | ||||
| ≥ 20 IU/L (n = 27) vs < 20 IU/L (n = 62) | 40.7 vs 17.7 | 0.021 | 37.0 vs 19.4 | 0.075 |
| γ-GTP at PLB | ||||
| ≥ 20 IU/L (n = 34) vs < 20 IU/L (n = 55) | 32.4 vs 20.0 | 0.189 | 29.4 vs 21.8 | 0.420 |
| Hyaluronic acid at PLB | ||||
| ≥ 20 ng/mL (n = 52) vs < 20 ng/mL (n = 37) | 32.7 vs 13.5 | 0.039 | 23.1 vs 27.0 | 0.671 |
| IgG at PLB | ||||
| ≥ 1200 mg/dL (n = 18) vs < 1200 mg/dL (n = 71) | 27.8 vs 23.9 | 0.737 | 33.3 vs 22.5 | 0.343 |
| ANA at PLB | ||||
| ≥ 20 × (n = 8) vs < 20 × (n = 81) | 12.5 vs 25.7 | 0.401 | 12.5 vs 25.9 | 0.401 |
| ASMA at PLB | ||||
| ≥ 20 × (n = 21) vs < 20 × (n = 68) | 23.8 vs 25.0 | 0.913 | 28.6 vs 23.5 | 0.640 |
| Trough of tacrolimus at PLB | ||||
| ≥ 3.0 ng/mL (n = 54) vs < 3.0 ng/mL (n = 32)1 | 25.9 vs 25.0 | 0.924 | 24.1 vs 25.0 | 0.924 |
Three cases which were used a cyclosporine were removed. LDLT: Living donor liver transplantation; PELD: Pediatric end-stage liver disease; MELD: Model for end-stage liver disease; GV/SLV: Ratio of graft volume to standard liver volume; PLB: Protocol liver biopsy; AST: Aspartate amino transferase; ALT: Alanine amino transferase; IgG: Immunoglobulin G; ANA: Antinuclear antibody; ASMA: Antismooth nuclear antibody.
Table 3.
Risk factors for ≥ A1 and ≥ F1 of protocol liver biopsy at two and five years after living donor liver transplantation: multivariate analysis
| Variables | OR | 95%CI | P value |
| Risk factors for ≥ A1 of PLB at two years after LDLT | |||
| HLA-A mismatch | |||
| Mismatch vs match | 0.46 | 0.145-1.479 | 0.194 |
| Cold ischemic time | |||
| ≥ 2 h vs < 2 h | 4.15 | 1.164-14.789 | 0.028 |
| Acute cellular rejection | |||
| Yes vs No | 0.20 | 0.046-0.878 | 0.033 |
| ALT | |||
| ≥ 20 IU/L vs < 20 IU/L | 4.64 | 1.409-15.306 | 0.012 |
| Hyaluronic acid | |||
| ≥ 20 ng/mL vs < 20 ng/mL | 3.30 | 0.982-11.076 | 0.054 |
| Risk factors for ≥ F1 of PLB at two years after LDLT | |||
| Recipient age | |||
| < 1 yr vs ≥ 1 yr | 1.54 | 0.506-4.706 | 0.446 |
| AST | |||
| ≥ 30 IU/L vs < 30 IU/L | 2.68 | 0.775-9.238 | 0.120 |
| ALT | |||
| ≥ 20 IU/L vs < 20 IU/L | 1.86 | 0.646-5.335 | 0.251 |
| Risk factors for ≥A1 of PLB at five years after LDLT | |||
| Cold ischemic time | |||
| ≥ 2 h vs < 2 h | 2.94 | 0.778-11.140 | 0.112 |
| Acute cellular rejection | |||
| Yes vs No | 2.26 | 0.728-7.035 | 0.158 |
| Risk factor for ≥ F1 of PLB at five years after LDLT | |||
| Acute cellular rejection | |||
| Yes vs No | 2.75 | 0.876-8.637 | 0.083 |
PLB: Protocol liver biopsy; LDLT: Living donor liver transplantation; ALT: Alanine amino transferase; AST: Aspartate amino transferase.
Figure 1.
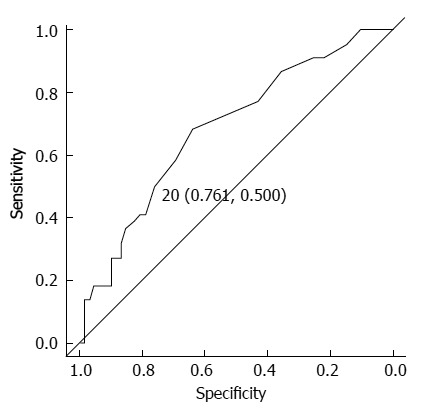
Receiver operating characteristic curve analysis of the alanine amino transaminase level at two years after living donor liver transplantation in the patients with a score of ≥ A1. The recommended cutoff value for diagnosing a score of ≥ A1 was set at 20 IU/L (sensitivity: 50.0%, specificity: 76.1%, area under the curve: 0.685 and 95%CI: 0.557-0.813).
The incidence of scores of ≥ A2 or ≥ F2 at two years after LDLT was 3.4% (three cases), and all patients had a score of ≥ A2 (Table 4). In all cases, the dose of immunosuppressants was increased after the PLB, and two patients who underwent a follow-up liver biopsy improved to scores of ≤ A1 and F1.
Table 4.
