Background: Vipp1 was previously thought to be essential for viability and biogenesis of thylakoid membranes.
Results: A vipp1 null mutant of cyanobacterium Synechococcus sp. PCC 7002 is viable and assembles thylakoid membranes but lacks Photosystem I.
Conclusion: Vipp1 is not essential but is required for biogenesis of Photosystem I.
Significance: Normal thylakoid biogenesis and structure requires Photosystem I but not Vipp1.
Keywords: Cyanobacteria, Electron Microscopy (EM), Membrane Biogenesis, Photosynthesis, Protein Translocation, Photosystem I
Abstract
The biogenesis of thylakoid membranes in cyanobacteria is presently not well understood, but the vipp1 gene product has been suggested to play an important role in this process. Previous studies in Synechocystis sp. PCC 6803 reported that vipp1 (sll0617) was essential. By constructing a fully segregated null mutant in vipp1 (SynPCC7002_A0294) in Synechococcus sp. PCC 7002, we show that Vipp1 is not essential. Spectroscopic studies revealed that Photosystem I (PS I) was below detection limits in the vipp1 mutant, but Photosystem II (PS II) was still assembled and was active. Thylakoid membranes were still observed in vipp1 mutant cells and resembled those in a psaAB mutant that completely lacks PS I. When the vipp1 mutation was complemented with the orthologous vipp1 gene from Synechocystis sp. PCC 6803 that was expressed from the strong PcpcBA promoter, PS I content and activities were restored to normal levels, and cells again produced thylakoids that were indistinguishable from those of wild type. Transcription profiling showed that psaAB transcripts were lower in abundance in the vipp1 mutant. However, when the yfp gene was expressed from the PpsaAB promoter in the presence and the absence of Vipp1, no difference in YFP expression was observed, which shows that Vipp1 is not a transcription factor for the psaAB genes. This study shows that thylakoids are still produced in the absence of Vipp1 and that normal thylakoid biogenesis in Synechococcus sp. PCC 7002 requires expression and biogenesis of PS I, which in turn requires Vipp1.
Introduction
Cyanobacteria are considered to be the first oxygen-evolving photolithoautotrophs on Earth. They may have evolved as early as 3.5 billion years ago and are thought to be responsible for the oxygenation of the atmosphere, which began ∼2.5 billion years ago (1). In cyanobacteria, photosynthesis antenna pigments capture solar energy, which is subsequently transformed into chemical energy using two reaction centers, Photosystem (PS)2 I and PS II, and an electron transport chain that connects them (2). Water is oxidized to O2 during this process, and atmospheric CO2 is reduced to cellular biomass and carbon storage compounds, such as glycogen, for longer term energy conservation. In cyanobacteria and in algal and plant chloroplasts, the photosynthetic electron transport chain is localized on intracytoplasmic membranes that form the thylakoid membrane network.
Targeting of proteins into and across the thylakoid membranes has been studied and is believed to occur through several pathways, but little is known about the origin of the thylakoid membrane system or how lipids are synthesized, transported, and inserted into this membrane system (3). Moreover, the relationship between the cytoplasmic and thylakoid membranes in cyanobacteria is still very poorly understood. Some researchers propose that these systems are interconnected, whereas others maintain that they are not, and there are arguments in favor of both viewpoints. There is general agreement that the biogenesis of thylakoid membranes is a complex, multidimensional process. During this process, lipids, proteins, and pigments, as well as other cofactors, must be synthesized, transported, assembled, and inserted into these membranes, but few mechanistic details are available.
Several genetic studies have implicated the product of the vipp1 (vesicle-inducing protein in plastids 1) gene as participating in the process of thylakoid biogenesis (4, 5). Vipp1 was first described as a chloroplast-localized protein in Pisum sativum, and further analyses showed that Vipp1 was located on both the inner envelope membrane and the thylakoids (5). This unique localization of Vipp1 on these two membranes led to the presumption that Vipp1 might be involved in the assembly of the thylakoid membrane system (5). This presumption was supported by the characterization of a mutant of Arabidopsis thaliana, in which the expression of the vipp1 gene was strongly reduced (4). However, a fully segregated null mutant for vipp1 could not be produced, and thus the product of vipp1 was believed to be essential for viability (4). The mutant plants expressed only about 20% of the Vipp1 protein levels that occur in wild type under normal growth conditions, and these knockdown mutants were incapable of photoautotrophic growth on soil (4).
Genes similar to vipp1 are also found in most cyanobacteria (6–8). Recently, attempts have been made to construct vipp1 mutants of Synechocystis sp. PCC 6803, but although the level of Vipp1 could be lowered, in none of these studies could null mutations of the vipp1 gene be fully segregated. These Synechocystis sp. PCC 6803 merodiploids had a phenotype similar to that of the knockdown strains of A. thaliana, and they exhibited a comparable loss of thylakoid membrane content and structure and also had reduced photosynthetic activity (6, 9). Thus, it was suggested that Vipp1 is also essential in cyanobacteria, because it apparently plays an essential role in thylakoid membrane biogenesis. However, because null alleles of vipp1 never fully segregated in these Synechocystis sp. PCC 6803 strains, the results obtained from the characterization of the merodiploid strains were inconclusive and must be interpreted cautiously.
By using an indirect route for the construction of a vipp1 mutant in the cyanobacterium Synechococcus sp. PCC 7002, we show here that a vipp1 null mutant can be constructed and that the fully segregated null mutant is viable. This mutant strain could not grow photoautotrophically, but it could be grown photoheterotrophically when supplied with glycerol under very low irradiance conditions. When this vipp1 mutant was complemented with the vipp1 gene from Synechocystis sp. PCC 6803 expressed from the strong PcpcBA promoter, the resulting strain regained the ability to grow photoautotrophically and regained all other phenotypic properties of the wild type. Characterization of these strains showed that Vipp1 is required for biogenesis of PS I and that PS I is required for the biogenesis of “normal” thylakoid membranes in Synechococcus sp. PCC 7002.
EXPERIMENTAL PROCEDURES
Strains, Culture Conditions, and Transformation Procedure
The wild-type strain of Synechococcus sp. PCC 7002 and the vipp1 mutant strain complemented with the vipp1 gene from Synechocystis sp. PCC 6803 (see below) were grown in liquid A+ medium under standard conditions (10): at 38 °C with an irradiance of 250 μmol photons m−2 s−1 provided by cool white fluorescent lights and with sparging with 1% (v/v) CO2 in air. Mutant strains were grown under low irradiance conditions (∼10 μmol photons m−2 s−1), and the A+ medium was supplemented with 20 mm glycerol, which served as the main carbon and energy source. For mutant strains, appropriate antibiotics were added as required at the following concentrations: spectinomycin (50 μg/ml); gentamycin (20 μg/ml); kanamycin (100 μg/ml); and erythromycin (20 μg/ml). Transformation of Synechococcus sp. PCC 7002 was performed as described previously (11).
Generation of vipp1 Deletion Mutant and a trans-Complemented Strain
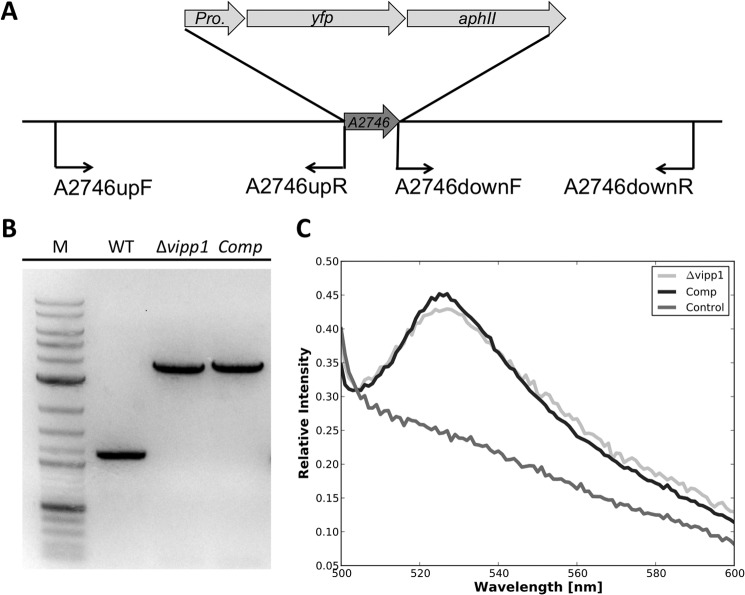
In agreement with the results of others and despite many attempts, direct deletion of vipp1 was never successful. Thus, a different strategy was employed. First, a PS I-less strain of Synechococcus sp. PCC 7002 was constructed by deleting the psaAB genes (12). The vipp1 gene in this strain was then inactivated by a homologous recombination strategy by deleting a part of the gene (bp 287–373 of the coding sequence); the deleted region was replaced with a DNA fragment encoding aacC1, which confers resistance to gentamycin (Fig. 1A). The PCR primers used to amplify the flanking sequence regions for this approach are given in Table 1. The ΔpsaAB::aadA mutant strain was rescued at the psaAB locus by transformation with plasmid pAQEEmr80 as described previously (13) to produce a PsaAB+ Vipp1− (vipp1::aacC1) mutant strain. Full segregation of the wild-type and vipp1::aacC1 alleles was verified by PCR analyses and DNA sequencing of amplicons derived from the DNA of the vipp1::aacC1 null mutant strain.
