Background: Reelin and F-spondin are important in brain development, signaling through apolipoprotein E receptor 2 (ApoER2).
Results: Reelin and F-spondin induce clustering of ApoER2 with itself and with other synaptic proteins, such as the amyloid precursor protein (APP).
Conclusion: Multimeric extracellular ligands induce cellular clustering of ApoER2 and APP.
Significance: Clustering of type I transmembrane proteins results from interactions with developmental proteins.
Keywords: Alzheimer Disease, Amyloid Precursor Protein (APP), Apolipoprotein E (ApoE), Receptor, Synapse
Abstract
ApoE Receptor 2 (ApoER2) and the very low density lipoprotein receptor (VLDLR) are type I transmembrane proteins belonging to the LDLR family of receptors. They are neuronal proteins found in synaptic compartments that play an important role in neuronal migration during development. ApoER2 and VLDLR bind to extracellular glycoproteins, such as Reelin and F-spondin, which leads to phosphorylation of adaptor proteins and subsequent activation of downstream signaling pathways. It is thought that ApoER2 and VLDLR undergo clustering upon binding to their ligands, but no direct evidence of clustering has been shown. Here we show strong clustering of ApoER2 induced by the dimeric ligands Fc-RAP, F-spondin, and Reelin but relatively weak clustering with the ligand apoE in the absence of lipoproteins. This clustering involves numerous proteins besides ApoER2, including amyloid precursor protein and the synaptic adaptor protein PSD-95. Interestingly, we did not observe strong clustering of ApoER2 with VLDLR. Clustering was modulated by both extracellular and intracellular domains of ApoER2. Together, our data demonstrate that several multivalent ligands for ApoER2 induce clustering in transfected cells and primary neurons and that these complexes included other synaptic molecules, such as APP and PSD-95.
Introduction
Apolipoprotein E Receptor 2 (ApoER2) and the very low density lipoprotein receptor (VLDLR)2 are type I transmembrane proteins belonging to the LDLR family of receptors (1, 2). They are neuronal proteins found in synaptic compartments (3, 4) that play an important role in neuronal migration during development (5, 6). Knockout of either ApoER2 or VLDLR leads to relatively mild impairments in neuronal migration, but double knockouts show gross neuronal migration abnormalities in the cortex, hippocampus, and cerebellum (3, 5). Both ApoER2 and VLDLR also affect synapses, increasing the density of dendritic spines in vitro and in vivo (4, 7).
The neuronal migration deficits of ApoER2 VLDLR double knockout mice are similar to deficits in mice with mutations in either the Reelin or Dab1 genes (3, 8). These molecules are associated mechanistically in that activation of ApoER2 and VLDLR by the extracellular matrix protein Reelin leads to phosphorylation of its intracellular adaptor protein Dab-1 (6, 8, 9). ApoER2 and VLDLR also bind extracellularly to a number of other molecules through ligand binding repeats in their N termini, such as apolipoprotein E (apoE) (10). One of the other extracellular ligands is F-spondin (11, 12), important in axon guidance during development (13). Intracellularly, ApoER2 and VLDLR also bind several other adaptor proteins, affecting numerous downstream signals, including Src tyrosine kinases and PKB/AKT pathways (14–18).
Little is known about the signaling mechanisms of Reelin and F-spondin. Reelin is a glycoprotein that is secreted in the embryonic cortex by Cajal-Retzius cells and in the adult by interneurons (2, 19). Reelin has an N-terminal domain important for dimerization, eight repeats of about 350 amino acids, and a C-terminal domain of 32 amino acids (20, 21). The Reelin repeats interact with the ligand-binding domain of ApoER2 (22). Reelin induces long term potentiation (LTP) in hippocampal neurons (23) and plays important roles in synaptic plasticity, memory, and learning (3, 24, 25). Similarly, F-spondin is a secreted glycoprotein. It has an N-terminal domain similar to Reelin, a central spondin domain, and six thrombospondin-type repeats (26, 27). The F-spondin thrombospondin repeats interact with the ligand-binding domain of ApoER2 (11). Besides ApoER2 and VLDLR, both Reelin and F-spondin also bind to the amyloid precursor protein (APP) and affect its processing (28–30). APP is transmembrane protein also present in synapses (25, 31). It undergoes regulated extracellular and intramembranous cleavage to generate the Aβ peptide that accumulates in Alzheimer disease brains (32).
Cell signaling through type I transmembrane proteins often requires receptor clustering (e.g. epidermal growth factor receptor (EGFR), Trk receptors, ephrins, and Toll-like receptors (33–36)). Many of these receptors have N-terminal domains that bind multivalent ligands and catalyze subsequent signaling through receptor autophosphorylation and phosphorylation of tyrosine kinase substrates. Reelin and F-spondin are both oligomeric/dimeric ligands (21, 27, 37), and both promote intracellular signaling cascades (12, 38–42). Here we show strong clustering of ApoER2 induced by F-spondin and Reelin but relatively weak clustering with the ligand apoE. This clustering involves numerous proteins besides ApoER2, including APP and the synaptic adaptor protein PSD-95. Interestingly, we did not observe strong clustering of ApoER2 with VLDLR.
EXPERIMENTAL PROCEDURES
Plasmids and Vectors
Constructs of murine ApoER2 and human VLDLR cDNAs are shown in Fig. 1. Construct 1 is full-length murine ApoER2 without a tag in the p3GFLAG vector under the CMV promoter. Constructs 2, 3, and 4 are full-length murine ApoER2 constructs fused at either the C or N terminus with myc or HA tags: construct 2, ApoER2 construct with C terminus HA tag (ApoER2-HA); construct 3, ApoER2 with C terminus myc tag (ApoER2-myc); and construct 4, N terminus HA tag and C terminus myc tag (HA-ApoER2-myc). Construct 5 is the human ApoER2 construct missing the ligand-binding repeats. This construct has the endogenous signal peptide, the EGF-like domain, the glycosylation domain, the transmembrane domain, and the C terminus cytoplasmic domain (ApoER2-ΔLBD). Construct 6 is an ApoER2 construct missing the C terminus cytoplasmic domain. It has only the first 11 amino acids of the cytoplasmic domain. This construct has an N terminus HA tag (ApoER2-HAΔICD). Construct 7 is human VLDLR-myc in the pSEC, tag2 hygro plasmid under the CMV promoter. All constructs have either the endogenous ApoER2 or VLDLR signal peptides (constructs 1–7) to direct protein expression to the endoplasmic reticulum. All DNA sequences were confirmed by sequencing. The plasmid expressing Fc-RAP containing a V5 tag was a gift from Joachim Herz (43). Full-length PSD-95 was expressed with a C-terminal GFP tag.
FIGURE 1.
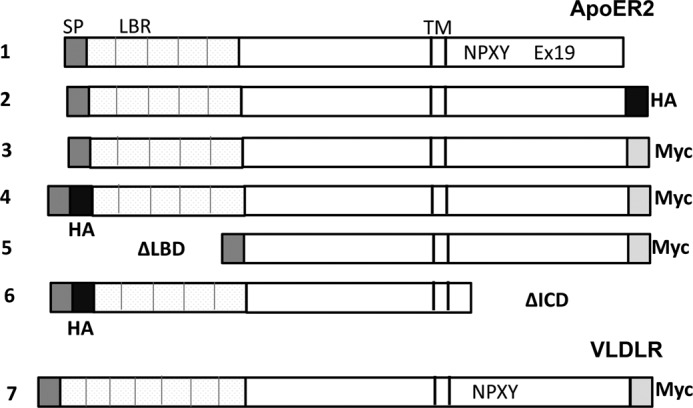
ApoER2 and VLDLR constructs. ApoER2 constructs (1–4 and 6) have murine ApoER2 in the p3GFLAG vector under the CMV promoter. Construct 5 is human ApoER2. Construct 7 is human VLDLR-myc in the pSEC, tag2 hygro plasmid under the CMV promoter. The HA tag is indicated in black and a myc tag in light gray. SP, signal peptide (dark gray); LBR, ligand-binding repeats; TM, transmembrane domain; Ex19, Exon 19.