Clinical and hitopathological findings of cases with ≥ A2 or ≥ F2 of protocol liver biopsy at two or five years after living donor liver transplantation
| Case | Original disease | Age at PLB/sex | Previous ACR | Post-transplant complications | IS at PLB | Tac trough at PLB | ALT/HA at PLB | A/F at PLB | IS at follow-up biopsy | A/F at follow-up biopsy |
| PLB at two years after LDLT | ||||||||||
| 1 | OTCD | 71/female | - | - | Tac (3.0) | 2.5 | 12/35 | 2/1 | Tac (1.0)/MMF (400) | 1/1 |
| 2 | OTCD | 164/female | - | BDS | Tac (2.0)/MMF (1000) | 5.2 | 34/13 | 2/1 | Tac (2.0)/MMF (1000) | 1/0 |
| 3 | OTCD | 44/male | - | - | Tac (0.8)/MMF (250) | 2 | 25/< 9 | 2/2 | Tac (0.8)/MMF (500) | N.E. |
| PLB at five years after LDLT | ||||||||||
| 4 | BA | 70/female | + | Bowel perforation | Tac (0.6) | 1.1 | 22/13 | 2/2 | Tac (2.0)/MMF (1000) | 0/0 |
| 5 | BA | 118/female | - | - | Tac (1.0) | 2.3 | 20/24 | 2/2 | Tac (2.0)/MMF (1000) | 1/0 |
| 6 | BA | 70/female | + | HAT/IHBDS | Tac (0.8)/MMF(500) | 3.6 | 32/28 | 1/2 | Tac (2.0)/MMF (500) | 1/1 |
| 7 | BA | 71/female | - | CMV-I | Tac (0.4) | 0 | 16/< 9 | 2/2 | Tac (1.6) | N.E. |
| 8 | FHF | 83/female | - | - | Tac (2.0)/MMF (500) | 2.2 | 26/< 9 | 1/2 | Tac (2.8)/MMF (500) | N.E. |
| 9 | BA | 77/female | - | CMV-I | Tac (0.4) | 2.6 | 14/29 | 2/3 | Tac (0.8) | 0/1 |
| 10 | BA | 84/female | + | Fungal infection | Tac (0.4) | 2.1 | 26/11 | 2/2 | Tac (0.4),MMF (500) | 1/1 |
| 11 | BA | 89/male | + | PVS | Tac (1.6)/MP (4.0)/MMF (1500) | 2.2 | 12/17 | 2/2 | Tac (1.6)/MP (2.0)/MMF (1500) | 1/1 |
| 12 | BA | 174/male | - | BDS | Tac (3.0) | 2.3 | 16/20 | 1/2 | Tac (4.0) | 0/1 |
| 13 | BA | 69/female | + | CMV-I | Tac (1.6) | 2.8 | 18/< 9 | 1/2 | Tac (2.0)/MMF (1000) | 0/1 |
| 14 | BA | 84/male | - | HVS | Tac (2.0)/MP (1.0)/MMF (1000) | 5.6 | 12/23 | 1/2 | Tac (2.0)/MP (1.0)/MMF (1000) | N.E. |
PLB: Protocol liver biopsy; LDLT: Living donor liver transplantation; ACR: Acute cellular rejection; IS: Immunosuppressants; Tac: Tacrolimus; ALT: Alanine amino transferase; HA: Hyaluronic acid; A: Activity; F: Fibrosis; OTCD: Ornithine transcarbamylase deficiency; BA: Biliary atresia; FHF: Fulminant hepatic failure; BDS: Biliary duct anastomotic stenosis; HAT: Hepatic artery thrombosis; IHBDS: Intrahepatic biliary duct stenosis; CMV-I: Cytomegalovirus infection; PVS: Portal vein stenosis; HVS: Hepatic vein stenosis; MMF: Mycophenolate mofetil: MP: Methylprednisolone.
Results of PLB at five years after LDLT
The incidence of scores of ≥ A1 and ≥ F1 at five years after LDLT was 42.3% and 34.5%, respectively. The activity score was A0 in 29 patients, A1 in 23 patients and A2 in three patients, and the fibrosis score was F0 in 36 patients, F1 in 12 patients and F2 in seven patients.
The impact of various recipient and graft variables on the scores of ≥ A1 and ≥ F1 was assessed, and the results are summarized in Table 5. A univariate analysis identified no risk factors for scores of ≥ A1 at five years after LDLT. The variables with P < 0.100 included ≥ 2 h of cold ischemic time and acute cellular rejection (P = 0.061 and P = 0.087, respectively). Multivariate analysis of these variables found none to be independent risk factors for a score of ≥ A1 at five years after LDLT (Table 3). Univariate analysis identified no risk factors for a score of ≥ F1 at five years after LDLT. The variable with P < 0.100 included acute cellular rejection (P = 0.079). Multivariate analysis of these variables found none to be independent risk factors for a score of ≥ F1 at five years after LDLT (Table 3).
Table 5.
Risk factors for ≥ A1 and ≥ F1 of protocol liver biopsy at five years after living donor liver transplantation: univariable analysis
| Variables | Incidence of ≥ A1 (%) | P-value | Incidence of ≥ F1 (%) | P-value |
| Recipient age at LDLT | ||||
| < 12 mo (n = 18) vs ≥ 12 mo (n = 37) | 38.9 vs 51.4 | 0.385 | 38.9 vs 32.4 | 0.637 |
| Recipient body weight at LDLT | ||||
| < 10 kg (n = 29) vs ≥ 10 kg (n = 26) | 41.4 vs 53.8 | 0.355 | 55.0 vs 30.8 | 0.577 |
| Original disease | ||||
| Cholestatic diseases (n = 45) vs others (n = 10) | 46.7 vs 50.0 | 0.850 | 35.6 vs 33.3 | 0.738 |
| PELD or MELD | ||||
| ≥ 20 (n = 12) vs < 20 (n = 43) | 41.7 vs 48.8 | 0.660 | 41.7 vs 52.6 | 0.558 |
| Donor age | ||||
| ≥ 35 yr (n = 22) vs < 35 yr (n = 33) | 40.9 vs 51.5 | 0.440 | 36.4 vs 33.3 | 0.816 |
| Gender combinations between donor and recipient | ||||
| Mismatch (n = 30) vs match (n = 25) | 53.3 vs 40.0 | 0.324 | 40.0 vs 28.0 | 0.352 |