FIGURE 1.
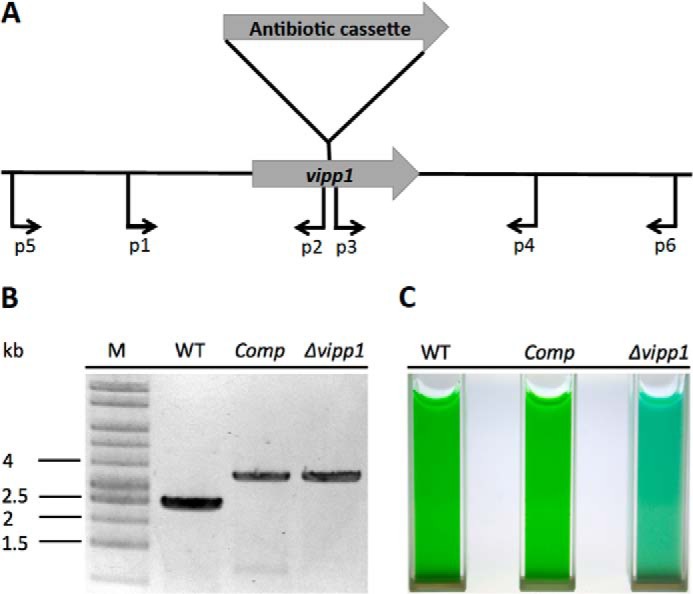
Construction and verification of vipp1 mutant as well as a trans-complemented strain. A, scheme showing the construction of a vipp1 mutant by homologous recombination by using primer set p1 and p2 to amplify the upstream region and primer set p3 and p4 to amplify the downstream region. An antibiotic resistance cassette was ligated into restriction sites added to the appropriate ends of the flanking sequences (see “Experimental Procedures” for other details). B, results of agarose gel electrophoresis of amplicons produced using primers p5 and p6, showing the fully segregated interruption mutant of vipp1. The template DNAs were isolated from the wild type (WT), the trans-complemented vipp1 mutant (Comp), and the vipp1 mutant strain (Δvipp1). The results clearly showed that the wild-type vipp1 and interrupted vipp1::aacC1 alleles had segregated completely in the vipp1 mutant and that the vipp1 gene was still mutated in the Comp strain. Lane M, DNA size markers. C, cultures of equivalent cell density (OD730 nm = 1.0) for WT, Comp, and Δvipp1.
TABLE 1.
Oligonucleotide primers used in this study
| Name | Sequence (5′–3′) |
|---|---|
| p1 | CATTGACTACCGAATACACCAAGGTGCAA |
| p2 | GGTGTCCGCAAAGCTTTTTTTCCGGACTAG |
| p3 | GAAAACCTCCTTGCTCTAGAAAGCAAAATC |
| p4 | AGACAGTTCCTTACGTTAACAGTTTTCGG |
| p5 | GCTAACTCGGCATCATTGGTATTCGTCAGC |
| p6 | TCAGTATCGGGATCAACGGCAACCCCATAA |
| ExF | GGTCGTCATATGGGATTATTTGACCGTTTA |
| ExR | GCAGCCGGATCCTTACAGATTATTTAACCG |
| psaABF | ATTCTGCCATGGCCTTTTCGGGTTAAACCT |
| psaABR | GACTGCCATATGAGGATTCTCCTCTCTAGA |
| chlLNF | CCTTTTGAATTCTTCAACATGACGGCAACG |
| chlLNR | TCATTGCCATGGGATTGCGTGCTCCTAAAA |
| A2746upF | TGCGACTCGTTTTGACATTCCGCCAAAACA |
| A2746upR | ACCATTGTCGACACCAGAGGAGACTTTAAA |
| A2746downF | CTTCGGGAATTCAGCAACGTTTTGAGGGAA |
| A2746downR | CCCCGCAAAATTTCCTTCGCGCCGCTTGTA |
For the construction of a strain in which the vipp1::aacC1 mutation was complemented in trans, the orthologous vipp1 gene (open reading frame sll0617) from Synechocystis sp. PCC 6803 was amplified by PCR using Phusion DNA polymerase (New England Biolabs) and was introduced into plasmid pAQ1Ex-PcpcBA using primer set ExR and ExF (Table 1) as described previously (14). The resulting plasmid, pAQ1Ex-PcpcBA::sll0617, was transformed into the vipp1::aacC1 mutant to generate a strain in which the vipp1 null mutation was heterologously complemented by expression of the sll0617 product from the strong cpcBA (phycocyanin) promoter (PcpcBA; also derived from Synechocystis sp. PCC 6803; see Ref. 14). The resulting complemented mutant strain (vipp1::aacC1 pAQ1Ex-PcpcBA::sll0617) was repeatedly streaked and grown photoautotrophically in A+ medium under standard irradiance conditions (i.e. 250 μmol of photons m−2 s−1).
Pigment Analysis
Chlorophyll (Chl) a, carotenoid, and phycobiliprotein (PBP) concentrations were measured as described (15). Pigment concentrations were compared on the basis of equal cell numbers, which were determined from the optical density at 730 nm (OD730 nm; 1.0 OD730 nm = 1.0 ± 0.2 × 108 cells ml−1; see Ref. 15). These measurements were made with cells that had been harvested by centrifugation from cultures grown to late exponential growth phase (OD730 nm = ∼0.6 to 0.7 ml−1) and resuspended in 50 mm Tris-HCl, pH 7.0 buffer. Chl a and carotenoids were extracted from cells with 100% methanol, and their concentrations were determined as described (15). To determine relative PBP levels, cells were incubated at 65 °C for 8 min, and a difference spectrum with untreated control cells was recorded as described previously (15).
Polyacrylamide Gel Electrophoresis and Immunoblotting
Polyacrylamide gel electrophoresis (PAGE) in the presence of sodium dodecyl sulfate (SDS) was performed as described (12) on 15% (w/v) polyacrylamide slab gels (30.0:0.8 acrylamide/bisacrylamide). Equal amounts of cells (100 μl of OD730 nm = 4) were centrifuged, and the cell pellets were collected and resuspended in 40 μl of BugBusterTM protein extraction reagent (Novagen, Madison, WI). Cells were disrupted after 20 min of incubation at room temperature. Aliquots (20 μl) of the whole cell extract were loaded to each lane. Rabbit antibodies against PsaA, PsaB, and Vipp1 were purchased from Agrisera (Vännäs, Sweden). Immunoblotting was performed as described previously (13).
Oxygen Evolution Assay
Whole-chain oxygen evolution as well as respiratory oxygen uptake in wild-type and mutant cells were measured using a Clark-type electrode as described (16). The temperature of the measuring chamber was maintained at 38 °C by a circulating water bath. Cells were washed once with fresh A+ medium and adjusted to equal final cell concentration on the basis of OD730 nm. For oxygen evolution measurements, 10 mm NaHCO3 was added to the cell suspension. Respiration rates were obtained under the same conditions but without illuminating the cells.
Fluorescence Emission Spectra at 77 K
Fluorescence emission spectra at 77 K were measured with an SLM 8000C spectrofluorometer, modified by OLIS, Inc., as described (12). Cells in exponential growth phase (OD730 nm = ∼0.6–0.7 ml−1) were collected and resuspended in 50 mm Tris-HCl, pH 7.0, buffer. Glycerol was added to a final concentration of 60% (v/v). Cells were adjusted to a concentration of ∼0.5 OD730 nm ml−1 and quickly frozen in liquid nitrogen. The excitation wavelength for Chl excitation was 440 nm. A long pass filter (transmitting at 600 nm) was used at the inlet of the emission monochromator to minimize contributions from scattered light.
Whole-cell P700 Activity Measurements
Cells (final OD730 nm = ∼0.5) were collected and resuspended in 50 mm Tris-HCl, pH 8.3, buffer. The absorbance change at 700 nm was monitored by a model JTS-10 LED pump-probe spectrometer (Bio-Logic). A high power red LED (680 ± 50 nm) provided the actinic illumination. A high power white LED, filtered through a 700-nm interference filter (Edmund Optics, Inc.), provided the measuring pulses.
Transmission Electron Microscopy
Thylakoid membranes from wild-type and mutant cells of Synechococcus sp. PCC 7002 were visualized by transmission electron microscopy of thin sections as described (17). The ultrathin sections were viewed with a JEM-1200 transmission electron microscope (JEOL Ltd.). Images were captured using TIETZ digital image capture software.