Proteins
Purified recombinant human F-spondin and mouse Reelin were obtained from R&D Biosystems (Minneapolis, MN). Recombinant human apoE was purchased from Sigma-Aldrich (St Louis, MO). These forms of apoE were primarily monomeric, as determined by immunoblots (44). Human plasma HDL was purchased from Millipore (Billerica, MA). Purified receptor-associated protein (RAP) was a gift from Dr. Guojun Bu (Mayo Clinic, Jacksonville, FL). Fc-RAP was isolated from the medium of COS-7 cells transiently transfected with the Fc-RAP construct for 48 h. The medium was collected, treated with protease inhibitors (Promega, Madison, WI), and incubated with protein A-Sepharose beads (Millipore) overnight to precipitate Fc-RAP. The beads were washed three times with TBS-T buffer (TBS with 1% Tween), and the Fc-RAP was eluted in 100 mm glycine buffer at pH 1.9 using Polyprep chromatography columns (Bio-Rad). Elution fractions were collected to identify the presence of Fc-RAP via immunoblot analyses with mouse V5 antibody (Invitrogen). The glycine buffer was dialyzed against PBS using Millipore dialysis cassettes.
Antibodies
The following antibodies were used: anti-ApoER2 (rabbit monoclonal, 1:1000) and anti-HA (mouse, monoclonal, 1:1000) from Abcam (Cambridge, MA); anti-Myc (rabbit polyclonal, 1:1000); anti-V5 tag; (mouse monoclonal, 1:1000) from Invitrogen; anti-VLDLR antibodies (5F3, mouse monoclonal), a gift from Dr. Dudley Strickland; Ab74 (mouse monoclonal), a gift from Dr. Johannes Nimpf; anti-Dab-1 (rabbit polyclonal, 1:1000) from Abcam; and anti-PSD95 (mouse polyclonal, 1:1000) from Millipore. APP C-terminal mouse monoclonal antibody C.1 was from Paul Mathews.
Cell Culture
For transient transfections, COS-7 cells were maintained in DMEM (Sigma-Aldrich) with 10% FBS (Invitrogen) at 37 °C and 5% CO2. For primary neuronal cultures, cortical neurons were isolated from Sprague-Dawley rats at embryonic days 18 and 19. The cortical cultures were plated in Neurobasal medium (Sigma-Aldrich) supplemented with 2% B-27, 1% penicillin/streptomycin, 0.25% 100 × glutamine, and 0.1% of 10 mm glutamate (Invitrogen) on poly-d-lysine-coated plates at density of 200,000 cells/ml. The medium was changed after 5 days in vitro and supplemented with Neurobasal medium minus glutamate. For cortical cells grown on coverslips, the coverslips were acid-treated, coated with poly-d-lysine, and plated at 150,000 cells/ml. Primary neurons were treated at 21 days in vitro for most assays.
Transfections
COS-7 cells were grown in DMEM with 10% fetal bovine serum prior to transfections and transiently transfected with ApoER2 and VLDLR constructs (1 μg of DNA) with FuGENE in serum-free medium (Promega) according to the protocol of the manufacturer. 24 h post-transfection, cells were treated with the noted concentrations of F-spondin, Reelin (500 ng/ml), recombinant apoE3, and apoE4 (5μg), purified Fc-RAP, and purified RAP (3 μg) in serum-free medium for 1 h.
Immunoprecipitation and Immunoblotting
Cultured cells were lysed in immunoprecipitation (IP) buffer (1 mm Tris-HCl (pH 8), 150 mm NaCl, 0.5% Triton X-100, 1× protease inhibitor, and phosphate inhibitor mixtures), and lysates were stored at −20 °C. Cellular protein was measured by BCA assay (Bio-Rad). For IPs from transfected COS7 cells, 50–100 μg of protein was used. HA-ApoER2 was immunoprecipitated using 45 μl of protein G-Sepharose beads (Millipore) diluted 1:1 in IP buffer and 1 μl of HA mouse monoclonal antibody (Abcam) at 4 °C overnight. For immunoprecipitations from primary neurons, 150 μg of cellular protein was used, with protein A-Sepharose beads (Millipore) diluted 1:1 in IP buffer and 1 μl of ApoER2 antibody. In both assays, the beads were centrifuged at 3000 rpm and washed four times in IP buffer at 4 °C. The beads were suspended in Laemmli buffer with 5% β-mercaptoethanol (65 μl), heated at 95 °C for 5 min, and then 20 μl were loaded on 8% polyacrylamide gels.
For immunoblot analyses, 25–50 μg of protein was separated on 8% polyacrylamide gels. Proteins were transferred to nitrocellulose membrane (Bio-Rad), blocked in 5% nonfat milk for 1 h, and incubated with primary antibody overnight. The blots were treated with secondary antibody and developed using SuperSignal West Pico or Dura luminol/enhancer solution (Thermo Scientific, Lafayette, CO) on x-ray films. Films were developed and quantified using ImageJ software.
Statistical Analysis
Data are plotted as mean ± S.D. Statistical analysis was performed using two-tailed unpaired Student's t test (*, p < 0.05; **, p <0.01; ***, p < 0.001 compared with controls).
RESULTS
Dimeric Ligands Induce ApoER2 Clustering
Reelin is thought to induce clustering of ApoER2 and VLDLR and subsequent phosphorylation of Dab-1 (43), but direct evidence of clustering has been lacking. To measure receptor clustering, we generated several different ApoER2 and VLDLR constructs with or without tags (Fig. 1). To measure whether ApoER2 formed homodimers, we used full-length ApoER2 without a tag and ApoER2 with a C-terminal HA tag (Fig. 2A). We first tested whether the antibodies against the C terminus of ApoER2 and against HA showed any cross-reactivity. COS-7 cells were transfected with either construct, and then cell lysates were probed with the two antibodies. As expected, the HA antibody detected only the species of ApoER2 with the HA tag, and the C-terminal ApoER2 antibody (α-ER2) detected only the species of ApoER2 with an untagged C terminus (Fig. 2B). ApoER2 appears as double bands in our blots, which is characteristic of ApoER2. The double band represents the differentially glycosylated form of ApoER2 (45, 46).
FIGURE 2.
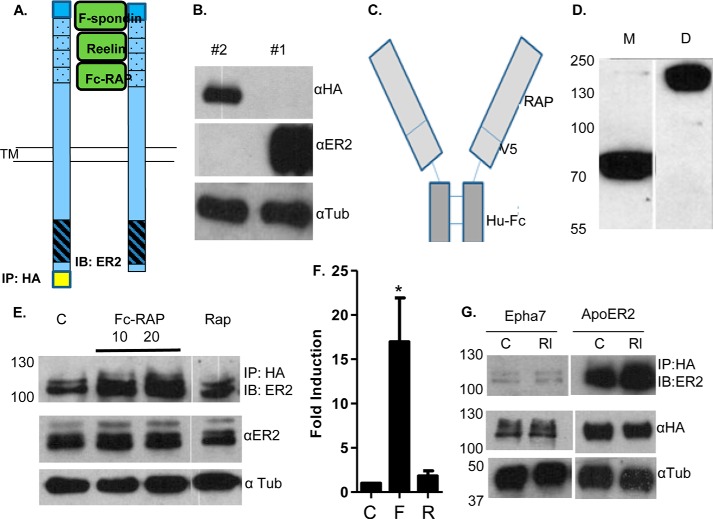
Assay to study ApoER2 and VLDLR clustering. A, model of proposed clustering of receptors by the dimeric ligands F-spondin, Reelin, and Fc-RAP. TM, transmembrane domain. IB, immunoblot. B, ApoER2 constructs 1 (#1) and 2 (#2) (from Fig. 1) were transfected in COS-7 cells and detected via Western blot analyses using an HA antibody (top panel) or C-terminal-specific ApoER2 antibody (center panel). α-Tubulin was used as a control (bottom panel). C, the dimeric Fc-RAP construct links RAP (containing a V5 tag) with the Fc portion of human immunoglobulin (Hu-Fc). D, Fc-RAP was expressed in COS-7 cells and immunoprecipitated from conditioned medium using protein A beads. Reduced monomeric (M, ∼80 kDa) and non-reduced dimeric (D, ∼160 kDa) Fc-RAP species were detected by immunoblotting with a V5 antibody. E, clustering of ApoER2 with Fc-RAP and monomeric RAP. Clustering measured by expressing ApoER2 HA and ApoER2 constructs in COS-7 cells and treating with vehicle control (C), purified Fc-RAP (20 μl), or purified monomeric RAP for 1 h. The cell lysates were immunoprecipitated with anti-HA antibody and immunoblotted with anti-ApoER2 antibody. F, quantitation of the clustering of ApoER2 by Fc-RAP (F) and RAP (R) (n = 3). *, p < 0.05. G, COS-7 cells were transfected with either Epha7-HA and ApoER2 or ApoER2-HA and ApoER2 constructs and treated with Reelin (Rl) for 1 h. The cell lysates were immunoprecipitated with HA antibody and immunoblotted with ApoER2 antibody. The total levels of Epha7 and ApoER2 were analyzed by HA antibody, and tubulin was used as a control.
