Background: The trans-molecular mechanism of Jak3 regulation is not known.
Results: p52ShcA regulated Jak3 dephosphorylation through direct interactions with both Jak3 and tyrosine phosphatases.
Conclusion: Results demonstrate the molecular mechanism of deregulation of Jak3 activation.
Significance: Understanding the regulation of Jak3 activation has implications in mucosal homeostasis, EMT, cancer metastasis, and immune cell migration.
Keywords: Apoptosis, Janus Kinase (JAK), Nonreceptor Tyrosine Kinase (nRTK), Phosphatase, Protein Phosphatase, Wound Healing
Abstract
Although constitutive activation of Janus kinase 3 (Jak3) leads to different cancers, the mechanism of trans-molecular regulation of Jak3 activation is not known. Previously we reported that Jak3 interactions with adapter protein p52ShcA (Shc) facilitate mucosal homeostasis. In this study, we characterize the structural determinants that regulate the interactions between Jak3 and Shc and demonstrate the trans-molecular mechanism of regulation of Jak3 activation by Shc. We show that Jak3 autophosphorylation was the rate-limiting step during Jak3 trans-phosphorylation of Shc where Jak3 directly phosphorylated two tyrosine residues in Src homology 2 (SH2) domain and one tyrosine residue each in calponin homology 1 (CH1) domain and phosphotyrosine interaction domain (PID) of Shc. Direct interactions between mutants of Jak3 and Shc showed that although FERM domain of Jak3 was sufficient for binding to Shc, CH1 and PID domains of Shc were responsible for binding to Jak3. Functionally Jak3 was autophosphorylated under IL-2 stimulation in epithelial cells. However, Shc recruited tyrosine phosphatases SHP2 and PTP1B to Jak3 and thereby dephosphorylated Jak3. Thus we not only characterize Jak3 interaction with Shc, but also demonstrate the molecular mechanism of intracellular regulation of Jak3 activation where Jak3 interactions with Shc acted as regulators of Jak3 dephosphorylation through direct interactions of Shc with both Jak3 and tyrosine phosphatases.
Introduction
Jak3, a nonreceptor tyrosine kinase, mediates signals initiated by cytokine through interactions with the common γ chain of cytokine receptors (1). Abnormal activation of Jak3 was associated with human hematologic and epithelial malignancies (2, 3), and its functions were essential for epithelial development (4). Jak3 contains seven Jak homology (JH)3 domains where JH3-JH4 regions have homology with SH2 domains and JH6-JH7 domains have homologies with FERM domain found in molecules such as Band 4.1, ezrin, radixin, and moesin (2). Although the FERM domain mediates intermolecular interactions with cytokine receptor (5), it is also involved in intramolecular binding to SH2 domain thereby maintaining the close conformation in Jak3 (6). Previously we reported that Jak3 regulated mucosal wound repair in human epithelial cells through interactions with villin and mucosal homeostasis through interactions with p52ShcA (7, 8).
Shc, identified as a proto-oncogenic protein with three members, viz. shcA, shcB, and shcC, is involved in regulation of growth factor signaling (9). Among these ShcA is ubiquitously expressed, whereas ShcB and ShcC are restricted to neuronal cells (10). ShcA is involved in motogenic signaling through activation of Ras/MAPK pathways (11), and KO of shcA gene results in embryonic lethality (12). The CH1 domain of p52ShcA contains three critical tyrosine residues, Tyr239, Tyr240, and Tyr317, that become phosphorylated upon engagement of a number of cell surface receptors. The SH2 domain of Grb2 binds to both Tyr239 and Tyr317, which leads to MAP kinase activation (9). The mechanism of Shc interactions with Jak3 is not known. In this study, we characterize the structural determinants responsible for p52ShcA (Shc) interactions with Jak3 and demonstrate the trans-molecular mechanism of Shc regulation of Jak3 activation.
EXPERIMENTAL PROCEDURES
Materials
Antibodies and other materials used were: P-Jak3 and Jak3 (Invitrogen); SHP2 (Cell Signaling); PTP1B, villin, and phosphotyrosine (MP Biomedicals); GST (Millipore); FLAG (Sigma); and FITC and rhodamine-phalloidin (Molecular Probes). Constructs for full-length SHP1, SHP2, and PTP1B cloned in pGEX2T were from Addgene.