Lipid Body Detection
Nile Red staining was performed to detect lipid bodies using a reported method with minor modifications (18). Cells were harvested, washed, and resuspended in 50 mm Tris-HCl, pH 8.0, buffer. Nile Red stock solution (1 μl of a 1 mg ml−1 stock solution in dimethyl sulfoxide) was added to an aliquot of washed cells (100 μl). After staining for 10 min, lipid bodies inside cells were visualized by fluorescence using a FluoView FV1000 confocal microscope (Olympus, Center Valley, PA) in scanning mode. The excitation wavelength was 488 nm, and an emission wavelength of 500–600 nm was selected for all experiments.
Total mRNA Profiling
Transcriptome profiling was performed as described (10). The vipp1 mutant strain and Comp strain were first adapted to low irradiance (∼10 μmol photons m−2 s−1) on medium A+ supplemented with glycerol (20 mm). Cells were reinoculated and harvested at OD730 nm = 0.7. Total RNA was then extracted as described (18). The construction of cDNA libraries and sequencing (SOLiDTM) were performed in the Genomic Core Facility at Pennsylvania State University. Mapping against the Synechococcus sp. PCC 7002 genome was performed using the BWA software package (19). The resulting alignment files were further analyzed with self-developed scripts to extract expression levels for each gene as described previously (10). The RNA sequencing data were deposited in the NCBI Sequence Read Archive under accession number SRP035555.
Yellow Fluorescent Protein (YFP) Detection
To determine whether Vipp1 plays a direct regulatory role in the transcription of the psaAB and chlLN operons, promoter regions for psaAB and chlLN were amplified and transcriptionally fused to yfp as well as aphII (conferring kanamycin resistance) separately. The primers used to amplify these promoter regions for psaAB (psaABF and psaABR) and chlLN (chlLNF and chlLNR) are listed in Table 1. The fused constructs were then used to replace open reading frame SYNPCC7002_A2746 (Fig. 2A) in both the vipp1 mutant and the complemented strain by homologous recombination as described (11). Primers used to amplify the upstream and downstream flanking regions of SYNPCC7002_A2746 are listed in Table 1 (A2746upF, A2746upR, A2746downF, and A2746downR). Transcription profiling analyses under many different conditions showed that ORF SYNPCC7002_A2746 produces few if any transcripts under most growth conditions (10, 20, 21). Additionally, a deletion mutant of SYNPCC7002_A2746 has been constructed (Fig. 2B). No detectable growth phenotype was observed for this mutant strain compared with wild type strain, and thus this gene site was used as a neutral site (Fig. 2C). Full segregation of the vipp1 mutant strain and the complemented strain containing the promoter fusions to yfp was verified using primer set A2746upF and A2746downR. The products were sequenced to verify that no inadvertent changes had occurred during strain construction. These strains were grown under low irradiance (∼10 μmol photons m−2 s−1) on medium A+ supplemented with glycerol (20 mm). Cells were harvested by centrifugation from exponential growth phase (OD730 nm = ∼0.6–0.7 ml−1), resuspended in A+ medium containing 20 mm glycerol, and adjusted to the same OD730 nm = 0.5 ml−1. YFP fluorescence in these cells was detected with an SLM 8000C spectrofluorometer, modified by OLIS, Inc. (Bogart, GA). The excitation wavelength was 488 nm, and emission spectra were recorded from 500 to 600 nm.
FIGURE 2.
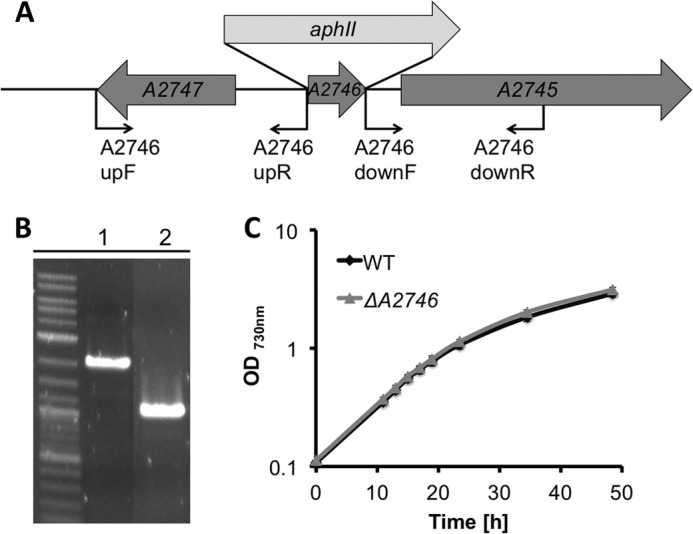
Construction and verification of a neutral site platform for Synechococcus sp. PCC 7002. A, diagram showing the construction of a SynPCC7002_A2746 mutant and positions of oligonucleotide primers (see Table 1). B, agarose gel electrophoresis of amplicons produced by polymerase chain reaction, demonstrating complete segregation of alleles for SynPCC7002_A2746::aphII and SynPCC7002_A2746, using primer set A2746upF and A2746downR. The template DNAs were isolated from the SynPCC7002_A2746 mutant (lane 1) and wild type (lane 2). C, comparison of growth rates for Synechococcus sp. PCC 7002 WT and a neutral site mutant strain constructed in open reading frame SynPCC7002_A2746 (ΔA2746). The growth rates were indistinguishable within experimental error. The data are the average of three biological replicates.
RESULTS
Generation of vipp1 Mutant
Genetic manipulations to generate a vipp1 null mutant, as well as a strain (denoted Comp) in which the resulting vipp1 mutation was complemented in trans, are described under “Experimental Procedures.” For construction of a vipp1 null mutant, Synechococcus sp. PCC 7002 was first adapted to grow on glycerol as a carbon/energy source, which allows one to construct mutants lacking PS I and/or PS II (12). Some observations made with vipp1 merodiploid strains suggested that these merodiploid strains had greatly reduced levels of PS I (data not shown, but see below), and thus a strain in which the psaAB genes had been deleted was used for inactivation of the vipp1 gene. After several attempts, a fully segregated vipp1::aacC1 strain, which was verified by PCR analysis as shown in Fig. 1B, was obtained. Although the vipp1 gene was believed to be essential in cyanobacteria (6, 9), the results in Fig. 1B show that the vipp1 product is not essential in Synechococcus sp. PCC 7002 and that a null mutant is viable. Because it was much easier to obtain a fully segregated vipp1 null mutation in a PS I-less strain, it appeared likely that Vipp1 was somehow involved in the biogenesis of PS I.
Characterization of the Mutant Strain
The vipp1 mutant strain could not grow photoautotrophically, but this strain could still grow photoheterotrophically under low irradiance conditions (∼10 μmol photons m−2 s−1) when cells were supplied with 20 mm glycerol. Immunoblotting showed that no Vipp1 protein was detectable in the mutant cells (Fig. 3). Previous studies had shown that a deficiency in Vipp1 led to impairment of the biosynthesis of thylakoid membranes in plants and Synechocystis sp. PCC 6803 (4, 9). Transmission electron microscopy of thin section cells was used to examine the membrane organization in the vipp1 mutant strain. As shown in Fig. 4B, cells of the vipp1 mutant had far fewer thylakoid membranes than the wild type (Fig. 4A). However, some vestigial thylakoid membranes were still present, and this indicated that Vipp1 is not essential for the biogenesis of thylakoid membranes. It should be noted, however, that the thylakoid membranes appeared to have a much simpler organization in the vipp1 mutant and that in some cells, the thylakoid membranes appeared to be directly connected to the cytoplasmic membrane (see Fig. 4B).
FIGURE 3.

Immunoblotting of whole cell extracts of Synechococcus sp. PCC 7002 strains with antibodies to Vipp1. Antibodies to Vipp1 were used to detect Vipp1 levels in WT, Comp, Δvipp1, and a PS I-less mutant (ΔpsaAB). Equal quantities of cells were used to produce the extracts for this experiment, so the Vipp1 levels detected in this experiment can be compared semiquantitatively.
FIGURE 4.

Thylakoid membrane morphology as revealed by electron microscopy. Thin sections of Synechococcus sp. PCC 7002 cells from various strains were examined by transmission electron microscopy. A, thylakoid membranes were normally assembled in WT (A) and in Comp (C). Thylakoids were greatly reduced in number and area in the vipp1 mutant (B), and these cells closely resembled cells for a psaAB mutant (D). The arrows in the bottom right of B show thylakoids that clearly appear to be directly connected to the cytoplasmic membrane. Bars, 500 nm.