To measure whether we could use these constructs to detect ligand-induced clustering of ApoER2, we first isolated Fc-RAP protein (Fig. 2C). The RAP protein binds ApoER2 avidly, and the RAP sequence was cloned behind the human Fc sequence to generate a dimeric RAP ligand (43). We purified Fc-RAP prepared from conditioned medium from transfected COS-7 cells using protein A-Sepharose beads and analyzed the purified protein by immunoblotting (Fig. 2D). Reduced Fc-RAP (Fig. 2D, first lane) was the expected 80 kDa in size, whereas non-reduced Fc-RAP (second lane) was greater than 150 kDa in size, indicative of the expected dimeric form. To measure whether Fc-RAP induced ApoER2 clustering, we transfected COS-7 cells with HA-tagged and untagged ApoER2 and, 24 h later, treated cells with Fc-RAP for 1 h. Cell lysates were immunoprecipitated with an HA antibody and immunoblotted with the α-ER2 antibody (Fig. 2E). We observed untagged ApoER2 molecules precipitating with HA-tagged ApoER2 in the absence of treatment (Fig. 2E, first lane, C), but the amount of coprecipitation increased dramatically in the presence of Fc-RAP (second and third lanes). Purified untagged (monomeric) RAP did not induce co-precipitation of ApoER2 constructs (Fig. 2E, fourth lane). Quantification of the blots showed that Fc-RAP treatment induced ∼10-fold more clustering compared with control or RAP treatment (Fig. 2F).
Because we detected some basal clustering in the absence of ligands, we hypothesized that spontaneous clustering may occur because the two proteins colocalized in the same cellular compartments. To test this hypothesis, we examined the Ephrin receptor A7 (EphA7), which is also localized in lipid rafts (47). COS-7 cells were either cotransfected with Epha7-HA and ApoER2 or transfected with ApoER2-HA and ApoER2 constructs and then treated with Reelin. Cell lysates were immunoprecipitated with an HA antibody and immunoblotted with the ApoER2 antibody (Fig. 2G). We did not detect any clustering of EphA7 with ApoER2, whereas ApoER2-HA clustered with ApoER2 (as before) (Fig. 2G).
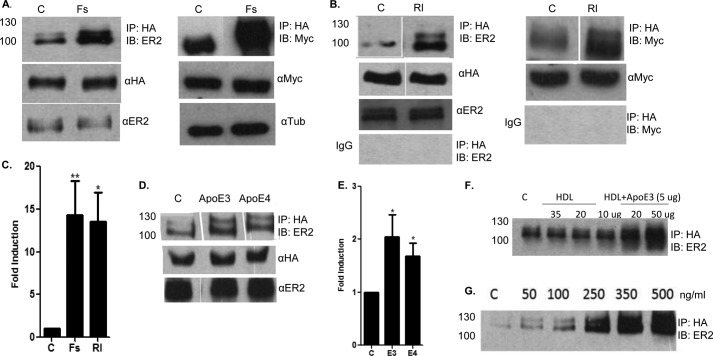
We next tested whether natural ligands for ApoER2 were also able to induce receptor clustering. Reelin is an extracellular glycoprotein that plays a role in neuronal migration (19, 48). F-spondin is a secreted protein involved in axonal growth (13). Both ligands have domains to induce dimerization (27, 37), and both bind ApoER2 and VLDLR and activate Dab-1 phosphorylation (6, 11, 12, 19, 39, 41). As above, we transfected COS-7 cells with HA-tagged and untagged ApoER2 and treated the cells for 1 h with ligand. F-spondin (Fig. 3A) induced coprecipitation of tagged and untagged ApoER2 (top panel) without affecting levels of HA-tagged ApoER2 (center panel) or untagged ApoER2 (bottom panel). Similarly, Reelin (Fig. 3B) induced receptor coprecipitation without affecting total ApoER2 levels. We conducted similar experiments using ApoER2 constructs tagged with either HA or myc (Fig. 1). Both F-spondin (Fig. 3A) and Reelin (Fig. 3B) induced coprecipitation of these forms of ApoER2. Negative controls with IgG antibody showed the specificity of HA antibody and the background level of coimmunoprecipitation. Quantification of these blots showed that F-spondin and Reelin both induced significant, 10- to 15-fold increases in clustering of ApoER2 (Fig. 3C).
FIGURE 3.
Clustering of ApoER2 with F-spondin (Fs), Reelin (Rl), ApoE3 (E3), and ApoE4 (E4). A, COS-7 cells were transfected with ApoER2-HA and ApoER2 constructs and treated with vehicle control (C) or F-spondin. The cell lysates were immunoprecipitated with HA antibody and immunoblotted (IB) with ApoER2 antibody. Similar ApoER2 clustering was observed between the ApoER2-HA and ApoER2-myc proteins with 500 ng/ml of F-spondin. No ApoER2 was immunoprecipitated using control IgG. Similar total levels of ApoER2-HA, ApoER2, and ApoER2-myc were found. B, COS-7 cells expressing ApoER2-HA and ApoER2 were treated with 500 ng/ml Reelin for 1 h. The cell lysates were immunoprecipitated with anti-HA and immunoblotted with ApoER2 antibody. Similar total levels of ApoER2 and ApoER2-HA were present with and without Reelin. ApoER2 clustering was also observed with ApoER2-HA and ApoER2 myc constructs. The total levels of ApoER2 constructs remained unchanged. C, levels of clustered ApoER2 were quantified using ImageJ software and plotted as fold induction to the untreated control. Data are mean ± S.D.; n = 3. *, p < 0.05; **, p < 0.005. D, COS-7 cells were transfected with ApoER2-HA and ApoER2 constructs and treated with recombinant ApoE3 and ApoE4 (5 μg/ml) for 1 h. The cell lysates were immunoprecipitated with HA antibody and immunoblotted with ApoER2 antibody. E, levels of clustered ApoER2 were quantified using ImageJ software and plotted as fold induction to the untreated control. Data are mean ± S.D.; n = 3. *, p < 0.05. F, COS-7 cells were transfected with ApoER2-HA and ApoER2 constructs and treated with different doses of HDL in the presence or absence of 5 μg of ApoE3. The cell lysates were immunoprecipitated with HA antibody and immunoblotted with ApoER2 antibody. G, dose response of F-spondin effects on clustering of ApoER2. COS-7 cells expressing ApoER2-HA and ApoER2 were treated for 1 h with various doses of F-spondin (50, 100, 250, 350, and 500 ng/ml). Cell lysate proteins were immunoprecipitated with anti HA antibody and immunoblotted with anti-ApoER2 antibody.
We tested whether the ApoER2 ligand apoE also induced clustering of ApoER2. We treated COS-7 cells transfected with HA-tagged and untagged ApoER2 with recombinant apoE3 or apoE4 for 1 h (Fig. 3D). The cell lysates were immunoprecipitated with HA antibody and immunoblotted with ApoER2 antibody. ApoE3 induced a 2-fold increase in clustering of ApoER2, whereas apoE4 induced a similar, 1.6-fold increase in clustering (Fig. 3E). However, compared with F-spondin and Reelin, recombinant apoE was a poor ligand for inducing clustering of ApoER2. These data suggest that apoE may not be as strong a signaling molecule as Reelin or F-spondin in this pathway.
We were interested in whether lipidated apoE might provoke a different response compared with purified apoE. Lipidated apoE binds with higher affinity to ApoER2, and the functional biological activity of apoE particle is optimum when it is lipidated (49). We treated COS7 cells transfected with HA-tagged and untagged ApoER2-HA with human plasma HDL with and without apoE3 (Fig. 3F). HDL alone did not induce ApoER2 clustering, but apoE in the presence of HDL strongly increased ApoER2 clustering. This clustering seemed dependent on the amount of HDL with apoE, suggesting that the stoichiometry of the lipoprotein-apoE complex is important for receptor clustering.
Next, we determined whether there was a dose-dependent, ligand-induced increase in the clustering of ApoER2. COS-7 cells transfected with HA-tagged and untagged ApoER2 constructs were treated with increasing doses of F-spondin (50–500 ng/ml) (Fig. 3G). We detected a dose-dependent effect of F-spondin on clustering of ApoER2. There was detectable clustering at the lowest dose of 50 ng/ml, with increasing clustering observed through the maximum dose of 500 ng/ml.