| ABO compatibility | ||||
| incompatible (n = 7) vs others (n = 48) | 42.9 vs 47.9 | 0.802 | 28.6 vs 35.4 | 0.722 |
| HLA-A | ||||
| Mismatch (n = 41) vs match (n = 14) | 51.2 vs 35.7 | 0.316 | 39.0 vs 21.4 | 0.232 |
| HLA-B | ||||
| Mismatch (n = 52) vs match (n = 3) | 48.1 vs 33.3 | 0.619 | 32.7 vs 66.7 | 0.229 |
| HLA-DRB1 | ||||
| Mismatch (n = 47) vs match (n = 8) | 51.1 vs 25.0 | 0.172 | 36.2 vs 25.0 | 0.539 |
| Lymphocyte cross-matching | ||||
| ≥ 4 × (n = 16) vs negative (n = 39) | 31.3 vs 53.8 | 0.127 | 18.8 vs 41.0 | 0.115 |
| GV/SLV | ||||
| < 40 % (n = 2) vs ≥ 40 % (n = 53) | 0.0 vs 49.1 | 0.173 | 0.0 vs 35.8 | 0.295 |
| Graft type | ||||
| Lateral segment graft (n = 43) vs others (n = 12) | 51.2 vs 33.3 | 0.274 | 39.5 vs 16.7 | 0.141 |
| Operation time | ||||
| ≥ 20 h (n = 16) vs < 20 h (n = 39) | 37.5 vs 51.3 | 0.352 | 31.3 vs 35.9 | 0.742 |
| Cold ischemic time | ||||
| ≥ 2 h (n = 40) vs < 2 h (n = 15) | 55.0 vs 26.7 | 0.061 | 40.0 vs 20.0 | 0.165 |
| Warm ischemic time | ||||
| ≥ 1 h (n = 42) vs < 1 h (n = 13) | 42.9 vs 61.5 | 0.238 | 31.0 vs 46.2 | 0.314 |
| Blood loss volume | ||||
| ≥ 150 mL/kg (n = 11) vs < 150 mL/kg (n = 44) | 27.3 vs 52.3 | 0.137 | 27.3 vs 36.4 | 0.57 |
| Transfusion volume | ||||
| ≥ 100 mL/kg (n = 15) vs < 100 mL/kg (n = 40) | 40.0 vs 50.0 | 0.508 | 40.0 vs 32.5 | 0.603 |
| Splenectomy | ||||
| Yes (n = 2) vs No (n = 53) | 100.0 vs 45.3 | 0.128 | 0.0 vs 35.8 | 0.295 |
| Portal vein complications | ||||
| Yes (n = 9) vs No (n = 46) | 44.4 vs 47.8 | 0.852 | 33.3 vs 34.8 | 0.933 |
| Hepatic arterial complications | ||||
| Yes (n = 4) vs No (n = 51) | 25.0 vs 49.0 | 0.354 | 25.0 vs 35.3 | 0.677 |
| Hepaticojejunostomic anastomotic stricture | ||||
| Yes (n = 16) vs No (n = 39) | 31.3 vs 53.8 | 0.127 | 25.0 vs 38.5 | 0.340 |
| Cytomegalovirus infection | ||||
| Yes (n = 17) vs No (n = 38) | 47.1 vs 47.4 | 0.999 | 47.1 vs 28.9 | 0.192 |
| Acute cellular rejection | ||||
| Yes (n = 23) vs No (n = 32) | 60.9 vs 37.5 | 0.087 | 47.8 vs 25.0 | 0.079 |
| Total bilirubin at PLB | ||||
| ≥ 0.7 mg/dL (n = 25) vs < 0.7 mg/dL (n = 30) | 48.0 vs 46.7 | 0.920 | 36.0 vs 33.3 | 0.836 |
| AST at PLB | ||||
| ≥ 30 IU/L (n = 22) vs < 30 IU/L (n = 33) | 54.5 vs 42.4 | 0.378 | 36.4 vs 33.3 | 0.816 |
| ALT at PLB | ||||
| ≥ 20 IU/L (n = 21) vs < 20 IU/L (n = 34) | 57.1 vs 41.2 | 0.249 | 28.6 vs 38.2 | 0.464 |
| γ-GTP at PLB | ||||
| ≥ 20 IU/L (n = 20) vs < 20 IU/L (n = 35) | 45.0 vs 48.6 | 0.799 | 30.0 vs 37.1 | 0.592 |
| Hyaluronic acid at PLB | ||||
| ≥ 20 ng/mL (n = 22) vs < 20 ng/mL (n = 33) | 50.0 vs 45.5 | 0.741 | 36.4 vs 33.3 | 0.816 |
| IgG at PLB | ||||
| ≥ 1200 mg/dL (n = 24) vs < 1200 mg/dL (n = 31) | 54.2 vs 41.9 | 0.368 | 41.7 vs 29.0 | 0.328 |
| ANA at PLB | ||||
| ≥ 20 × (n = 14) vs < 20 × (n = 41) | 35.7 vs 51.2 | 0.316 | 28.6 vs 36.6 | 0.586 |
| ASMA at PLB | ||||
| ≥ 20 × (n = 10) vs < 20 × (n = 45) | 70.0 vs 42.2 | 0.111 | 40.0 vs 33.3 | 0.688 |
| Trough of tacrolimus at PLB | ||||
| ≥ 3.0 ng/mL (n = 19) vs < 3.0 ng/mL (n = 33)1 | 52.6 vs 42.4 | 0.477 | 36.8 vs 33.3 | 0.797 |
Three cases which were used a cyclosporine were removed. LDLT: Living donor liver transplantation; PELD: Pediatric end-stage liver disease; MELD: Model for end-stage liver disease; GV/SLV: Ratio of graft volume to standard liver volume; PLB: Protocol liver biopsy; AST: Aspartate amino transferase; ALT: Alanine amino transferase; IgG: Immunoglobulin G; ANA: Antinuclear antibody; ASMA: Antismooth nuclear antibody.
The incidence of scores of ≥ A2 or ≥ F2 at five years after LDLT was 20.0% (11 cases), and all patients had a score of ≥ F2 (Table 4). In all cases, the dose of immunosuppressants was increased after the PLB, and all eight patients who underwent a follow-up liver biopsy improved to scores of ≤ A1 and F1.
Clinical and histopathological findings in the patients who underwent PLB at both two and five years after LDLT
PLBs were performed at both two and five years after LDLT in 21 cases; the results are summarized in Table 6. The activity and fibrosis scores at two years after LDLT were A0 and F0 in 14 patients, A1 or F1 in six patients and ≥ A2 or ≥ F2 in one patient. Seven patients with scores of A0 and F0 at two years after LDLT maintained scores of A0 and F0 at five years; however, the remaining patients exhibited worse scores of ≥ A1 or ≥ F1. Three patients with a score of A1 or F1 at two years after LDLT maintained a score of A1 or F1 at five years; however, the remaining patients exhibited worse a score of ≥ A2 or ≥ F2.
Table 6.