Complementation of the vipp1 Mutation in Trans
Because of the multistep procedure employed to construct the vipp1 mutant strain, it was important to demonstrate that the resulting vipp1 mutant could be complemented to rescue a wild-type phenotype. To avoid potential problems arising from homologous recombination of vipp1 alleles, the orthologous vipp1 gene (locus tag, sll0617) from Synechocystis sp. PCC 6803 was used for complementation instead of vipp1 from Synechococcus sp. PCC 7002. Vipp1 from Synechocystis sp. PCC 6803 is 54% identical and 71% similar in sequence to Vipp1 from Synechococcus sp. PCC 7002. A plasmid was constructed in which ORF sll0617 from Synechocystis sp. PCC 6803 was placed under the control of the strong PcpcBA promoter (also from Synechocystis sp. PCC 6803 (14)), and this plasmid was transformed into the vipp1 null mutant. As shown in Fig. 1B, PCR analysis using primer set p5 and p6 (Table 1) showed that the vipp1 gene was still interrupted in the resulting complemented strain, which was denoted as strain “Comp.” Immunoblotting (Fig. 3) showed that the complemented mutant strain accumulated much more Vipp1 protein than the wild type. The complemented strain could grow photoautotrophically (Fig. 1C) and was no longer sensitive to high light conditions. Analysis of thin sections by transmission electron microscopy showed that the thylakoid membranes of the Comp strain cells were indistinguishable from those in wild-type cells (Fig. 4C). These results demonstrate that vipp1 (sll0617) from Synechocystis sp. PCC 6803 can fully complement the vipp1 mutant of Synechococcus sp. PCC 7002 and could restore a wild-type phenotype (see additional results, and see “Discussion”).
Pigments and Oxygen Evolution Rate
The vipp1 mutant was noticeably bluer in color than the wild type (Fig. 1C), so we next analyzed the pigment content and oxygen evolution behavior of the vipp1 mutant and the Comp strain (Table 2). The Chl a content of the vipp1 mutant was reduced to about 10% and the total carotenoid content was reduced to about 20% of the levels in the wild type. The PBP content of the vipp1 mutant was approximately half that of the wild type (Table 2). The increased PBP content relative to the Chl a explains the bluish color of the cells. These values returned to nearly wild-type levels when the vipp1 mutation was complemented in trans. Interestingly, the Chl a and carotenoid levels in the vipp1 mutant were similar to those in a PS I-less mutant, although the PBP content of the PS I-less mutant was essentially the same as that of the wild type (Table 2).
TABLE 2.
Pigment contents of Synechococcus sp. PCC 7002 WT, Comp, ΔpsaAB, and Δvipp1
The data shown are averages and S.D. values for three biological replicates.
| WT | Comp | ΔpsaAB | Δvipp1 | |
|---|---|---|---|---|
| Chlorophyll a (μg ml−1 OD730 nm−1) | 7.15 ± 0.16 | 7.11 ± 0.22 | 0.88 ± 0.02 | 0.71 ± 0.06 |
| Carotenoids (μg ml−1 OD730 nm−1) | 2.25 ± 0.05 | 2.62 ± 0.07 | 0.68 ± 0.01 | 0.41 ± 0.04 |
| Phycobiliproteins (relative amount) | 372 ± 4 | 306 ± 8 | 365 ± 3 | 202 ± 2 |
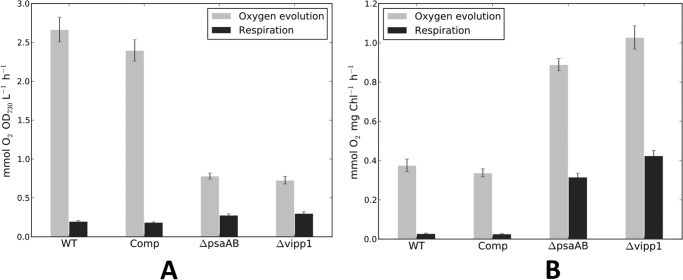
Oxygen evolution rates and respiratory oxygen uptake rates were measured for these strains (Fig. 5). The measurements were either compared on the basis of equal cell number, as determined by the optical density at 730 nm (Fig. 5A), or on the basis of the Chl a content (Fig. 5B). As shown in Fig. 5A, the vipp1 mutant evolved oxygen, but this activity rapidly declined after several min of illumination (data not shown). The initial oxygen evolution rate on the basis of Chl a was roughly 2.5-fold higher than that of the wild-type strain but was only about 25% of the wild-type rate on a per cell basis. These behaviors are very similar to those of a strain lacking PS I (12). When the vipp1 mutation was complemented in trans, the oxygen evolution and respiratory uptake rates for the resulting Comp strain were very similar to those of the wild type. Thus, PS II complexes in the vipp1 mutant and the PS I-less strain were assembled and were functional. The similar pigment contents and oxygen evolution behaviors of these two strains further suggested that the vipp1 mutant probably had fewer PS I complexes per cell.
FIGURE 5.
Oxygen evolution and respiration rates for WT, Comp, ΔpsaAB, and Δvipp1, based on equal cell numbers (based on OD730 nm) (A) or equal Chl a (B). The vipp1 mutant strain as well as the psaAB mutant strain had much higher oxygen evolution rates than the WT strain when rates were compared on the basis of Chl but much lower oxygen evolution rates when rates were compared on the basis of equal cell numbers. Note that these values were derived from the initial rates of oxygen evolution for the vipp1 and the psaAB mutant strains, because oxygen evolution rates rapidly declined for these two strains that had no PS I activity to drive the reoxidation of the plastoquinone pool. The data shown are averages values for three biological replicates, and the error bars show the standard deviation.
Low Temperature Fluorescence Emission Spectroscopy
Fig. 6 shows the low temperature (77 K) fluorescence emission spectra of various Synechococcus sp. PCC 7002 strains. When the excitation wavelength was 440 nm to excite Chl a, three major emission peaks were observed at 685, 695, and 715 nm for the wild type. The first two peaks principally arise from PS II, whereas the emission peak at 715 nm arises from PS I (13). The fluorescence emission spectrum of the vipp1 mutant shows no emission peak from PS I and only shows emission peaks associated with PS II. The emission spectrum for the vipp1 mutant after trans-complementation with sll0617 from Synechocystis sp. PCC 6803 was nearly indistinguishable from that of the wild type (Fig. 6). These data strongly implicate Vipp1 in the expression or biogenesis of PS I.
FIGURE 6.

Low temperature (77 K) fluorescence emission spectra of whole cells of WT, Comp, and Δvipp1. In the Δvipp1 mutant, PS I fluorescence emission at ∼715 nm was completely absent, but PS II was still assembled and exhibited normal fluorescence emission at 685 and 695 nm. The excitation wavelength was 440 nm.
PS I Activities in Whole Cells
The low temperature fluorescence emission spectrum of the vipp1 mutant showed that the PS I content of this strain was severely reduced. To verify that the PS I activity was similarly reduced in the vipp1 mutant, photobleaching of P700 was directly measured at 700 nm in whole cells with a pump-probe spectrophotometer as described under “Experimental Procedures.” As shown in Fig. 7, photobleaching of P700 occurred when whole cells were illuminated with actinic light, and a slight increase in photobleaching occurred over a 10-s period of illumination. The absorption change at 700 nm was fully reversible when the actinic light was switched off. No photobleaching at 700 nm was detectable for the vipp1 mutant, but the photobleaching of P700 in the trans-complemented strain was similar in magnitude and kinetics to that of the wild type. PS I complexes were isolated from the wild type and from the trans-complemented Comp strain. Time-resolved optical spectroscopy on the millisecond time scale showed that the PS I complexes from these two strains had similar extents of photobleaching and lifetimes of charge separation (data not shown). These spectroscopic studies show that the vipp1 mutant does not assemble functional PS I complexes. Heterologous complementation of vipp1 with ORF sll0617 from Synechocystis sp. PCC 6803 was sufficient to restore normal PS I biogenesis and functionality to the vipp1 mutant of Synechococcus sp. PCC 7002.
FIGURE 7.
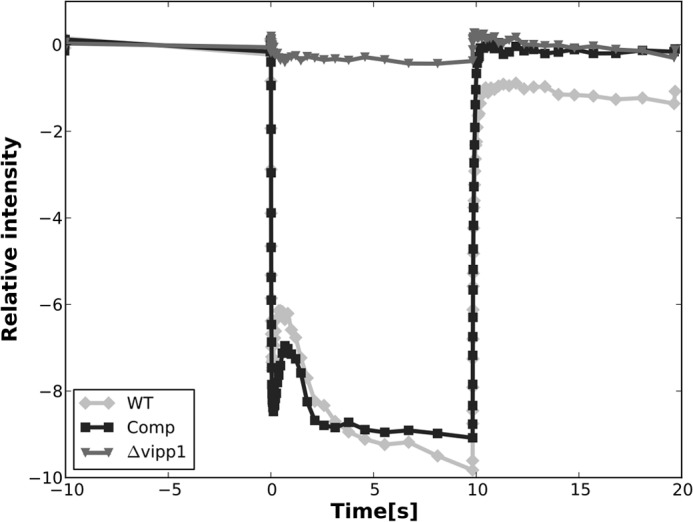
Photobleaching of P700 in whole cells of WT (light gray line), Comp (black line), and Δvipp1 (dark gray line). The PS I activity in the trans-complemented vipp1 mutant strain was almost the same as that of the WT. No P700 photobleaching activity was detected in the vipp1 mutant strain. The actinic light was turned on at 0 s and turned off after 10 s, and absorption difference was measured at 700 nm.