Domains of ApoER2 in Clustering
To determine which domains of ApoER2 are required for clustering, we generated two deletion mutants of ApoER2. One construct, ApoER2-ΔLBD, has a C-terminal myc tag and lacks the ligand binding repeats (Fig. 1). The second construct, ApoER2-HAΔICD, has an N-terminal HA tag, the entire extracellular domains, and transmembrane domains but has only the first 11 amino acids of the cytoplasmic domain of ApoER2. Cells were transfected with these ApoER2 constructs or full-length ApoER2 constructs, along with Dab-1, and treated with F-spondin or Reelin for 1 h. Each version of ApoER2 was well expressed in cells, demonstrating the expected sizes, and the levels did not vary with treatments (Fig. 4B). As expected, F-spondin and Reelin induced clustering of full-length ApoER2 (Fig. 4A, first panel). F-spondin and Reelin also induced clustering of full-length ApoER2, with ApoER2 lacking the ligand-binding domain (Fig. 4A, second panel), or ApoER2 lacking the cytoplasmic domain (Fig. 4A, third panel). Finally, we observed a small amount of ligand-induced clustering of the form of ApoER2 lacking the ligand binding domain clustered with the version lacking the cytoplasmic domain (Fig. 4A, fourth panel). These results are quantified in Fig. 4C. F-spondin and Reelin induced a 9- to 12-fold induction of clustering of full-length ApoER2 but only a 5- to 6-fold induction of clustering of full-length ApoER2 with the form lacking the cytoplasmic domain, and an ∼4- to 5-fold induction of clustering of full-length ApoER2 with the form lacking the ligand-binding repeats. These two deletion mutants of ApoER2 clustered with each other, but only at levels of about 3-fold above the background. Thus, removal of either the extracellular ligand-binding domain or the intracellular domain still allows ligand-induced clustering with full-length ApoER2.
FIGURE 4.
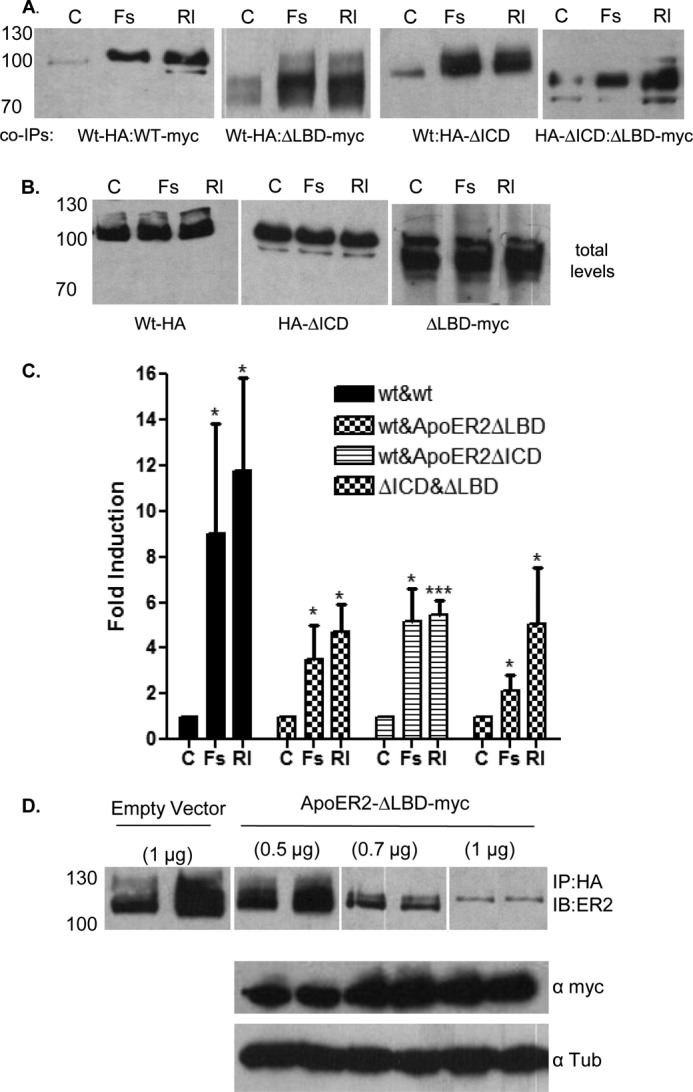
Role of the N terminus and C terminus of ApoER2 in clustering. A, COS-7 cells were transfected with ApoER2-HA and ApoER2-myc, with ApoER2-HA and ApoER2ΔLBD, with the ApoER2 and ApoER2-HA-ΔICD constructs, or with the ApoER2-HAΔICD and ApoER2-ΔLBD constructs and treated with vehicle control (C), F-spondin (Fs), or Reelin (Rl) for 1 h. The cell lysates were immunoprecipitated with HA antibody and blotted with myc antibody or ApoER2 antibody. B, total levels of full-lengthApoER2, HAΔICD, and ApoER2-ΔLBD were unchanged with treatments. C, levels of clustered ApoER2 and ApoER2 mutants were quantified using ImageJ software and plotted as fold induction to the untreated control. Data are mean ± S.D.; n = 3. *, p < 0.05; ***, p < 0.0005. D, COS-7 cells were transfected with ApoER2-HA and ApoER2 constructs with increasing concentrations of ApoER2ΔLBD (0.5, 0.7, and 1 μg), and the cells were treated with Reelin for 1 h. Increasing concentrations of empty vector (0.5, 0.7, and 1 μg) were used as a control. Cell lysate proteins were immunoprecipitated with anti HA antibody and immunoblotted (IB) with anti-ApoER2 antibody. The total levels of ApoER2-ΔLBD were determined by anti-myc antibody, and tubulin was used as a control.
As the construct of ApoER2 lacking a large part of the LBD clustered with the full-length ApoER2, we decided to test whether this construct inhibited the clustering of full-length ApoER2 constructs. COS-7 cells were transfected with full-length ApoER-HA and increasing concentrations of ApoER2-ΔLBD. Transfected cells were treated with Reelin for 1 h. At lower concentrations, ApoER2-ΔLBD did not inhibit clustering of full-length ApoER2 (Fig. 4D). Increasing the concentration of ApoER2-ΔLBD significantly reduced the clustering of full-length ApoER2 by Reelin compared with the empty vector. ApoER2-ΔLBD also reduced the basal level of ApoER2 clustering (Fig. 4D). These data suggest that ApoER2-ΔLBD acts as a dominant negative molecule to inhibit clustering of ApoER2. It would be interesting to see how the ApoER2-ΔLBD construct would affect downstream signaling of ApoER2 receptors.
Clustering Partners of ApoER2
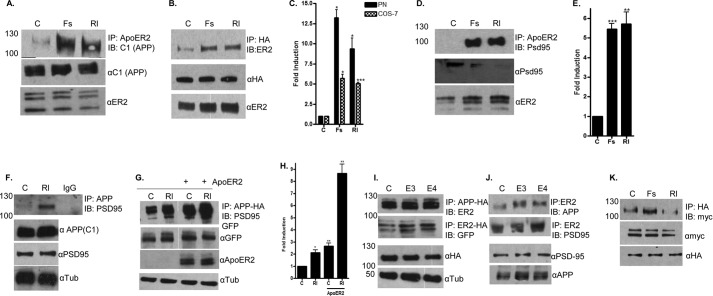
Using similar assays, we tested whether APP, VLDLR, or PSD-95 could cluster with ApoER2. We tested the interactions between APP and ApoER2 first because several studies have found that these transmembrane molecules interact (11, 28, 30). Initially, we treated primary cortical neurons with F-spondin or Reelin for 1 h and immunoprecipitated the endogenous ApoER2. Probing for endogenous APP revealed that F-spondin and Reelin induced ∼12-fold and 10-fold clustering of ApoER2 and APP (Fig. 5, A and C). We tested whether this clustering also occurred in COS-7 cells after transfection with ApoER2 and APP-HA constructs. In COS-7 cells, F-spondin and Reelin each similarly induced an ∼6-fold induction of clustering of ApoER2 and APP (Fig. 5, B and C).
FIGURE 5.