Clinical and histopathological findings of cases who performed protocol liver biopsy at both two and five years after living donor liver transplantation
| Case | Original disease | Age at LT/sex | Previous ACR | Post-transplant complications | IS at two years PLB | Tac trough at PLB | ALT/HA at PLB | A/F at PLB | IS at five years PLB | Tac trough at PLB | ALT/HA at PLB | A/F at PLB |
| 1 | OTCD | 46/female | - | - | Tac (3.0) | 2.5 | 12/35 | 2/1 | Tac (1.0)/MMF (400) | 0.5 | 11/52 | 1/1 |
| 11 | BA | 26/male | + | PVS | Tac (0.8)/MP (4.0)/MMF (500) | 3.2 | 20/11 | 0/1 | Tac (1.6)/MP (4.0)/MMF (1500) | 2.2 | 12/17 | 2/2 |
| 12 | BA | 114/male | - | BDS | Tac (2.0) | 2.6 | 14/21 | 1/0 | Tac (3.0) | 2.3 | 16/20 | 1/2 |
| 13 | BA | 10/female | + | CMV-I | Tac (0.4) | 3.8 | 19/11 | 0/0 | Tac (1.6) | 2.8 | 18/< 9 | 1/2 |
| 14 | BA | 30/male | - | HVS | Tac (1.2) | 5.3 | 18/29 | 1/1 | Tac (2.0)/MP (1.0)/MMF (1000) | 5.6 | 12/23 | 1/2 |
| 15 | BA | 120/female | - | BDS | Tac (1.5)/PSL (2.5) | 4.4 | 15/14 | 0/0 | Tac (4.0) | 7.0 | 17/< 9 | 0/0 |
| 16 | BA | 163/male M | + | BDS/CMV-I | CsA (150) | CsA 50 | 9/27 | 0/0 | CsA (150)/MMF (1000) | CsA 83 | 91020 | 0/1 |
| 17 | BA | 8/female | +/OKT3 | PVS/CMV-I | Tac (0.8)/MP (0.5) | 2.4 | 30/36 | 0/0 | Tac (2.0) | 5.3 | 15/18 | 1/1 |
| 18 | BA | 12/male | + | - | Tac (0.8) | 3.8 | 14/58 | 0/0 | Tac (0.8) | 0.2 | 8/20 | 1/1 |
| 19 | BA | 13/female | + | CMV-I | Tac (1.6)/MP (2.0) | 9.3 | 22/11 | 0/0 | Tac (1.4)/MP (3.0)/MMF (500) | 2.1 | 15/15 | 0/1 |
| 20 | AD | 19/female | + | CMV-I | Tac (0.8)/MMF (500) | 2.3 | 19/13 | 0/1 | Tac (2.4)/MMF (500) | 3.8 | 14/15 | 1/1 |
| 21 | WD | 112/male | - | - | Tac (4.0) | 1.3 | 16/16 | 0/0 | Tac (5.0) | 1.4 | 19/13 | 0/0 |
| 22 | BA | 170/female | + | BDS | Tac (2.0) | 6.3 | 17/16 | 0/1 | Tac (6.0)/MP (12)/MMF (2000) | 15.5 | 39/22 | 1/0 |
| 23 | BA | 33/F | + | HVS | Tac (1.0)/MP (2.0)/MMF (400) | 3.7 | 10/< 9 | 0/0 | Tac (1.5)/MMF (1000) | 5.4 | 41/19 | 1/0 |
| 24 | BA | 9/female | - | HAT | Tac (0.6) | 0.3 | 14/24 | 0/0 | Tac (0.8) | 0 | 10/10 | 0/0 |
| 25 | BA | 28/female | - | - | Tac (0.4) | 2.8 | 18/< 9 | 0/0 | Tac (1.0) | 0.9 | 15/10 | 1/0 |
| 26 | BA | 9/female | - | IHBDS | Tac (0.4) | 2.1 | 23/< 9 | 0/1 | Tac (2.0)/MP (0.5)/MMF (500) | 5.3 | 41/19 | 1/1 |
| 27 | AD | 19/male | - | - | Tac (0.6) | 3.3 | 12/17 | 0/0 | Tac (2.0)/MMF (500) | 1.5 | 11/59 | 0/0 |
| 28 | BA | 45/female | - | BDS | Tac (1.2) | 3.6 | 13/15 | 0/0 | Tac (1.5) | 4.6 | 11/10 | 0/0 |
| 29 | BA | 9/female | - | - | Tac (0.4) | 0.9 | 11/17 | 0/0 | Tac (0.8) | 1.1 | 13/25 | 0/0 |
| 30 | CEPS | 37/male | - | - | Tac (2.0)/MP (2.5)/MMF (500) | 2.5 | 13/< 9 | 0/0 | Tac (2.0)/MP (1.5)/MMF (500) | 3.9 | 11/< 9 | 0/0 |
PLB: Protocol liver biopsy; LDLT: Living donor liver transplantation; ACR: Acute cellular rejection; IS: Immunosuppressants; Tac: Tacrolimus; ALT: Alanine amino transferase; HA: Hyaluronic acid; A: Activity; F: Fibrosis; OTCD: Ornithine transcarbamylase deficiency; BA: Biliary atresia; AD: Alagille syndrome; WD: Wilson disease; CEPS: Congenital extrahepatic portsystemic shunt; OKT3: Muromonab-CD3; PVS: Portal vein stenosis; BDS: Biliary duct anastomotic stenosis; CMV-I: Cytomegalovirus infection; HVS: Hepatic vein stenosis; HAT: Hepatic artery thrombosis; IHBDS: Intrahepatic biliary duct stenosis; MP: Methylprednisolone; MMF: Mycophenolate mofetil; PSL: Prednisolone; CsA: Cyclosporin A.
Complications of PLB
Complications related to the PLB occurred in only one patient (0.7%) who developed acute cholangitis. This complication resolved following the administration of antibiotics for three days.
Case reports
We described two representative liver transplant recipients with abnormal histopathological findings and normal LFT results in whom the dose of immunosuppressants was increased, which led to improvements in the histopathological findings (Table 4).
Case 4: A seven-month-old female infant with biliary atresia underwent ABO-identical LDLT using a left lateral segment graft. Tac and MP were administered as the standard postoperative immunosuppressive regimen. The patient’s postoperative course included an episode of small intestine perforation requiring surgical repair and acute cellular rejection requiring steroid pulse treatment; however, she was discharged from the hospital on POD 28 after LDLT. MP was withdrawn at 18 mo after LDLT, and thereafter, only Tac was administered for immunosuppression. In postoperative year (POY) 5, a PLB was performed; the LFT data were normal, but the Metavir scores were A2 and F2 (Figure 2A). The immunosuppression was subsequently strengthened by increasing the dose of Tac and adding MMF because the PLB histopathology was considered to be abnormal. A follow-up liver biopsy was performed 18 mo after the PLB, at which time the scores were A0 and F0 (Figure 2B).