Immunoblotting to Detect PS I Polypeptides
Whole-cell extracts of the wild type, the vipp1 mutant strain, and the trans-complemented vipp1 mutant strain were prepared and subjected to SDS-PAGE, and the resolved proteins were transferred to membrane filters for immunoblotting. Previous studies have shown that PsaC, PsaD, and PsaE, which are water-soluble and form the stromal ridge of PS I (2, 12), do not accumulate in PS I-less mutants (12). Immunoblotting showed that PsaA, PsaB, PsaC, PsaD, and PsaE do not accumulate in the vipp1 mutant, which is consistent with all other evidence indicating that PS I is missing in the vipp1 mutant (Fig. 8). However, all of these proteins were detected in the wild type and in the Comp strain. Interestingly, the integral membrane protein, PsaL, which is responsible for trimerization of PS I (22), accumulated in the absence of PsaA and PsaB in the vipp1 mutant (Fig. 8). This observation is consistent with observations made for Synechocystis sp. PCC 6803, for which it was also found that PsaL accumulated in membranes independently of the levels of PsaA and PsaB (23).
FIGURE 8.
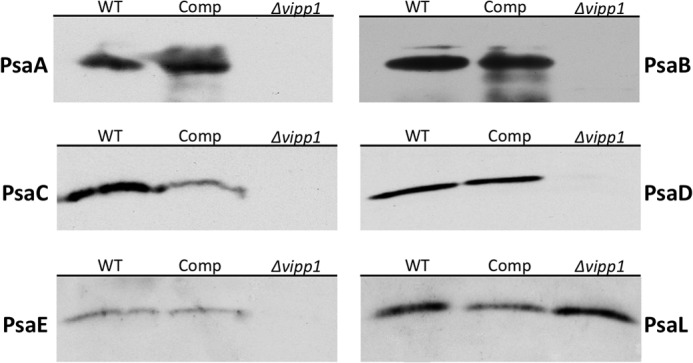
Immunoblotting of PS I subunits. Antibodies to PsaA, PsaB, PsaC, PsaD, PsaE, and PsaL were used to detect the presence of the PS I subunits in whole cell extracts of WT, Comp, and Δvipp1. PsaL was the only PS I subunit detected in the vipp1 mutant strain, but all subunits were detected in the WT control and Comp strains. Note that these immunoblots were performed with whole cell extracts of cells scraped from plates; thus, the results are only qualitative and should not be compared quantitatively.
Thylakoid Membranes in a psaAB Deletion Mutant
Although previous studies had suggested that Vipp1 was required for thylakoid membrane biogenesis, the results shown above suggested that Vipp1 is required for psaAB expression or the biogenesis of PS I. The presence of vestigial thylakoid membranes in the vipp1 mutant indicated that normal thylakoid biogenesis might require PS I assembly rather than Vipp1. A natural hypothesis arising from these observations is that a PS I-less mutant should produce vestigial thylakoids that closely resemble those in the vipp1 mutant. Fig. 4D shows images of cells of a psaAB deletion mutant of Synechococcus sp. PCC 7002 that is unable to assemble any functional PS I complexes (or subcomplexes (12)). Immunoblotting showed that the PS I-less mutant cells accumulated wild-type levels of Vipp1 (Fig. 3). Like the vipp1 mutant, cells of the psaAB deletion mutant produce vestigial thylakoids that closely resemble those of the vipp1 mutant. Therefore, these results clearly demonstrate that the capacity to produce wild-type thylakoid membranes requires the normal biogenesis of PS I rather than Vipp1.
Detection of Lipid Bodies
Previous studies in plant chloroplasts and Synechocystis sp. PCC 6803 suggested that vipp1 might play a role in membrane biogenesis and might more specifically affect the insertion of membrane lipids (24). However, the results presented here conclusively demonstrate that cyanobacterial Vipp1 plays a role in the expression or biogenesis of PS I. Nile red is a lipid-soluble fluorescent dye that can be used to detect lipids in microorganisms (18). To determine whether lipid bodies were produced in the strains constructed here, Nile red staining was performed to detect lipid bodies. The vipp1 mutant accumulated numerous lipid bodies in the cytoplasm (Fig. 9B), but lipid bodies were not observed in wild type (Fig. 9A). This result excludes the possibility that Vipp1 is involved in the biogenesis of lipid bodies, but it suggests that Vipp1 is directly or indirectly involved in the assembly of lipids into thylakoid membranes. The inability to produce normal amounts of PS I apparently interferes with lipid insertion into the thylakoid membranes, and the lipids apparently then accumulate as lipid bodies in the cytoplasm. Consistent with the results described above, the PS I-less strain also accumulated lipid bodies in the cytoplasm that resembled those in the vipp1 strain (Fig. 9D). When the ability to produce PS I was restored by trans-complementation of the vipp1 mutation, lipids no longer accumulated as lipid bodies in the cytoplasm of the Comp strain (Fig. 9C).
FIGURE 9.
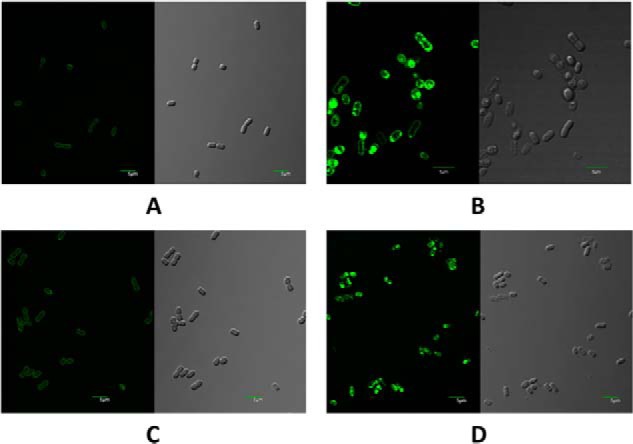
Confocal fluorescence microscopy images (left) and differential interference contrast images (right) of Synechococcus sp. PCC 7002 cells stained with Nile red. For the fluorescence images, the excitation wavelength was 488 nm, and emitted light in the wavelength range from 500 to 600 nm was detected. Scale bars, 5 μm. A, cells of wild-type Synechococcus sp. PCC 7002. B, cells of the vipp1 mutant strain. C, trans-complemented cells of the vipp1 mutant strain. D, cells of the psaAB mutant strain.
Vipp1 Is Not Required for Transcription of psaAB
In the experiments described above, we showed that PS I is not detectable in cells that lack Vipp1 and that restoration of Vipp1 by trans-complementation reverses all known phenotypic defects associated with the absence of Vipp1. Fig. 10 shows a scatter plot that compares the transcript abundances for each gene in the vipp1 deletion strain in comparison with their abundances in the Comp strain. Four genes, psaAB and chlLN, which occur in two dicistronic operons, showed significantly lower transcript abundances in the vipp1 mutant compared with the trans-complemented strain. These data suggested that Vipp1 might regulate psaAB transcript levels.
FIGURE 10.
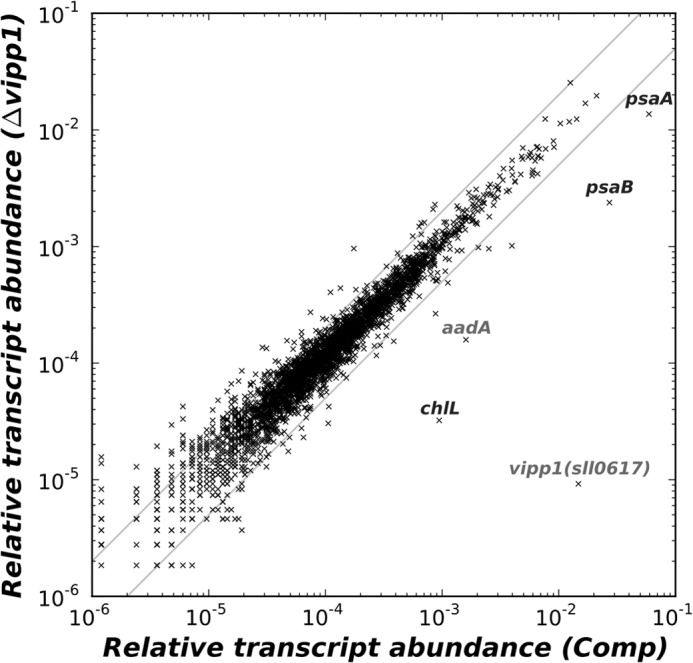
Scatter plot comparing the relative transcript abundances for mRNAs of the vipp1 mutant to those in the trans-complemented vipp1 mutant strain. Transcripts for psaA and psaB were specifically higher in the trans-complemented vipp1 strain, which also had enhanced mRNA levels for the vipp1 (sll0617) gene from Synechocystis sp. PCC 6803. Transcript levels for chlL were also more abundant in the trans-complemented strain, but nearly all other transcripts were unchanged. The gray lines indicate 2-fold changes in transcript levels.