Clustering partners of ApoER2. A, primary neurons at day 21 were treated with vehicle control (C), F-spondin (Fs), or Reelin (Rl) for 1 h (500 ng/ml). The cell lysates were immunoprecipitated using anti-ApoER2 antibody and blotted (IB) with anti APP (C1) antibody. B, COS-7 cells were transfected with a full-length ApoER2 construct and an APP-HA construct. The cell lysates were immunoprecipitated with HA antibody and immunoblotted with ApoER2 antibody. The total levels of ApoER2 and APP were unchanged in both primary neurons and COS-7 cells. C, levels of clustered ApoER2 and APP from primary neurons (PN) or COS-7 cells were quantified using ImageJ software and plotted as fold induction to the untreated control. Data are mean ± S.D.; n = 3. *, p < 0.05; ***, p < 0.0005. D, primary neurons at day 21 in vitro were treated with F-spondin or Reelin for 1 h. The cell lysates were immunoprecipitated using anti-ApoER2 antibody and immunoblotted using anti-PSD95 antibody. E, levels of clustered ApoER2 and PSD95 were quantified using ImageJ software and plotted as fold induction to the untreated control. Data are mean ± S.D.; n = 3. **, p < 0.005; ***, p < 0.0005. F, primary neurons at day 21 were treated with Reelin for 1 h. The cell lysates were immunoprecipitated using anti-APP (C1) antibody and immunoblotted with anti PSD-95 antibody. α-Tub, α-tubulin. G, COS-7 cells were transfected with APP-HA and PSD-95-GFP constructs in the presence or absence of ApoER2 and treated with Reelin for 1 h. Cell lysate proteins were immunoprecipitated with anti HA antibody and immunoblotted with anti-ApoER2 antibody. Total levels of PSD-95 and ApoER2 were detected by GFP and ApoER2 antibodies. H, levels of clustered APP-HA and PSD-95-GFP in the presence or absence of ApoER2 were quantified using ImageJ software and plotted as fold induction to the untreated control. Data are mean ± S.D.; n = 3. *, p <0.05; **, p < 0.005. I, COS-7 cells were transfected with APP-HA and ApoER2 or ApoER2-HA and PSD-95-GFP constructs and treated with ApoE3 (E3) and ApoE4 (E4) for 1 h (5 μg). Cell lysate proteins were immunoprecipitated with anti HA antibody and immunoblotted with anti-ApoER2 or anti-GFP antibody. J, primary neurons at day 21 were treated with ApoE3 and ApoE4 for 1 h. The cell lysates were immunoprecipitated using ApoER2 antibody and blotted with anti-APP (C1) antibody or with anti PSD-95 antibody. K, COS-7 cells transfected with ApoER2-HA and VLDLR-myc-tagged constructs were treated with F-spondin and Reelin for 1 h. The cell lysates were immunoprecipitated with anti-HA antibody and immunoblotted with anti-myc antibody.
ApoER2 and PSD-95 are known to interact with each other through the cytoplasmic domain of ApoER2 (3, 18). Using our cortical neurons, we tested whether F-spondin and Reelin promoted the interaction of these two proteins. Both of these ligands induced an ∼6-fold greater interaction of ApoER2 with PSD-95 (Fig. 5, D and E). These results suggest that ApoER2 forms clusters with itself and may form larger clusters that can include other proteins, such as APP and PSD95.
We also determined whether Reelin could induce the interaction of APP and PSD95 because Reelin binds directly to APP (50, 51). Reelin induced the coimmunoprecipitation of APP and PSD95 in primary neurons (Fig. 5F). We determined whether this interaction between APP and PSD95 was dependent on ApoER2. COS-7 cells expressing APP-HA and PSD95-GFP, with or without full-length ApoER2, were treated for 1 h with Reelin. Reelin induced APP interaction with PSD95 by ∼2.5-fold independently of ApoER2 (Fig. 5, G and H), although the presence of ApoER2 alone increased the basal interaction of APP and PSD95 by a similar amount. In the presence of ApoER2, Reelin induced the interaction of APP and PSD95 by an additional 8-fold (Fig. 5, G and H). These data also suggest that clustering induced by Reelin includes ApoER2 and other receptors to form larger complexes.
Next we determined whether recombinant ApoE3 and ApoE4 could induce clustering of APP and PSD-95 with ApoER2. In COS-7 cells expressing APP-HA and ApoER2, ApoE3 and ApoE4 induced a nearly 2-fold increase in clustering of APP and ApoER2 (Fig. 5I). This increase in clustering was similar to the clustering of ApoER2 alone (Fig. 3). Primary neurons treated with recombinant ApoE3 and ApoE4 also showed an ∼2-fold increase in clustering of ApoER2 with APP and a 1.6-fold increase in clustering of ApoER2 with PSD-95 (Fig. 5J).
Finally, we investigated the clustering of ApoER2 with VLDLR because of their interactions in promoting the Reeler-like phenotype in development. To determine whether ApoER2 clustered with VLDLR, we used COS-7 cells and transfected them with ApoER2-HA and VLDLR-myc constructs. Surprisingly, F-spondin and Reelin did not induce a significant increase in clustering of ApoER2 and VLDLR (Fig. 5K). We also examined primary cortical neurons using an antibody against the endogenous VLDLR. Similarly, we did not detect clustering of ApoER2 and VLDLR in primary cortical neurons (data not shown). It is possible that ApoER2 and VLDLR are present in different membrane domains and, hence, do not cluster. For example, ApoER2 is found mostly in membrane lipid rafts, whereas VLDLR is not (52).
DISCUSSION
Our data demonstrate that clustering of ApoER2 is induced by the multivalent ligands F-spondin and Reelin. We initially examined clustering of ApoER2 by the synthetic dimeric ligand Fc-RAP, which has been found previously to induce phosphorylation and oligomerization of Dab-1 (43). We observed strong clustering of ApoER2 by Fc-RAP, suggesting that our assay was sensitive for detecting regulated receptor clustering. We next tested F-spondin and Reelin, both of which bind to ApoER2 (11, 12, 19) and are important in embryonic development (2, 5, 6, 13). Both F-spondin and Reelin induced clustering of ApoER2, a potential signaling mechanism related to the function of these molecules in the developing brain.
Clustering of receptors allows activation of intracellular signaling cascades. This method of signaling is used by a number of transmembrane molecules, such as EGFR, Trk, and Ephrin receptors (33, 34, 36). These receptors have C-terminal domains that catalyze autophosphorylation and subsequent phosphorylation of other tyrosine kinase substrates. Like ApoER2, these receptors can be activated by multivalent ligands, such as EGF, neurotrophins, and ephrins. Interestingly, Reelin also induces the homotypic clustering of ephrin receptors (53). Activation of ApoER2 and VLDLR by Reelin leads to phosphorylation of Dab-1 and activation of downstream signaling pathways (6, 14). However, because ApoER2 has no intrinsic tyrosine kinase activity, it must signal through a kinase independent of the receptor. One possibility is the tyrosine kinase Fyn, which interacts with the cytosolic domain of ApoER2 (39, 54, 55). Fyn knockout mice show subtle abnormalities in embryonic neuronal migration (56), further supporting a role of Fyn in ApoER2 signaling during development.
Activation of Src family tyrosine kinases and the PKB/AKT pathway causes deactivation of glycogen synthase kinase (GSK3β) and prevents hyperphosphorylation of Tau (57–59). Hyperphosphorylated Tau is increased in Reelin-deficient mice, in ApoER2, in VLDLR double knockout mice, and in Dab-1 mutant mice, suggesting that Tau phosphorylation is involved in this Reelin/apoER2/VLDLR/Dab-1 pathway (60). Like Reelin, F-spondin activation leads to phosphorylation of Dab-1 (12), although the subsequent downstream signaling is less defined. The connection between downstream signaling and clustering is very complex.
Ligand binding is thought to induce clustering of many cell surface receptors, including EGFR, T cell receptors, Ephrin receptors, and Toll-like receptors (33, 34, 61, 62). Receptor clustering is dependent on many aspects, such as ligand affinity, ligand dissociation, the size of ligands, and the concentration of coactivators (63–66). In the case of the B cell receptor and T cell receptor clusters, the mobility of the ligand also affects the clustering of these receptors (67, 68). The receptor clustering creates centers of signaling, with larger clusters corresponding to increased signaling events (69–71). We observed the formation of complex clusters of molecules by the oligomeric ligands Reelin and F-spondin. EGFR dimerization leads to the formation of higher order clusters through extracellular and intracellular interactions (70), as we propose here for ApoER2. In addition to homotypic EGFR clustering, there is heterotypic clustering involving other receptors and proteins in larger clusters (70, 72). Recent studies have highlighted the importance and physiological significance of clustering and downstream signaling in many diseases (62, 65, 73).