Figure 2.
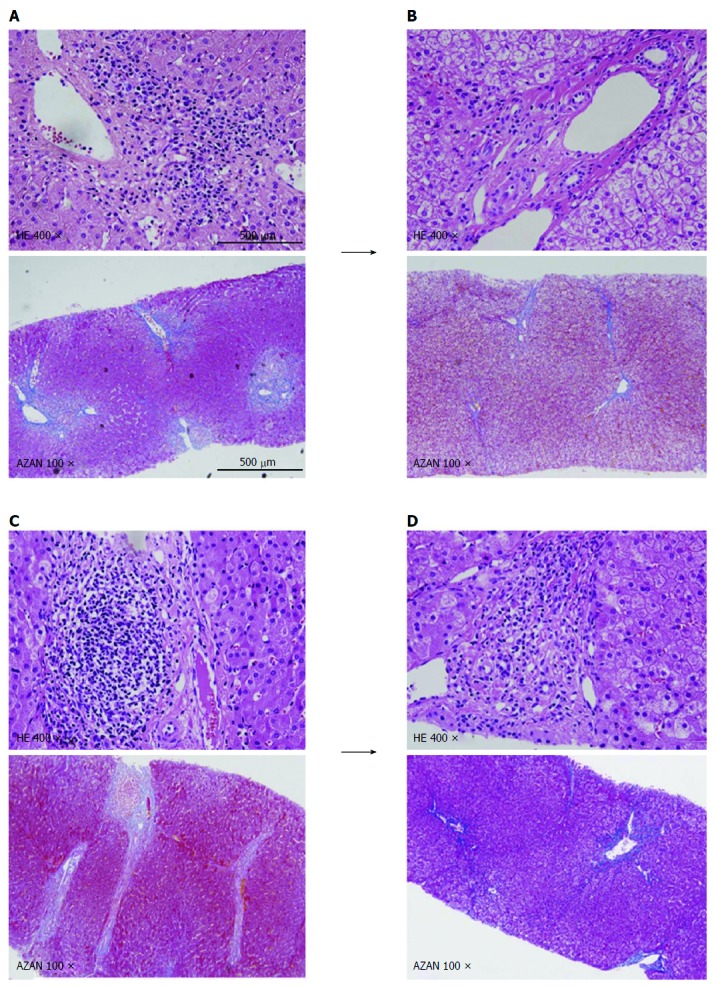
In postoperative year 5, a protocol liver biopsy was performed. A: At which time the Metavir scores were abnormal: A2 (portal inflammation) and F2 (portal and pericellular fibrosis); B: Follow-up liver biopsy was performed at 18 mo after the protocol liver biopsy (PLB), at which time the scores were A0 and F0; C: At which time the Metavir scores were abnormal: A2 (portal inflammation) and F2 (portal fibrosis); D: A follow-up liver biopsy was performed at 20 mo after the PLB, at which time the scores were A1 (portal inflammation) and F0. HE: Hematoxylin and eosin stain; AZAN: Azan stain.
Case 5: A 58-month-old female girl with biliary atresia underwent ABO-identical LDLT using a left lateral segment graft. Tac and MP were administered as the standard postoperative immunosuppressive regimen. The patient’s postoperative course was uneventful, except for an episode of acute respiratory distress, and she was discharged from the hospital on POD 56 after LDLT. MP was withdrawn at 18 mo after LDLT, and thereafter, only Tac was administered for immunosuppression. In POY 5, PLB was performed; the LFT data were normal, but the Metavir scores were A2 and F2 (Figure 2C). The immunosuppression was then strengthened by increasing the dose of Tac and adding MMF because the PLB histopathology was considered to be abnormal. A follow-up liver biopsy was performed 20 mo after the PLB, at which time the scores were A1 and F0 (Figure 2D).
DISCUSSION
LT is an established curative treatment for pediatric patients with end-stage liver disease or acute liver failure[1-3]. However, histopathological assessments performed during the mid- and long-term period after LT remain insufficient, and it is necessary to further improve the prognosis by maintaining the function of the liver graft using a minimum degree of immunosuppression to obtain an optimal balance between the effectiveness and side effects of individual immunosuppressants.
Histopathological assessments using PLB have recently been reported to be important in adult recipient[12-15], because the occurrence of graft fibrosis or the recurrence of the original disease cannot be predicted using standard LFTs alone. However, in pediatric recipients, the need for PLB is controversial due to the low incidence of recurrent original diseases. Liver graft fibrosis has recently been reported to be present in 43%-65% and 25%-69% of patients at two and five years after LT, respectively, even if the LFT data are normal[4-6]. Moreover, there is a relationship between liver graft fibrosis and chronic rejection[4,5], and the progression to severe fibrosis has been reported to occur in 14%-25% of patients at ten years after LT[4,6]. Furthermore, the development of liver graft fibrosis after pediatric LT occurs in 69%-97% of cases, including cases of mild fibrosis[4-8]. The risk factors for fibrosis include an increasingly long interval after LT[4,7], positivity for antinuclear antibodies[4], long cold ischemic time[6], young age at LT[6], a high donor to recipient graft ratio[6] and partial LT[6]. In the present study, independent risk factors in a multivariate analysis of a score of ≥ A1 at two years after LDLT included ≥ 2 h of cold ischemic time, no acute cellular rejection and an ALT level of ≥ 20 IU/L (P = 0.028, P = 0.033 and P = 0.012, respectively); however, no risk factors were identified for a score of ≥ F1. Furthermore, no independent risk factors were identified in a multivariate analysis of scores of ≥ A1 and ≥ F1 at five years. We believe that ≥ 2 h of cold ischemic time was found to be an independent risk factor for a score of ≥ A1 at two years after LDLT because a prolonged cold ischemic time may induce an immune response by affecting graft liver dysfunction. In addition, we believe that no acute cellular rejection was found to be an independent risk factor for a score of ≥ A1 at two years after LDLT because acute cellular rejection may cause an immune response due to the use of less immunosuppression. However, as a result of the ROC curve analysis of ALT at two years after LDLT in the patients with a score of ≥ A1, the recommended cutoff value for diagnosing a score of ≥ A1 was set at 20 IU/L (sensitivity: 50.0% and specificity: 76.1%). Therefore, the serum ALT level reflects the degree of portal inflammation in PLB patients at two years after LDLT with an ALT level of ≥ 20 IU/L.