Cell Culture, IL-2 Treatment, Stable Transfection, and Wound Closure
Methods for HT-29 cell maintenance, treatment, transfection, and wound closure were reported before (6–8). DNA constructs for pCDNA-FLAG-ShcA-wt and mutants were stably transfected into HT-29 cells as reported before (7).
Site-directed Mutagenesis, Expression, and Purification of the Recombinant Protein
Wild type (wt) and mutant constructs were transformed in Escherichia coli BL21 and TKX1 cells to produce the nonphosphorylated and phosphorylated forms, respectively, of recombinant purified proteins using the methods reported before (13). Mutations in full-length p52ShcA cDNA were done using methods as reported (6).
In Vitro Kinase Assay, Phosphatase Assay, and Protein-Protein Interaction
In vitro kinase and phosphatase assays and protein-protein interaction were done as reported (6, 7).
Immunoprecipitations (IP), Immunoblotting (IB), Immunofluorescence Microscopy, and Apoptosis Assay
Methods for IP, IB, immunofluorescence microscopy, and apoptosis assay were used as reported before (7).
RESULTS
Shc
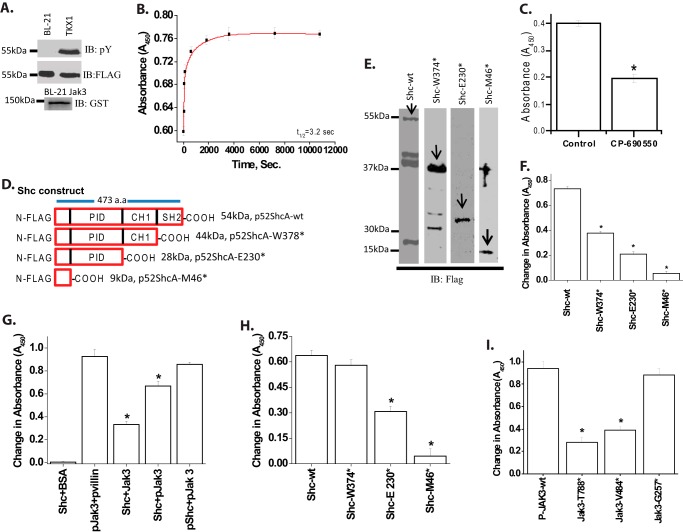
Previously we reported that Jak3 played an essential role in mucosal homeostasis through its interactions with Shc (7, 8). To determine the mechanism of Jak3 interactions with Shc and the structural determinants that regulate these interactions, we expressed and purified the phosphorylated (P) and nonphosphorylated forms of FLAG-tagged Shc-wt using TKX1 and BL-21 expression systems, respectively (Fig. 1A, first and second panels). Because recombinant Jak3-wt (Fig. 1A, third panel) autophosphorylates itself in a time-dependent manner with a t½ (the time taken to reach half of the maximum phosphorylation) of autophosphorylation of 135 s (6), we determined whether the autophosphorylated Jak3-wt could trans-phosphorylate the nonphosphorylated forms of Shc-wt. Fig. 1B shows that Jak3 trans-phosphorylates recombinant Shc-wt in a time-dependent manner with a t½ of trans-phosphorylation of 3.2 s. To further confirm the trans-phosphorylation of Shc-wt by Jak3-wt, the kinase reaction was carried out in the presence of CP-690550, a previously reported potent Jak3 inhibitor (6). Fig. 1C shows that CP-690550 inhibited the phosphorylation of Shc-wt by Jak3-wt. Because in a reaction, the slowest step is considered as rate-limiting, these results showed that Shc-wt was not only a direct substrate for Jak3-wt but also that the autophosphorylation of Jak3 was the rate-limiting step during tyrosine phosphorylation of Shc-wt by Jak3.
FIGURE 1.