We found that Reelin or F-spondin could induce the clustering of ApoER2. In development, the Reelin-ApoER2/VLDLR-Dab1 pathway is necessary for the inside-out layering of the neocortex and the proper neuronal organization of the hippocampus and cerebellum (5). ApoER2 and VLDLR have distinct role in these developmental pathways. ApoER2 knockout mice showed that early layers of cortex were formed normally, whereas the formation of superficial late layers was affected severely. These data suggest that ApoER2 signaling plays a role in the formation of late generation neurons (5). VLDLR knockout mice showed neurons migrating in the marginal zone, suggesting that VLDLR may act as a stop signal for migrating neurons (5). The lack of evidence of ApoER2-VLDLR heterodimers here suggests that the receptors may mediate separate signaling cascades important for specific migration functions. These functions could be mediated through different intracellular adaptor proteins that bind to VLDLR or ApoER2, such as JNK-interacting protein to ApoER2 or Lis1 to VLDLR (10).
We found that ApoER2 lacking its cytoplasmic domain clustered with full-length ApoER2, which was not unexpected, and that ApoER2 lacking the extracellular ligand binding domain clustered with full-length ApoER2, which was unexpected. Clustering of receptors occurs in cellular microdomains such as lipid rafts (74) and can, thus, involve many proteins beyond the ligand-bound receptors that are clustered. Ephrin receptors can be induced by ephrin to cluster with versions of the receptors lacking kinase activity, the entire cytoplasmic domain, or the receptor hetero-oligomerization domain (34). Similarly, EGFR can be clustered in the absence of its cytoplasmic domain (33). We propose that binding of Reelin or F-spondin can promote clustering of full-length ApoER2, which then forms clusters with other mutants of ApoER2 to form large complexes. Preliminary results from phosphorylation studies suggest that the ApoER2 HAΔICD and the ApoER2-ΔLBD cluster and have a higher basal level phosphorylation of ApoER2 compared with full-length constructs. Our data suggest that APP and PSD-95 may also be part of these clusters. These proteins, like ApoER2, are synaptic proteins (31, 32, 75, 76) and may comprise part of a complex of postsynaptic molecules.
APP and its homologues share a number of both extracellular and intracellular interacting proteins with the family of lipoprotein receptors containing ApoER2 and VLDLR (77). Both families are type I transmembrane proteins with signaling functions. Both also undergo regulated extracellular and intramembranous cleavages (30). Members of the APP and lipoprotein receptor families are synaptic proteins playing roles in modulating neuronal migration (8, 78, 79). Understanding the ways in which each family affects the expression, cleavage, and interactions of the other will be vital to understanding the functions of these proteins in the developing brain and in the aging brain.
ApoER2 affects LTP in hippocampal neurons through interactions with Reelin (80). ApoER2 modulation of LTP requires the ApoER2 cytoplasmic domain, specifically the alternatively spliced exon 19 (3). Fc-RAP also enhances LTP induction in hippocampal neurons (43), suggesting that a multivalent ligand of ApoER2 is important for synaptic plasticity. ApoER2 exon 19 binds PSD-95 as well as an NMDA receptor subunit and modulates NMDA receptor conductance (3, 18), which may contribute to the mechanism of LTP modulation. These effects seem to continue throughout life because exogenously applied Reelin has long lasting effects on learning and synaptic plasticity in adult mice (80).
ApoE is another ligand for ApoER2 and VLDLR. Each of the three isoforms of human apoE (E2, E3, and E4) bind to ApoER2 (81, 82). Both ApoE3 and ApoE4 induced clustering of ApoER2, but the clustering was significantly less than that induced by F-spondin or Reelin. Thus, ApoE may not be a strong clustering ligand for ApoER2. It is also possible that recombinant ApoE, which we used, may function differently than apoE associated with lipoprotein particles. In the presence of human plasma HDL, we observed that ApoE3 promoted more ApoER2 clustering. It will be important to test the effects of ApoE on lipoproteins found in the brain, i.e. lipoproteins secreted from glia or those present in cerebrospinal fluid. Furthermore, it will be interesting to test whether induction of ApoER2 clustering correlates with the induction of intracellular signaling cascades.
ApoE is thought to also induce signaling through its receptors. ApoE induces phosphorylation of the AKT and ERK pathways and inhibition of the JNK pathway (83–85). Activation of the AKT and ERK pathways was through the LRP1 receptor (83). A tandem repeat ApoE mimetic peptide also induced phosphorylation of Dab-1 independent of effects on ERK and AKT signaling (84). ApoE signaling may also inhibit normal Reelin signaling (19). Our data suggest that various signaling ligands should be considered in light of their ability to promote homotypic and heterotypic ApoER2 clustering.
In summary, our data demonstrate that several multivalent ligands for ApoER2 induce clustering in transfected cells and primary neurons. This clustering was not wholly dependent on either the extracellular ligand-binding domain of ApoER2 or the cytoplasmic domain. However, clustering by Reelin and F-spondin included complexes containing other synaptic molecules, such as APP and PSD-95. The regulation of clustering of type I transmembrane proteins, such as ApoER2, may be important as the initial step in the downstream signaling important in developmental and neuronal plasticity pathways.
Acknowledgments
We thank Dudley Strickland, Johannes Nimpf, Paul Mathews, and Dan Pak for antibodies; Joachim Herz for the ApoER2 and Fc-RAP constructs, and Maria Donoghue for the EphA7-HA.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 AG030128 and R01 AG035379.
- VLDLR
- very low density lipoprotein receptor
- ApoE
- apolipoprotein E
- LTP
- long term potentiation
- APP
- amyloid precursor protein
- EGFR
- EGF receptor
- IP
- immunoprecipitation
- LBD
- ligand-binding domain
- RAP
- receptor-associated protein.
REFERENCES
- 1. Beffert U., Stolt P. C., Herz J. (2004) Functions of lipoprotein receptors in neurons. J. Lipid Res. 45, 403–409 [DOI] [PubMed] [Google Scholar]
- 2. Tissir F., Goffinet A. M. (2003) Reelin and brain development. Nat. Rev. Neurosci. 4, 496–505 [DOI] [PubMed] [Google Scholar]
- 3. Beffert U., Weeber E. J., Durudas A., Qiu S., Masiulis I., Sweatt J. D., Li W. P., Adelmann G., Frotscher M., Hammer R. E., Herz J. (2005) Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor ApoER2. Neuron 47, 567–579 [DOI] [PubMed] [Google Scholar]
- 4. Dumanis S. B., Cha H. J., Song J. M., Trotter J. H., Spitzer M., Lee J. Y., Weeber E. J., Turner R. S., Pak D. T., Rebeck G. W., Hoe H. S. (2011) ApoE receptor 2 regulates synapse and dendritic spine formation. PloS ONE 6, e17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hack I., Hellwig S., Junghans D., Brunne B., Bock H. H., Zhao S., Frotscher M. (2007) Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development 134, 3883–3891 [DOI] [PubMed] [Google Scholar]
- 6. Niu S., Renfro A., Quattrocchi C. C., Sheldon M., D'Arcangelo G. (2004) Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron 41, 71–84 [DOI] [PubMed] [Google Scholar]
- 7. Niu S., Yabut O., D'Arcangelo G. (2008) The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 28, 10339–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R. E., Richardson J. A., Herz J. (1999) Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 [DOI] [PubMed] [Google Scholar]
- 9. Howell B. W., Gertler F. B., Cooper J. A. (1997) Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 16, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy S. S., Connor T. E., Weeber E. J., Rebeck W. (2011) Similarities and differences in structure, expression, and functions of VLDLR and ApoER2. Mol. Neurodegener. 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoe H. S., Wessner D., Beffert U., Becker A. G., Matsuoka Y., Rebeck G. W. (2005) F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol. Cell. Biol. 25, 9259–9268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blake S. M., Strasser V., Andrade N., Duit S., Hofbauer R., Schneider W. J., Nimpf J. (2008) Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 27, 3069–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burstyn-Cohen T., Tzarfaty V., Frumkin A., Feinstein Y., Stoeckli E., Klar A. (1999) F-Spondin is required for accurate pathfinding of commissural axons at the floor plate. Neuron 23, 233–246 [DOI] [PubMed] [Google Scholar]