With respect to concrete assessment methods for evaluating graft liver fibrosis, portal fibrosis-based liver fibrosis staging systems, such as those reported by Ishak et al[16] and the Metavir Study Group[11], are widely used, even in studies of pediatric LT recipients[7,8,17]. Therefore, we applied histopathological assessments using the Metavir score in the present study. Recent reports have indicated that centrilobular perisinusoidal fibrosis occurs in pediatric LT recipients in association with tacrolimus withdrawal or in the presence of donor-specific anti-human leukocyte antigen antibodies[18,19]. Venturi et al[17] recently developed a novel histopathological scoring system based on the detection of fibrosis in three areas: portal tracts, sinusoids and centrilobular veins. However, the significance of these histopathological findings with respect to morbidity has yet to be clarified and is the most important issue that should be addressed in the future. In the present study, using the Metavir scoring system, the incidence of the scores of ≥ F1 at two and five years after LDLT was 24.7% and 34.5%, respectively. However, no risk factors for graft fibrosis were identified, and no serum markers reflected the degree of graft fibrosis. Therefore, detecting graft fibrosis by performing a histopathological assessment using a liver biopsy is important. Furthermore, the PLB represents an important periodic examination in long-term recipients after LDLT because it enables the assessment of the effectiveness of the current immunosuppressive regimen, even when the PLB histopathology is normal. Therefore, at present, PLB is an indispensable examination for the management of patients who have undergone LDLT.
Potential problems associated with PLB include the following: (1) timing; (2) invasiveness; and (3) the obscure definition of abnormal PLB histopathology. The timing of PLB after LT is not definitive. In our department, we performed PLB at two, five, ten and 15 years after LT, considering the examination’s effectiveness, invasiveness and potential complications. In the present study, the PLB performed two years after LDLT was found to be an unnecessary examination because the serum ALT level reflected the degree of portal inflammation. At the time, the immunosuppressive therapy should be modulated to maintain the ALT concentration at a level less than 20 IU/L. Gelson et al[20] reported that the histological inflammatory index is correlated with the ALT level. A PLB performed at five years is an excellent examination for the detection of early reversible graft fibrosis because no serum markers reflect the degree of graft fibrosis.
PLB suffers, however, from a disadvantage. PLB is an invasive procedure that is potentially associated with severe complications, with an incidence of 0.57%[21]. In the present study, although the rate of PLB-associated complications was only 0.7%, this rate may nevertheless be considered high. Non-invasive examinations, such as imaging, may be used instead of PLB if such examinations become more effective than PLB in the future. Acoustic radiation force impulse and transient elastography imaging have been reported to exhibit good accuracy in the noninvasive diagnosis of liver fibrosis in the setting of pediatric LT[22,23].
The most problematic aspect of PLB is the obscure definition of abnormal histopathology. The histopathological findings of PLB after LT include idiopathic post-transplantation hepatitis (4.4%-64.0%)[4,24-26], central venulitis (16.0%-27.0%)[13,27], interface hepatitis (14.0%-24.4%)[28-30] and fibrosis (69.0%-97.0%)[4-8]. However, the indication for treatment with respect to each histopathological finding is unclear and controversial. In general, liver fibrosis is thought to be irreversible and resistant to treatment. However, in the present cases, the liver fibrosis was reversible, and portal inflammation was ameliorated after strengthening the immunosuppressive regimen. Immunosuppression can be strengthened effectively by increasing the dose of Tac and introducing MMF, given concerns about the side effects of MP[31-34] and the proven effectiveness of MMF[35,36]. Our present findings suggest that the early detection of graft liver fibrosis can be achieved using a liver biopsy and that liver fibrosis may be reversible if early treatment is initiated. In our department, we initially defined a histopathological abnormality as a Metavir score of ≥ A2 or ≥ F2. However, among 21 patients who underwent PLB at both two and five years after LDLT, the activity and fibrosis scores at two years after LDLT were A0 and F0 in 14 patients, A1 or F1 in six patients and ≥ A2 or ≥ F2 in one patient. Seven patients with scores of A0 and F0 at two years after LDLT exhibited worse a score of ≥ A1 or ≥ F1. Three patients with a score of A1 or F1 at two years after LDLT exhibited worse a score of ≥ A2 or ≥ F2. Therefore, we currently define a histopathological abnormality as a Metavir score of ≥ A1 or ≥ F1 and consider such scores to indicate the need for treatment because liver fibrosis is reversible if early treatment is initiated. Both further investigations and the accumulation of more LT cases are required to confirm our present findings.
In a conclusion, A PLB performed at two years after LDLT is an unnecessary examination because the serum ALT level reflects the degree of portal inflammation. In addition, immunosuppressive therapy should be modulated to maintain the ALT concentration at a level less than 20 IU/L. A PLB at five years is an excellent examination for the detection of early reversible graft fibrosis because no serum markers reflect the degree of graft fibrosis.
COMMENTS
Background
Histopathological assessments using protocol liver biopsy (PLB) after liver transplantation (LT) have recently been reported to be important. However, few studies have investigated the impact of the timing and effectiveness of PLBs in the field of pediatric LT. This retrospective study assessed the clinical significance of PLBs in pediatric LT.
Research frontiers
The development of liver graft fibrosis after pediatric LT has been reported to occur in 69%-97% of cases, including cases of mild fibrosis. Because graft dysfunction does not occur unless the fibrosis becomes advanced and because the occurrence of graft liver fibrosis or portal inflammation cannot be predicted using standard liver function test (LFT) alone, histopathological assessments using PLB have recently been reported to be important. However, the significance of mild to severe fibrosis is unknown, and the indications for the treatment of abnormal PLB findings are controversial. In addition, few studies have investigated the impact of the timing and effectiveness of PLB.