To ascertain whether Vipp1 was acting as an activator or repressor of transcription of the psaAB operon, we introduced a promoter fusion of PpsaAB to yfp (PpsaAB::yfp) into a neutral site (SYNPCC7002_A2746) in the Synechococcus sp. PCC 7002 chromosome in the vipp1 mutant as well as the trans-complemented Comp strain (Fig. 11, A and B). The Yfp fluorescence in these two strains was equal within error (Fig. 11C). Similar results were observed for the PchlLN fused to (PchlLN::yfp; data not shown). These results show that Vipp1 is not directly involved in regulating the transcription of psaAB. However, it is possible that Vipp1 plays a role in PS I biogenesis that indirectly influences the stability of the psaAB mRNA (e.g. by influencing translation of this transcript). The presence or absence of Vipp1 in cells did not cause a change in the amount of YFP expression. Because the vipp1 mutant strain can only be grown at very low irradiance levels, it is possible that the ChlLN operon, which encodes two of the three subunits of the light-independent protochlorophyllide reductase (25), is specifically transcribed at higher levels in the Comp strain. This strain, which is derived from the vipp1 mutant, has a much greater demand for Chl a than the parental strain because of restoration of PS I. Low irradiance growth conditions might cause a limitation for Chl a biosynthesis at the level of protochlorophyllide a reduction, which typically is catalyzed by the light-dependent protochlorophyllide a reductase (26). The low light levels might lead to derepression of the light-independent protochlorophyllide a reductase, ChlNBL, in the Comp strain because of an increased demand for Chl a to assemble PS I. This would not occur in the vipp1 mutant because it does not assemble PS I and requires only 10% of the Chl found in the wild type (Table 2).
FIGURE 11.
Construction (A) and verification (B) of insertion of PpsaAB or PchlLN promoters fused to yfp into a neutral site in the chromosome of Synechococcus sp. PCC 7002 strains and relative YFP fluorescence emission in the resulting strains (C). A, scheme showing the replacement of open reading frame SynPCC7002_A2746 by yfp reporter gene fusions by homologous recombination and selection with kanamycin (aphII gene cassette confers resistance to kanamycin). Promoter regions (Pro.) for psaAB or chlLN were transcriptionally fused to the yfp gene, which was placed upstream from an aphII gene, which encodes aminoglycoside phosphotransferase II and confers resistance to kanamycin. B, agarose gel electrophoresis of PCR amplicons using primer set A2746upF and A2746downR to verify the introduction of the promoter-yfp-aphII constructions in the respective strains. Template DNAs came from the WT strain, the vipp1 mutant, and the Comp. C, fluorescence emission spectra showing YFP emission in the constructed reporter strains. The control spectrum shows fluorescence emission from the vipp1 strain, which does not contain the yfp gene. Very similar levels of YFP fluorescence emission were detected in the vipp1 and Comp strains, which carried the yfp gene fused to the PpsaAB promoter. These data indicate that Vipp1 does not modify transcription from the PpsaAB promoter.
DISCUSSION
The exact role of Vipp1 has been a longstanding mystery that has been investigated in several organisms over about 2 decades. However, its function is still unclear. A vipp1 merodiploid knockdown strain of Synechocystis sp. PCC 6803 had reduced levels of PS I (27) and fewer thylakoid membranes. However, the authors of that study concluded that the reduction in thylakoid membrane content affected the ratio of PS I and PS II. Other studies suggested that Vipp1 was essential because it played a major role in the biogenesis of thylakoid membranes (4, 24). However, based on the results obtained in this study, precisely the opposite is the case in Synechococcus sp. PCC 7002. Vipp1 is clearly required for the biogenesis of PS I, and the absence of PS I leads to a greatly decreased level of thylakoid membranes in Synechococcus sp. PCC 7002 cells.
In contrast to previous studies in Synechocystis sp. PCC 6803, a fully segregated null mutation was constructed in the vipp1 gene of Synechococcus sp. PCC 7002 in this study, and we were able to complement this mutant heterologously in trans with the Synechocystis sp. PCC6803 vipp1 gene to restore the wild-type phenotype. These experiments clearly demonstrate that the function of Vipp1 is highly conserved, but Vipp1 is clearly not required for viability in the cyanobacterium Synechococcus sp. PCC 7002. Since 1996, the genomes of many cyanobacteria have been sequenced (e.g. see Refs. 28 and 29). Comparative analysis of these genomic data shows that some cyanobacteria, such as certain Prochlorococcus species, lack the vipp1 gene. However, these cyanobacteria clearly still assemble thylakoids and produce functional PS I complexes (30). Thus, Vipp1 is not required for the formation of thylakoid membranes in Prochlorococcus spp.; nor is it essential in all cases for PS I biogenesis. These observations suggest that cyanobacteria must have redundant mechanisms to assemble PS I, probably PS II, and thylakoids. This is almost certainly one of the reasons why it has been so difficult to establish mechanistic details for the biogenesis of PS I and PS II.
It has been reported that reduced expression of Vipp1 in Synechocystis sp. PCC 6803 resulted in a decreased PS I content and an altered PS I/PS II ratio, reduced thylakoid content, and a reduced percentage of trimeric versus monomeric PS I complexes (27). Mutants lacking phosphatidylglycerol (PG) synthase, encoded by the pgsA gene, in Synechocystis sp. PCC6803 are not viable unless PG is added to the growth medium (23). When cells of a pgsA mutant were deprived of PG over many days, a phenotype similar to that for Vipp1 depletion was noted with respect to PS I complexes. Depletion of PG not only led to decreased PS I activity but also caused a depletion of PS I trimers and an increase in PS I monomers. However, PsaL was still inserted into membranes and could reassemble trimeric complexes in the absence of protein synthesis when PG was added back to cells (23). The crystal structure of trimeric PS I complexes from Thermosynechococcus elongatus showed that three PG molecules are tightly associated with each monomeric PS I complex and thus may play a role in PS I biogenesis (2). An A. thaliana mutant strain unable to synthesize PG was no longer able to grow photoautotrophically and had a severe reduction in Chl and thylakoid membranes (31), which could potentially be due at least in part to a loss of functional PS I complexes. Collectively, these results suggest that a relationship exists among Vipp1, PG biosynthesis, and biogenesis of PS I complexes and that collectively Vipp1, PG synthesis, and PS I biogenesis strongly influence thylakoid membrane structure and biogenesis.
Analysis of the trans-complemented vipp1 mutant and the psaAB deletion mutant strains further indicated that Vipp1 is more likely involved primarily in the biogenesis of PS I complexes rather than thylakoid membrane biogenesis, because PS I complexes and activity are restored to wild-type levels in the trans-complemented vipp1 mutant strain. In contrast, in the vipp1 null mutant, no PS I complexes accumulated, and no PS I activity was detected. Previous studies also suggested that a critical Vipp1 concentration might be required for thylakoid membrane protein complex formation (27). Vipp1 has been reported to form rodlike structures in vivo (32), and it is possible that these Vipp1 structures could assist in the translation, transport, and/or insertion of membrane-associated subunits into thylakoids. Thus, the loss of Vipp1 might interfere with one or more of these processes and therefore interfere with the insertion of the PsaA and PsaB polypeptides into the thylakoid membranes. Although it seems clear that the loss of Vipp1 interferes with one or more of these processes, it certainly is not yet clear at which level Vipp1 acts to interfere with PS I biogenesis. However, the reduced level of psaAB transcripts in vipp1 mutant cells suggests that Vipp1 probably acts directly or indirectly at the level of translation (see below).