- 14. Morimura T., Ogawa M. (2009) Relative importance of the tyrosine phosphorylation sites of Disabled-1 to the transmission of Reelin signaling. Brain Res. 1304, 26–37 [DOI] [PubMed] [Google Scholar]
- 15. Gotthardt M., Trommsdorff M., Nevitt M. F., Shelton J., Richardson J. A., Stockinger W., Nimpf J., Herz J. (2000) Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 275, 25616–25624 [DOI] [PubMed] [Google Scholar]
- 16. Stockinger W., Brandes C., Fasching D., Hermann M., Gotthardt M., Herz J., Schneider W. J., Nimpf J. (2000) The Reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J. Biol. Chem. 275, 25625–25632 [DOI] [PubMed] [Google Scholar]
- 17. Hoe H. S., Magill L. A., Guenette S., Fu Z., Vicini S., Rebeck G. W. (2006) FE65 interaction with the ApoE receptor ApoEr2. J. Biol. Chem. 281, 24521–24530 [DOI] [PubMed] [Google Scholar]
- 18. Hoe H. S., Pocivavsek A., Chakraborty G., Fu Z., Vicini S., Ehlers M. D., Rebeck G. W. (2006) Apolipoprotein E receptor 2 interactions with the N-methyl-d-aspartate receptor. J. Biol. Chem. 281, 3425–3431 [DOI] [PubMed] [Google Scholar]
- 19. D'Arcangelo G., Homayouni R., Keshvara L., Rice D. S., Sheldon M., Curran T. (1999) Reelin is a ligand for lipoprotein receptors. Neuron 24, 471–479 [DOI] [PubMed] [Google Scholar]
- 20. Royaux I., Lambert de Rouvroit C., D'Arcangelo G., Demirov D., Goffinet A. M. (1997) Genomic organization of the mouse reelin gene. Genomics 46, 240–250 [DOI] [PubMed] [Google Scholar]
- 21. Jossin Y., Ignatova N., Hiesberger T., Herz J., Lambert de Rouvroit C., Goffinet A. M. (2004) The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J. Neurosci. 24, 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yasui N., Nogi T., Takagi J. (2010) Structural basis for specific recognition of Reelin by its receptors. Structure 18, 320–331 [DOI] [PubMed] [Google Scholar]
- 23. Weeber E. J., Beffert U., Jones C., Christian J. M., Forster E., Sweatt J. D., Herz J. (2002) Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 277, 39944–39952 [DOI] [PubMed] [Google Scholar]
- 24. Rogers J. T., Weeber E. J. (2008) Reelin and apoE actions on signal transduction, synaptic function and memory formation. Neuron Glia Biol. 4, 259–270 [DOI] [PubMed] [Google Scholar]
- 25. Durakoglugil M. S., Chen Y., White C. L., Kavalali E. T., Herz J. (2009) Reelin signaling antagonizes beta-amyloid at the synapse. Proc. Natl. Acad. Sci. U.S.A. 106, 15938–15943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feinstein Y., Borrell V., Garcia C., Burstyn-Cohen T., Tzarfaty V., Frumkin A., Nose A., Okamoto H., Higashijima S., Soriano E., Klar A. (1999) F-spondin and mindin: two structurally and functionally related genes expressed in the hippocampus that promote outgrowth of embryonic hippocampal neurons. Development 126, 3637–3648 [DOI] [PubMed] [Google Scholar]
- 27. Feinstein Y., Klar A. (2004) The neuronal class 2 TSR proteins F-spondin and Mindin: a small family with divergent biological activities. Int. J. Biochem. Cell Biol. 36, 975–980 [DOI] [PubMed] [Google Scholar]
- 28. Hoe H. S., Tran T. S., Matsuoka Y., Howell B. W., Rebeck G. W. (2006) DAB1 and Reelin effects on amyloid precursor protein and ApoE receptor 2 trafficking and processing. J. Biol. Chem. 281, 35176–35185 [DOI] [PubMed] [Google Scholar]
- 29. Ho A., Südhof T. C. (2004) Binding of F-spondin to amyloid-β precursor protein: a candidate amyloid-β precursor protein ligand that modulates amyloid-β precursor protein cleavage. Proc. Natl. Acad. Sci. U.S.A., 101, 2548–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoe H. S., Rebeck G. W. (2008) Regulated proteolysis of APP and ApoE receptors. Mol. Neurobiol. 37, 64–72 [DOI] [PubMed] [Google Scholar]
- 31. Hoe H. S., Fu Z., Makarova A., Lee J. Y., Lu C., Feng L., Pajoohesh-Ganji A., Matsuoka Y., Hyman B. T., Ehlers M. D., Vicini S., Pak D. T., Rebeck G. W. (2009) The effects of amyloid precursor protein on postsynaptic composition and activity. J. Biol. Chem. 284, 8495–8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nalivaeva N. N., Turner A. J. (2013) The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett. 587, 2046–2054 [DOI] [PubMed] [Google Scholar]
- 33. Ichinose J., Murata M., Yanagida T., Sako Y. (2004) EGF signalling amplification induced by dynamic clustering of EGFR. Biochem. Biophys. Res. Commun. 324, 1143–1149 [DOI] [PubMed] [Google Scholar]
- 34. Wimmer-Kleikamp S. H., Janes P. W., Squire A., Bastiaens P. I., Lackmann M. (2004) Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J. Cell Biol. 164, 661–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindsay R. M. (1996) Role of neurotrophins and Trk receptors in the development and maintenance of sensory neurons: an overview. Philos. Trans. R Soc. Lond. B Biol. Sci. 351, 365–373 [DOI] [PubMed] [Google Scholar]
- 36. Triantafilou M., Gamper F. G., Haston R. M., Mouratis M. A., Morath S., Hartung T., Triantafilou K. (2006) Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 281, 31002–31011 [DOI] [PubMed] [Google Scholar]
- 37. Yasui N., Kitago Y., Beppu A., Kohno T., Morishita S., Gomi H., Nagae M., Hattori M., Takagi J. (2011) Functional importance of covalent homodimer of Reelin protein linked via its central region. J. Biol. Chem. 286, 35247–35256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ballif B. A., Arnaud L., Arthur W. T., Guris D., Imamoto A., Cooper J. A. (2004) Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr. Biol. 14, 606–610 [DOI] [PubMed] [Google Scholar]
- 39. Ballif B.A., Arnaud L., Cooper J. A. (2003) Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res. Mol. Brain Res. 117, 152–159 [DOI] [PubMed] [Google Scholar]
- 40. Arnaud L., Ballif B. A., Cooper J. A. (2003) Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol. Cell. Biol. 23, 9293–9302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheng Y. C., Chen T. A., Chen C. Y., Liang C. M., Liang S. M. (2012) 3′ poly-G-tailed ODNs inhibit F-spondin to induce cell death and neurite retraction in rat embryonic neurons. Mol. Neurobiol. 45, 536–549 [DOI] [PubMed] [Google Scholar]
- 42. Cheng Y. C., Liang C. M., Chen Y. P., Tsai I. H., Kuo C. C., Liang S. M. (2009) F-spondin plays a critical role in murine neuroblastoma survival by maintaining IL-6 expression. J. Neurochem. 110, 947–955 [DOI] [PubMed] [Google Scholar]
- 43. Strasser V., Fasching D., Hauser C., Mayer H., Bock H. H., Hiesberger T., Herz J., Weeber E. J., Sweatt J. D., Pramatarova A., Howell B., Schneider W. J., Nimpf J. (2004) Receptor clustering is involved in Reelin signaling. Mol. Cell. Biol. 24, 1378–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tamboli I. Y., Heo D., Rebeck G. W. (2014) Extracellular proteolysis of apolipoprotein E (apoE) by secreted serine neuronal protease. PLoS ONE 9, e93120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brandes C., Kahr L., Stockinger W., Hiesberger T., Schneider W. J., Nimpf J. (2001) Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding Reelin but not α 2-macroglobulin. J. Biol. Chem. 276, 22160–22169 [DOI] [PubMed] [Google Scholar]
- 46. May P., Bock H. H., Nimpf J., Herz J. (2003) Differential glycosylation regulates processing of lipoprotein receptors by γ-secretase. J. Biol. Chem. 278, 37386–37392 [DOI] [PubMed] [Google Scholar]
- 47. Chadwick W., Brenneman R., Martin B., Maudsley S. (2010) Complex and multidimensional lipid raft alterations in a murine model of Alzheimer's disease. Int. J. Alzheimers Dis. 2010, 604792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levenson J. M., Qiu S., Weeber E. J. (2008) The role of Reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene. Biochim. Biophys. Acta 1779, 422–431 [DOI] [PubMed] [Google Scholar]
- 49. Ruiz J., Kouiavskaia D., Migliorini M., Robinson S., Saenko E. L., Gorlatova N., Li D., Lawrence D., Hyman B. T., Weisgraber K. H., Strickland D. K. (2005) The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J. Lipid Res. 46, 1721–1731 [DOI] [PubMed] [Google Scholar]