Innovations and breakthroughs
The development of liver graft fibrosis after pediatric LT has been reported to occur in 69%-97% of cases, including cases of mild fibrosis. Because graft liver dysfunction does not occur unless the fibrosis becomes advanced and because the occurrence of graft liver fibrosis or portal inflammation cannot be predicted using standard LFT alone, histopathological assessments using PLB have recently been reported to be important. However, the significance of mild to severe fibrosis is unknown, and the indications for the treatment of abnormal PLB findings are controversial. In addition, few studies have investigated the impact of the timing and effectiveness of PLB. This retrospective study assessed the clinical significance of the timing and effectiveness of PLB after pediatric living donor liver transplantation (LDLT). In conclusion, a PLB performed at two years after LDLT is an unnecessary examination because the serum ALT level reflects the degree of portal inflammation. In addition, immunosuppressive therapy should be modulated to maintain the ALT concentration at a level less than 20 IU/L. A PLB at five years is an excellent examination for the detection of early reversible graft fibrosis because no serum markers reflect the degree of graft fibrosis.
Applications
The study results suggest the following contents. A PLB performed at two years after LDLT is an unnecessary examination because the serum ALT level reflects the degree of portal inflammation. In addition, immunosuppressive therapy should be modulated to maintain the ALT concentration at a level less than 20 IU/L. A PLB at five years is an excellent examination for the detection of early reversible graft fibrosis because no serum markers reflect the degree of graft fibrosis.
Terminology
Protocol liver biopsy: Protocol liver biopsy is a liver biopsy that is periodically performed at two and five years after LT.
Peer review
This is a good descriptive study in which the authors analyzed the histopathological findings using the Metavir scoring system and identified factors associated with scores of ≥ A1 and ≥ F1. They, thereafter, clarified the timing and effectiveness of PLB. The results are interesting and suggest the following. A PLB performed at two years after LDLT is an unnecessary examination because the serum ALT level reflects the degree of portal inflammation. In addition, immunosuppressive therapy should be modulated to maintain the ALT concentration at a level less than 20 IU/L. A PLB at five years is an excellent examination for the detection of early reversible graft fibrosis because no serum markers reflect the degree of graft fibrosis.
Footnotes
P- Reviewers: Alsolaiman MM, Cichoz-Lach H, Hubscher SG, Li JD, Marino IR, Morioka D, Schuurman HJ S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
References
- 1.Ueda M, Oike F, Ogura Y, Uryuhara K, Fujimoto Y, Kasahara M, Ogawa K, Kozaki K, Haga H, Tanaka K. Long-term outcomes of 600 living donor liver transplants for pediatric patients at a single center. Liver Transpl. 2006;12:1326–1336. doi: 10.1002/lt.20826. [DOI] [PubMed] [Google Scholar]
- 2.Wallot MA, Mathot M, Janssen M, Hölter T, Paul K, Buts JP, Reding R, Otte JB, Sokal EM. Long-term survival and late graft loss in pediatric liver transplant recipients--a 15-year single-center experience. Liver Transpl. 2002;8:615–622. doi: 10.1053/jlts.2002.34149. [DOI] [PubMed] [Google Scholar]
- 3.Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, McDiarmid S, Cohen G, Anand R. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a north american multicenter registry. Pediatrics. 2008;122:e1128–e1135. doi: 10.1542/peds.2008-1363. [DOI] [PubMed] [Google Scholar]
- 4.Evans HM, Kelly DA, McKiernan PJ, Hübscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43:1109–1117. doi: 10.1002/hep.21152. [DOI] [PubMed] [Google Scholar]
- 5.Fouquet V, Alves A, Branchereau S, Grabar S, Debray D, Jacquemin E, Devictor D, Durand P, Baujard C, Fabre M, et al. Long-term outcome of pediatric liver transplantation for biliary atresia: a 10-year follow-up in a single center. Liver Transpl. 2005;11:152–160. doi: 10.1002/lt.20358. [DOI] [PubMed] [Google Scholar]
- 6.Scheenstra R, Peeters PM, Verkade HJ, Gouw AS. Graft fibrosis after pediatric liver transplantation: ten years of follow-up. Hepatology. 2009;49:880–886. doi: 10.1002/hep.22686. [DOI] [PubMed] [Google Scholar]
- 7.Ekong UD, Melin-Aldana H, Seshadri R, Lokar J, Harris D, Whitington PF, Alonso EM. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver Transpl. 2008;14:1582–1587. doi: 10.1002/lt.21549. [DOI] [PubMed] [Google Scholar]
- 8.Ueno T, Tanaka N, Ihara Y, Takama Y, Yamada H, Mushiake S, Fukuzawa M. Graft fibrosis in patients with biliary atresia after pediatric living-related liver transplantation. Pediatr Transplant. 2011;15:470–475. doi: 10.1111/j.1399-3046.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibelli NE, Tannuri U, Mello ES, Cançado ER, Santos MM, Ayoub AA, Maksoud-Filho JG, Velhote MC, Silva MM, Pinho-Apezzato ML, et al. Successful treatment of de novo autoimmune hepatitis and cirrhosis after pediatric liver transplantation. Pediatr Transplant. 2006;10:371–376. doi: 10.1111/j.1399-3046.2005.00470.x. [DOI] [PubMed] [Google Scholar]
- 10.Foster PF, Sankary HN, Williams JW, Bhattacharyya A, Coleman J, Ashmann M. Morphometric inflammatory cell analysis of human liver allograft biopsies. Transplantation. 1991;51:873–876. doi: 10.1097/00007890-199104000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 12.Sebagh M, Rifai K, Féray C, Yilmaz F, Falissard B, Roche B, Bismuth H, Samuel D, Reynès M. All liver recipients benefit from the protocol 10-year liver biopsies. Hepatology. 2003;37:1293–1301. doi: 10.1053/jhep.2003.50231. [DOI] [PubMed] [Google Scholar]
- 13.Mells G, Neuberger J. Protocol liver allograft biopsies. Transplantation. 2008;85:1686–1692. doi: 10.1097/TP.0b013e318176b1fd. [DOI] [PubMed] [Google Scholar]