In Synechococcus sp. PCC 7002, a psaAB deletion mutant lacking PS I had normal transcript levels for vipp1 but greatly reduced thylakoid membrane content, and similarly, the vipp1 mutant that lacks PS I had reduced thylakoid membrane content. Restoration of PS I levels in cells caused thylakoids to return to wild-type levels and overall thylakoid structure, which implies that PS I plays an important role in the formation of structurally normal and functional thylakoid membranes. These observations generally agree with observations that depletion of PS II had only minor effects on thylakoid membrane formation (33) and that intracytoplasmic membranes were much less abundant in a mutant depleted of both PS I and PS II (34). It has been suggested that PS I plays a role in the early steps to form thylakoid membranes and that PS II is then involved in forming highly ordered tubular structures of thylakoid membranes together with PS I (34). A direct interaction of Vipp1 with Albino3.2 protein in Chlamydomonas reinhardtii also implicated Vipp1 in the integration of thylakoid membrane proteins (35). The thylakoid-localized protein, Albino3.2, belongs to the conserved YidC/Oxa1p/Alb3 protein family, and it plays an essential role during the insertion of photosystem reaction center polypeptides (such as PsaA, PsbA (D1), and PsbD (D2)) into the thylakoid membranes (35, 36). Overproduction of Vipp1 occurred when Albino3.2 was depleted in C. reinhardtii (35). This observation suggests that Vipp1 is involved in stabilizing the membrane structure during the Albino3.2-mediated protein insertion into thylakoid membranes and that Vipp1 may deliver photosystem polypeptides to Albino3.2 for insertion into thylakoid membranes. If PsaA and PsaB were cotranslationally inserted into thylakoids during or after Vipp1 action, this could explain why transcripts for psaAB were reduced in the vipp1 mutant. Transcript levels for yidC were similar in Synechococcus sp. PCC 7002 cells in the presence or absence of Vipp1 (see supplemental Table S1). Furthermore, we observed no changes in transcript levels for genes encoding other PS I polypeptides, PS I-specific chaperones (e.g. rubA, ycf3, ycf4), or other general chaperones or proteases. Correspondingly, the transcript levels for all of these components were similar in the wild type, the vipp1 mutant, the complemented vipp1 mutant strain, and the psaAB deletion strain.
Abundant lipid bodies were detected in the vipp1 mutant strain, which suggests that Vipp1 is not directly involved in lipid synthesis and accumulation. However, the abnormal localization of lipids in vipp1 mutant indicates that Vipp1 directly or indirectly affects the insertion of lipids into thylakoid membranes. Previous studies suggested that Vipp1 played a stimulatory role in the cpTat transport system, potentially by enhancing protein binding interactions with lipid-rich regions of thylakoid membranes (37). Interestingly, it appears that lipid synthesis still occurs, but normal lipid insertion into membranes is apparently greatly reduced when PS I complexes are not inserted into the membranes in Synechococcus sp. PCC 7002. As observed previously in other organisms, it appears that there is a relationship between membrane biogenesis, lipid insertion, and PS I complex biogenesis and membrane insertion and that Vipp1 is necessary for all of these processes to proceed normally (24).
Nordhues et al. (38) recently studied the role of Vipp1 in C. reinhardtii by RNA interference. They found that core complexes for PS I and PS II as well as the cytochrome b6f complex and ATP synthase were reduced 14–20% in Vipp1-depleted cells, but light-harvesting complex II levels increased by 30%. These authors proposed a highly speculative hypothesis that Vipp1 provides structural lipids for the biogenesis of some protein complexes of the thylakoid membrane. However, the obvious differences in their results in C. reinhardtii and those presented here for Synechococcus sp. PCC 7002 strongly suggest that Vipp1 may play different roles in prokaryotes and eukaryotes. Alternatively, redundant pathways may exist in some but not all organisms that could explain the substantial phenotypic differences observed in different organisms.
Transcription profiling suggested that Vipp1 might function as a potential transcriptional regulator of the chlLN and psaAB operons. The phage shock protein A (PspA), a key transcription regulatory protein in bacteria (39), has significant sequence similarity with Vipp1 and is thus is structurally related to Vipp1 as well. In other studies, the translocation of proteins by TAT complexes in Escherichia coli was blocked in a pspA mutant, but this phenotype could be relieved by expression of the vipp1 gene from Synechocystis sp. PCC 6803 in the mutant (40). These findings indicate that cyanobacterial Vipp1 can functionally replace bacterial PspA. Subsequent studies suggested that the α-helical, PspA-like domain of Vipp1 plays a crucial role in forming high molecular mass complexes (41). Phylogenetic analyses of Vipp1 and PspA clearly suggest that these proteins form separate clades (24), and most cyanobacteria contain a protein from each family. A possible explanation for these results is that Vipp1 evolved from cyanobacterial PspA by gene duplication and may have gained a novel function in cyanobacteria in thylakoid biogenesis or perhaps stress responses. In Synechocystis sp. PCC 6803, vipp1 expression increased under high salt conditions (42), and in C. reinhardtii, vipp1 expression increased under high irradiance (43). These findings suggest that Vipp1 may play a role in responses to these stress conditions, although its expression might also increase if PS I levels increase under these same conditions. However, our results with reporter strains clearly showed that Vipp1 is not directly involved in the transcriptional regulation of either the psaAB or chlLN genes of Synechococcus sp. PCC 7002. We propose that psaAB transcripts decreased because of an effect of Vipp1 on translation or co-translational insertion of these PS I polypeptides into the thylakoid membrane. The decreased transcript levels for chlLN are probably due to the reduced demand for Chl a in the Vipp1 mutant, which does not accumulate PS I (Table 1). It is also interesting that the marine symbiont strain UCYN-A, which lacks PS II and cannot fix CO2 but has retained PS I complexes (44), has also retained two copies of the vipp1 gene like Trichodesmium erythraeum, another nitrogen-fixing marine cyanobacterium.
In conclusion, our results show that Vipp1 is not essential for viability of the cyanobacterium Synechococcus sp. PCC 7002 and that it is most likely involved in the biogenesis of PS I, possibly by participating in the insertion of PS I polypeptides into thylakoid membranes. Our results further suggest that normal thylakoid membrane biogenesis is dependent upon assembly of PS I but is not directly dependent on Vipp1. Further studies to elucidate the precise role of Vipp1 in PS I and lipid insertion into membranes will enhance our knowledge of the underlying mechanisms of thylakoid membrane biogenesis and photosynthetic protein assembly.
Supplementary Material
Acknowledgments
We thank Missy Hazen for assistance with the electron microscopy and confocal fluorescence microscopy at the Microscopy and Cytometry Facility of the Huck Institutes of the Life Sciences (The Pennsylvania State University). We also thank Candace Price and Dr. Craig Praul for assistance with the preparation and sequencing of the cDNA libraries.
This work was supported by National Science Foundation Grant MCB-1021725 (to D. A. B. and J. H. G.) and by Air Force Office of Scientific Research Grant FA9550-11-1-0148 (to D. A. B.).

This article contains supplemental Table S1.
- PS
- Photosystem
- Chl
- chlorophyll
- PBP
- phycobiliproteins
- PG
- phosphatidylglycerol
- Comp
- trans-complemented vipp1 mutant strain.
REFERENCES
- 1. De Marais D. J. (2000) When did photosynthesis emerge on Earth? Science 289, 1703–1705 [PubMed] [Google Scholar]
- 2. Jordan P., Fromme P., Witt H. T., Klukas O., Saenger W., Krauss N. (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411, 909–917 [DOI] [PubMed] [Google Scholar]
- 3. Vothknecht U. C., Westhoff P. (2001) Biogenesis and origin of thylakoid membranes. Biochim. Biophys. Acta 1541, 91–101 [DOI] [PubMed] [Google Scholar]
- 4. Kroll D., Meierhoff K., Bechtold N., Kinoshita M., Westphal S., Vothknecht U. C., Soll J., Westhoff P. (2001) VIPP1, a nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. Proc. Natl. Acad. Sci. U.S.A. 98, 4238–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H.-M., Kaneko Y., Keegstra K. (1994) Molecular cloning of a chloroplastic proteinassociated with both the envelope and thylakoid membranes. Plant Mol. Biol. 25, 619–632 [DOI] [PubMed] [Google Scholar]
- 6. Westphal S., Heins L., Soll J., Vothknecht U. C. (2001) Vipp1 deletion mutant of Synechocystis: a connection between bacterial phage shock and thylakoid biogenesis? Proc. Natl. Acad. Sci. U.S.A. 98, 4243–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang F., Parmryd I., Nilsson F., Persson A. L., Pakrasi H. B., Andersson B., Norling B. (2002) Proteomics of Synechocystis sp. strain PCC 6803: identification of plasma membrane proteins. Mol. Cell Proteomics 1, 956–966 [DOI] [PubMed] [Google Scholar]