- 50. Hoe H. S., Lee K. J., Carney R. S., Lee J., Markova A., Lee J. Y., Howell B. W., Hyman B. T., Pak D. T., Bu G., Rebeck G. W. (2009) Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J. Neurosci. 29, 7459–7473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rice H. C., Young-Pearse T. L., Selkoe D. J. (2013) Systematic evaluation of candidate ligands regulating ectodomain shedding of amyloid precursor protein. Biochemistry 52, 3264–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duit S., Mayer H., Blake S. M., Schneider W. J., Nimpf J. (2010) Differential functions of ApoER2 and very low density lipoprotein receptor in Reelin signaling depend on differential sorting of the receptors. J. Biol. Chem. 285, 4896–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bouché E., Romero-Ortega M. I., Henkemeyer M., Catchpole T., Leemhuis J., Frotscher M., May P., Herz J., Bock H. H. (2013) Reelin induces EphB activation. Cell Res. 23, 473–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bock H. H., Herz J. (2003) Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13, 18–26 [DOI] [PubMed] [Google Scholar]
- 55. Hoe H. S., Minami S. S., Makarova A., Lee J., Hyman B. T., Matsuoka Y., Rebeck G. W. (2008) Fyn modulation of Dab1 effects on amyloid precursor protein and ApoE receptor 2 processing. J. Biol. Chem. 283, 6288–6299 [DOI] [PubMed] [Google Scholar]
- 56. Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. (1992) Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258, 1903–1910 [DOI] [PubMed] [Google Scholar]
- 57. Tsujio I., Tanaka T., Kudo T., Nishikawa T., Shinozaki K., Grundke-Iqbal I., Iqbal K., Takeda M. (2000) Inactivation of glycogen synthase kinase-3 by protein kinase C δ: implications for regulation of Tau phosphorylation. FEBS Lett. 469, 111–117 [DOI] [PubMed] [Google Scholar]
- 58. Tanaka T., Tsujio I., Nishikawa T., Shinosaki K., Kudo T., Takeda M. (2000) Significance of Tau phosphorylation and protein kinase regulation in the pathogenesis of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 14, S18–24 [DOI] [PubMed] [Google Scholar]
- 59. Liu S. J., Zhang A. H., Li H. L., Wang Q., Deng H. M., Netzer W. J., Xu H., Wang J. Z. (2003) Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of Tau and impairment of spatial memory. J. Neurochem. 87, 1333–1344 [DOI] [PubMed] [Google Scholar]
- 60. Hiesberger T., Trommsdorff M., Howell B. W., Goffinet A., Mumby M. C., Cooper J. A., Herz J. (1999) Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates Tau phosphorylation. Neuron 24, 481–489 [DOI] [PubMed] [Google Scholar]
- 61. Schamel W. W., Alarcón B. (2013) Organization of the resting TCR in nanoscale oligomers. Immunol. Rev., 251, 13–20 [DOI] [PubMed] [Google Scholar]
- 62. Kumar R., Ferez M., Swamy M., Arechaga I., Rejas M. T., Valpuesta J. M., Schamel W. W., Alarcon B., van Santen H. M. (2011) Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity 35, 375–387 [DOI] [PubMed] [Google Scholar]
- 63. Manz B. N., Groves J. T. (2010) Spatial organization and signal transduction at intercellular junctions. Nat. Rev. Mol. Cell Biol. 11, 342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Manz B. N., Jackson B. L., Petit R. S., Dustin M. L., Groves J. (2011) T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. Proc. Natl. Acad. Sci. U.S.A. 108, 9089–9094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mossman K. D., Campi G., Groves J. T., Dustin M. L. (2005) Altered TCR signaling from geometrically repatterned immunological synapses. Science 310, 1191–1193 [DOI] [PubMed] [Google Scholar]
- 66. Bustos F. J., Varela-Nallar L., Campos M., Henriquez B., Phillips M., Opazo C., Aguayo L. G., Montecino M., Constantine-Paton M., Inestrosa N. C., van Zundert B. (2014) PSD95 suppresses dendritic arbor development in mature hippocampal neurons by occluding the clustering of NR2B-NMDA receptors. PloS ONE 9, e94037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ketchum C., Miller H., Song W., Upadhyaya A. (2014) Ligand mobility regulates B cell receptor clustering and signaling activation. Biophys. J. 106, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hsu C. J., Hsieh W. T., Waldman A., Clarke F., Huseby E. S., Burkhardt J. K., Baumgart T. (2012) Ligand mobility modulates immunological synapse formation and T cell activation. PloS ONE 7, e32398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Caré B. R., Soula H. A. (2013) Receptor clustering affects signal transduction at the membrane level in the reaction-limited regime. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 87, 012720 [DOI] [PubMed] [Google Scholar]
- 70. Kozer N., Barua D., Orchard S., Nice E. C., Burgess A. W., Hlavacek W. S., Clayton A. H. (2013) Exploring higher-order EGFR oligomerisation and phosphorylation: a combined experimental and theoretical approach. Mol. Biosyst. 9, 1849–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arneja A., Johnson H., Gabrovsek L., Lauffenburger D. A., White F. M. (2014) Qualitatively different T cell phenotypic responses to IL-2 versus IL-15 are unified by identical dependences on receptor signal strength and duration. J. Immunol. 192, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kozer N., Barua D., Henderson C., Nice E. C., Burgess A. W., Hlavacek W. S., Clayton A. H. (2014) Recruitment of the adaptor protein Grb2 to EGFR tetramers. Biochemistry 53, 2594–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lohmüller T., Xu Q., Groves J. T. (2013) Nanoscale obstacle arrays frustrate transport of EphA2-Ephrin-A1 clusters in cancer cell lines. Nano Lett. 13, 3059–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Head B. P., Patel H. H., Insel P. A. (2014) Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta 1838, 532–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kornau H. C., Schenker L. T., Kennedy M. B., Seeburg P. H. (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269, 1737–1740 [DOI] [PubMed] [Google Scholar]
- 76. Kim E., Niethammer M., Rothschild A., Jan Y. N., Sheng M. (1995) Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature 378, 85–88 [DOI] [PubMed] [Google Scholar]
- 77. Dieckmann M., Dietrich M. F., Herz J. (2010) Lipoprotein receptors: an evolutionarily ancient multifunctional receptor family. Biol. Chem. 391, 1341–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Young-Pearse T. L., Bai J., Chang R., Zheng J. B., LoTurco J. J., Selkoe D. J. (2007) A critical function for β-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J. Neurosci. 27, 14459–14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rice H. C., Townsend M., Bai J., Suth S., Cavanaugh W., Selkoe D. J., Young-Pearse T. L. (2012) Pancortins interact with amyloid precursor protein and modulate cortical cell migration. Development 139, 3986–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rogers J. T., Rusiana I., Trotter J., Zhao L., Donaldson E., Pak D. T., Babus L. W., Peters M., Banko J. L., Chavis P., Rebeck G. W., Hoe H. S., Weeber E. J. (2011) Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn. Mem. 18, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li X., Kypreos K., Zanni E. E., Zannis V. (2003) Domains of apoE required for binding to apoE receptor 2 and to phospholipids: implications for the functions of apoE in the brain. Biochemistry 42, 10406–10417 [DOI] [PubMed] [Google Scholar]
- 82. He X., Cooley K., Chung C. H., Dashti N., Tang J. (2007) Apolipoprotein receptor 2 and X11 α/β mediate apolipoprotein E-induced endocytosis of amyloid-β precursor protein and β-secretase, leading to amyloid-β production. J. Neurosci. 27, 4052–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Laffont I., Takahashi M., Shibukawa Y., Honke K., Shuvaev V. V., Siest G., Visvikis S., Taniguchi N. (2002) Apolipoprotein E activates Akt pathway in neuro-2a in an isoform-specific manner. Biochem. Biophys. Res. Commun. 292, 83–87 [DOI] [PubMed] [Google Scholar]
- 84. Hoe H. S., Harris D. C., Rebeck G. W. (2005) Multiple pathways of apolipoprotein E signaling in primary neurons. J. Neurochem. 93, 145–155 [DOI] [PubMed] [Google Scholar]
- 85. Hoe H. S., Pocivavsek A., Dai H., Chakraborty G., Harris D. C., Rebeck G. W. (2006) Effects of apoE on neuronal signaling and APP processing in rodent brain. Brain Res. 1112, 70–79 [DOI] [PubMed] [Google Scholar]