- 14.Mells G, Mann C, Hubscher S, Neuberger J. Late protocol liver biopsies in the liver allograft: a neglected investigation? Liver Transpl. 2009;15:931–938. doi: 10.1002/lt.21781. [DOI] [PubMed] [Google Scholar]
- 15.Abraham SC, Poterucha JJ, Rosen CB, Demetris AJ, Krasinskas AM. Histologic abnormalities are common in protocol liver allograft biopsies from patients with normal liver function tests. Am J Surg Pathol. 2008;32:965–973. doi: 10.1097/PAS.0b013e3181622490. [DOI] [PubMed] [Google Scholar]
- 16.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 17.Venturi C, Sempoux C, Bueno J, Ferreres Pinas JC, Bourdeaux C, Abarca-Quinones J, Rahier J, Reding R. Novel histologic scoring system for long-term allograft fibrosis after liver transplantation in children. Am J Transplant. 2012;12:2986–2996. doi: 10.1111/j.1600-6143.2012.04210.x. [DOI] [PubMed] [Google Scholar]
- 18.Egawa H, Miyagawa-Hayashino A, Haga H, Teramukai S, Yoshizawa A, Ogawa K, Ogura Y, Okamoto S, Kaido T, Uemoto S. Non-inflammatory centrilobular sinusoidal fibrosis in pediatric liver transplant recipients under tacrolimus withdrawal. Hepatol Res. 2012;42:895–903. doi: 10.1111/j.1872-034X.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 19.Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, Egawa H, Yurugi K, Masuda S, Minamiguchi S, Maekawa T, Uemoto S, Haga H. Progressive graft fibrosis and donor-specific human leukocyte antigen antibodies in pediatric late liver allografts. Liver Transpl. 2012;18:1333–1342. doi: 10.1002/lt.23534. [DOI] [PubMed] [Google Scholar]
- 20.Gelson W, Hoare M, Unitt E, Palmer C, Gibbs P, Coleman N, Davies S, Alexander GJ. Heterogeneous inflammatory changes in liver graft recipients with normal biochemistry. Transplantation. 2010;89:739–748. doi: 10.1097/TP.0b013e3181c96b32. [DOI] [PubMed] [Google Scholar]
- 21.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 22.Tomita H, Hoshino K, Fuchimoto Y, Ebinuma H, Ohkuma K, Tanami Y, Du W, Masugi Y, Shimojima N, Fujino A, et al. Acoustic radiation force impulse imaging for assessing graft fibrosis after pediatric living donor liver transplantation: a pilot study. Liver Transpl. 2013;19:1202–1213. doi: 10.1002/lt.23708. [DOI] [PubMed] [Google Scholar]
- 23.Goldschmidt I, Stieghorst H, Munteanu M, Poynard T, Schlue J, Streckenbach C, Baumann U. The use of transient elastography and non-invasive serum markers of fibrosis in pediatric liver transplant recipients. Pediatr Transplant. 2013;17:525–534. doi: 10.1111/petr.12116. [DOI] [PubMed] [Google Scholar]
- 24.Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, Czaja A, Daskal I, El-Monayeri MS, Fontes P, Fung J, et al. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489–501. doi: 10.1002/hep.21280. [DOI] [PubMed] [Google Scholar]
- 25.Miyagawa-Hayashino A, Haga H, Egawa H, Hayashino Y, Uemoto S, Manabe T. Idiopathic post-transplantation hepatitis following living donor liver transplantation, and significance of autoantibody titre for outcome. Transpl Int. 2009;22:303–312. doi: 10.1111/j.1432-2277.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- 26.Hübscher SG. What is the long-term outcome of the liver allograft? J Hepatol. 2011;55:702–717. doi: 10.1016/j.jhep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Krasinskas AM, Ruchelli ED, Rand EB, Chittams JL, Furth EE. Central venulitis in pediatric liver allografts. Hepatology. 2001;33:1141–1147. doi: 10.1053/jhep.2001.23938. [DOI] [PubMed] [Google Scholar]
- 28.Herzog D, Soglio DB, Fournet JC, Martin S, Marleau D, Alvarez F. Interface hepatitis is associated with a high incidence of late graft fibrosis in a group of tightly monitored pediatric orthotopic liver transplantation patients. Liver Transpl. 2008;14:946–955. doi: 10.1002/lt.21444. [DOI] [PubMed] [Google Scholar]
- 29.Nagai S, Ito M, Kamei H, Nakamura T, Ando H, Kiuchi T. Indirect immunohistochemical evaluation of graft fibrosis and interface hepatitis after pediatric liver transplantation. Pediatr Transplant. 2010;14:342–350. doi: 10.1111/j.1399-3046.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 30.Hübscher S. What does the long-term liver allograft look like for the pediatric recipient? Liver Transpl. 2009;15 Suppl 2:S19–S24. doi: 10.1002/lt.21902. [DOI] [PubMed] [Google Scholar]
- 31.Al-Sinani S, Dhawan A. Corticosteroids usage in pediatric liver transplantation: To be or not to be! Pediatr Transplant. 2009;13:160–170. doi: 10.1111/j.1399-3046.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 32.Diem HV, Sokal EM, Janssen M, Otte JB, Reding R. Steroid withdrawal after pediatric liver transplantation: a long-term follow-up study in 109 recipients. Transplantation. 2003;75:1664–1670. doi: 10.1097/01.TP.0000063938.49112.C2. [DOI] [PubMed] [Google Scholar]
- 33.Campsen J, Zimmerman MA, Trotter JF, Wachs M, Bak T, Steinberg T, Kaplan M, Wright F, Kam I. Liver transplantation for autoimmune hepatitis and the success of aggressive corticosteroid withdrawal. Liver Transpl. 2008;14:1281–1286. doi: 10.1002/lt.21525. [DOI] [PubMed] [Google Scholar]
- 34.Trouillot TE, Shrestha R, Kam I, Wachs M, Everson GT. Successful withdrawal of prednisone after adult liver transplantation for autoimmune hepatitis. Liver Transpl Surg. 1999;5:375–380. doi: 10.1002/lt.500050514. [DOI] [PubMed] [Google Scholar]
- 35.Junge G, Neuhaus R, Schewior L, Klupp J, Guckelberger O, Langrehr JM, Tullius S, Neuhaus P. Withdrawal of steroids: a randomized prospective study of prednisone and tacrolimus versus mycophenolate mofetil and tacrolimus in liver transplant recipients with autoimmune hepatitis. Transplant Proc. 2005;37:1695–1696. doi: 10.1016/j.transproceed.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 36.Klupp J, Pfitzmann R, Langrehr JM, Neuhaus P. Indications of mycophenolate mofetil in liver transplantation. Transplantation. 2005;80:S142–S146. doi: 10.1097/01.tp.0000187133.53916.8f. [DOI] [PubMed] [Google Scholar]