- 8. Srivastava R., Battchikova N., Norling B., Aro E. M. (2006) Plasma membrane of Synechocystis PCC 6803: a heterogeneous distribution of membrane proteins. Arch. Microbiol. 185, 238–243 [DOI] [PubMed] [Google Scholar]
- 9. Gao H., Xu X. (2009) Depletion of Vipp1 in Synechocystis sp. PCC 6803 affects photosynthetic activity before the loss of thylakoid membranes. FEMS Microbiol. Lett. 292, 63–70 [DOI] [PubMed] [Google Scholar]
- 10. Ludwig M., Bryant D. A. (2011) Transcription profiling of the model cyanobacterium Synechococcus sp. strain PCC 7002 by Next-Gen (SOLiD) sequencing of cDNA. Front. Microbiol. 2, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frigaard N. U. (2004) Gene inactivation in the cyanobacterium Synechococcus sp. PCC 7002 and the green sulfur bacterium Chlorobium tepidum using in vitro-made DNA constructs and natural transformation. Methods Mol. Biol. 274, 325–340 [DOI] [PubMed] [Google Scholar]
- 12. Shen G., Bryant D. A. (1995) Characterization of a Synechococcus sp. strain PCC 7002 mutant lacking Photosystem I. Protein assembly and energy distribution in the absence of the Photosystem I reaction center core complex. Photosynth. Res. 44, 41–53 [DOI] [PubMed] [Google Scholar]
- 13. Shen G., Zhao J., Reimer S. K., Antonkine M. L., Cai Q., Weiland S. M., Golbeck J. H., Bryant D. A. (2002) Assembly of photosystem I. I. Inactivation of the rubA gene encoding a membrane-associated rubredoxin in the cyanobacterium Synechococcus sp. PCC 7002 causes a loss of photosystem I activity. J. Biol. Chem. 277, 20343–20354 [DOI] [PubMed] [Google Scholar]
- 14. Xu Y., Alvey R. M., Byrne P. O., Graham J. E., Shen G., Bryant D. A. (2011) Expression of genes in cyanobacteria: adaptation of endogenous plasmids as platforms for high-level gene expression in Synechococcus sp. PCC 7002. Methods Mol. Biol. 684, 273–293 [DOI] [PubMed] [Google Scholar]
- 15. Sakamoto T., Bryant D. A. (1998) Growth at low temperature causes nitrogen limitation in the cyanobacterium Synechococcus sp. PCC 7002. Arch. Microbiol. 169, 10–19 [DOI] [PubMed] [Google Scholar]
- 16. Nomura C. T., Persson S., Shen G., Inoue-Sakamoto K., Bryant D. A. (2006) Characterization of two cytochrome oxidase operons in the marine cyanobacterium Synechococcus sp. PCC 7002: inactivation of ctaDI affects the PS I:PS II ratio. Photosynth. Res. 87, 215–228 [DOI] [PubMed] [Google Scholar]
- 17. Spence E., Bailey S., Nenninger A., Møller S. G., Robinson C. (2004) A homolog of Albino3/OxaI is essential for thylakoid biogenesis in the cyanobacterium Synechocystis sp. PCC6803. J. Biol. Chem. 279, 55792–55800 [DOI] [PubMed] [Google Scholar]
- 18. Greenspan P., Mayer E. P., Fowler S. D. (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100, 965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H., Durbin R. (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ludwig M., Bryant D. A. (2012) Synechococcus sp. strain PCC 7002 transcriptome: acclimation to temperature, salinity, oxidative stress, and mixotrophic growth conditions. Front. Microbiol. 3, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludwig M., Bryant D. A. (2012) Acclimation of the global transcriptome of the cyanobacterium Synechococcus sp. strain PCC 7002 to nutrient limitations and different nitrogen sources. Front. Microbiol. 3, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chitnis V. P., Chitnis P. R. (1993) PsaL subunit is required for the formation of photosystem I trimers in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 336, 330–334 [DOI] [PubMed] [Google Scholar]
- 23. Domonkos I., Malec P., Sallai A., Kovács L., Itoh K., Shen G., Ughy B., Bogos B., Sakurai I., Kis M., Strzalka K., Wada H., Itoh S., Farkas T., Gombos Z. (2004) Phosphatidylglycerol is essential for oligomerization of photosystem I reaction center. Plant Physiol. 134, 1471–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vothknecht U. C., Otters S., Hennig R., Schneider D. (2012) Vipp1: a very important protein in plastids? J. Exp. Bot. 63, 1699–1712 [DOI] [PubMed] [Google Scholar]
- 25. Suzuki J. Y., Bauer C. E. (1992) Light-independent chlorophyll biosynthesis: involvement of the chloroplast gene chlL (frxC). Plant Cell 4, 929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reinbothe S., Reinbothe C., Lebedev N., Apel K. (1996) PORA and PORB, two light-dependent protochlorophyllide-reducing enzymes of angiosperm chlorophyll biosynthesis. Plant Cell 8, 763–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fuhrmann E., Gathmann S., Rupprecht E., Golecki J., Schneider D. (2009) Thylakoid membrane reduction affects the photosystem stoichiometry in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 149, 735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaneko T., Sato S., Kotani H., Tanaka A., Asamizu E., Nakamura Y., Miyajima N., Hirosawa M., Sugiura M., Sasamoto S., Kimura T., Hosouchi T., Matsuno A., Muraki A., Nakazaki N., Naruo K., Okumura S., Shimpo S., Takeuchi C., Wada T., Watanabe A., Yamada M., Yasuda M., Tabata S. (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3, 109–136 [DOI] [PubMed] [Google Scholar]
- 29. Stanley D. N., Raines C. A., Kerfeld C. A. (2013) Comparative analysis of 126 cyanobacterial genomes reveals evidence of functional diversity among homologs of the redox-regulated CP12 protein. Plant Physiol. 161, 824–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dufresne A., Salanoubat M., Partensky F., Artiguenave F., Axmann I. M., Barbe V., Duprat S., Galperin M. Y., Koonin E. V., Le Gall F., Makarova K. S., Ostrowski M., Oztas S., Robert C., Rogozin I. B., Scanlan D. J., Tandeau de Marsac N., Weissenbach J., Wincker P., Wolf Y. I., Hess W. R. (2003) Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc. Natl. Acad. Sci. U.S.A. 100, 10020–10025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagio M., Sakurai I., Sato S., Kato T., Tabata S., Wada H. (2002) Phosphatidylglycerol is essential for the development of thylakoid membranes in Arabidopsis thaliana. Plant Cell Physiol. 43, 1456–1464 [DOI] [PubMed] [Google Scholar]
- 32. Fuhrmann E., Bultema J. B., Kahmann U., Rupprecht E., Boekema E. J., Schneider D. (2009) The vesicle-inducing protein 1 from Synechocystis sp. PCC 6803 organizes into diverse higher-ordered ring structures. Mol. Biol. Cell 20, 4620–4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nilsson F., Simpson D. J., Jansson C., Andersson B. (1992) Ultrastructural and biochemical characterization of a Synechocystis 6803 mutant with inactivated psbA genes. Arch. Biochem. Biophys. 295, 340–347 [DOI] [PubMed] [Google Scholar]
- 34. van de Meene A. M., Sharp W. P., McDaniel J. H., Friedrich H., Vermaas W. F., Roberson R. W. (2012) Gross morphological changes in thylakoid membrane structure are associated with photosystem I deletion in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1818, 1427–1434 [DOI] [PubMed] [Google Scholar]
- 35. Göhre V., Ossenbühl F., Crèvecoeur M., Eichacker L. A., Rochaix J. D. (2006) One of two alb3 proteins is essential for the assembly of the photosystems and for cell survival in Chlamydomonas. Plant Cell 18, 1454–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang P., Dalbey R. E. (2011) Inserting membrane proteins: the YidC/Oxa1/Alb3 machinery in bacteria, mitochondria, and chloroplasts. Biochim. Biophys. Acta 1808, 866–875 [DOI] [PubMed] [Google Scholar]
- 37. Lo S. M., Theg S. M. (2012) Role of vesicle-inducing protein in plastids 1 in cpTat transport at the thylakoid. Plant J. 71, 656–668 [DOI] [PubMed] [Google Scholar]
- 38. Nordhues A., Schöttler M. A., Unger A.-K., Geimer S., Schönfelder S., Schmollinger S., Rütgers M., Finazzi G., Soppa B., Sommer F., Mülhaus T., Roach T., Krieger-Liszkay A., Lokstein H., Crespo J. L., Schroda M. (2012) Evidence for a role of VIPP1 in the structural organization of the photosynthetic apparatus in Chlamydomonas. Plant Cell 24, 637–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Darwin A. J. (2005) The phage-shock-protein response. Mol. Microbiol. 57, 621–628 [DOI] [PubMed] [Google Scholar]
- 40. DeLisa M. P., Lee P., Palmer T., Georgiou G. (2004) Phage shock protein PspA of Escherichia coli relieves saturation of protein export via the Tat pathway. J. Bacteriol. 186, 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aseeva E., Ossenbühl F., Eichacker L. A., Wanner G., Soll J., Vothknecht U. C. (2004) Complex formation of Vipp1 depends on its α-helical PspA-like domain. J. Biol. Chem. 279, 35535–35541 [DOI] [PubMed] [Google Scholar]
- 42. Huang F., Fulda S., Hagemann M., Norling B. (2006) Proteomic screening of salt-stress-induced changes in plasma membranes of Synechocystis sp. strain PCC 6803. Proteomics 6, 910–920 [DOI] [PubMed] [Google Scholar]
- 43. Im C. S., Grossman A. R. (2002) Identification and regulation of high light-induced genes in Chlamydomonas reinhardtii. Plant J. 30, 301–313 [DOI] [PubMed] [Google Scholar]
- 44. Tripp H. J., Bench S. R., Turk K. A., Foster R. A., Desany B. A., Niazi F., Affourtit J. P., Zehr J. P. (2010) Metabolic streamlining in an open-ocean nitrogen-fixing cyanobacterium. Nature 464, 90–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.