Recombinant Jak3 trans-phosphorylates adapter proteins p52ShcA. A, the expression and tyrosine phosphorylation of recombinant proteins were detected through Western analysis using FLAG, phosphotyrosine (pY), and GST antibody. B, changes in tyrosine trans-phosphorylation of ShcA-wt by Jak3-wt were detected in the presence or absence (control) of ATP using 96-well microtiter plates precoated with FLAG-ShcA-wt proteins, and the phosphorylation was induced by the addition of activated Jak3 (P-Jak3-wt) (6), where P-Jak3-wt alone and FLAG-ShcA-wt alone were taken as controls. The phosphorylation was detected as reported before (6). Curve-fitting was done as reported before (25) using the Hyperbol-fit program in MicroCal Origin to calculate t½. C, similar experiments were performed as in B except in the presence of Jak3 inhibitor CP-690505 and a fixed reaction time of 5 min. D, schematic representation of FLAG-p52ShcA-wt and mutants. E, wt and mutants were expressed and purified as in A, and Western analysis of the expressed proteins was done using anti-GST antibody. Arrows indicate recombinant protein expression. F, similar experiments were performed as in C using different truncation mutants except that the 96-well microtiter plates were precoated with p52ShcA-wt or its truncation mutants and the phosphorylation was induced by the addition of P-Jak3-wt where P-Jak3-wt alone and Shc-wt or mutants alone were taken as controls. G, direct interactions between Jak3-wt and Shc-wt were determined by a pairwise binding assay (detailed in supplemental Fig. S3) except using equimolar concentrations of the indicated proteins with BSA as negative and villin as positive control. H, direct interactions between Jak3-wt and Shc mutants were determined as in G. I, direct interactions between P-Shc-wt and the indicated Jak3 mutants were determined as in H. A and E, blots are representative from n = 3 experiments. B and C and F–I, values are mean ± S.E.* indicates statistically significant differences from control (in C), Shc-wt (in F and H), P-Shc-P-Jak3 (in G), and P-Jak3 (in I); p < 0.05, n = 3 experiments.
Jak3 Phosphorylates the SH2, CH1, and PID Domains of Shc
Because Jak3-wt phosphorylated Shc-wt, we determined the structural determinants of Shc-wt phosphorylated by Jak3-wt. Fig. 1D shows the schematic diagram for the truncation mutants of Shc. The wild type Shc and these mutants were expressed and purified using the BL21 expression system (Fig. 1E) and were used as substrates for in vitro kinase assays using autophosphorylated Jak3-wt (6, 7) as an enzyme. Fig. 1F (first and second bar from the left) shows that there was 2-fold decrease in absorbance (as a measure of tyrosine phosphorylation) when SH2 domain of ShcA was deleted. Moreover, deletion of CH1 and PID domain further decreased the tyrosine phosphorylation of Shc by Jak3 (third and fourth bar from the left). To determine the number of tyrosine residues of Shc phosphorylated by Jak3, we analyzed the tyrosine residues present in each domain of Shc and calculated the effect of deletion of each domain on the decrease in absorbance. These analyses show that Jak3 phosphorylated four tyrosine residues in Shc where two of them were present in SH2 domain, whereas one each was present in CH1 and PID domains (supplemental Fig. S1 and supplemental Table ST1). The contribution of a single tyrosine residue toward absorbance during Jak3-mediated phosphorylation was also confirmed using purified SH2 domain of Shc with one or both tyrosine residues mutated, which showed an average decrease in absorbance by 0.175 (data not shown). Because SH2 domain of ShcA has only two tyrosine residues (Tyr410 and Tyr448), we further confirmed these as Jak3-mediated phosphorylation sites in a cell model by mutating both into phenylalanine in full-length Shc (supplemental Fig. S2), which showed decreased phosphorylation of full-length Shc by Jak3.
CH1 and PID Domains of Shc and FERM Domain of Jak3 Facilitate Jak3 Interactions with Shc
Because Jak3-wt phosphorylated Shc, we determined the binding kinetics of P-Jak3-wt to P-Shc-wt. Pairwise binding studies showed that P-Jak3-wt interacted with P-Shc-wt in a dose-dependent manner with a Kd of 0.22 μm and a Hill coefficient of 1.07 (supplemental Figs. S3 and S4). This showed that the binding between P-Jak3 and P-Shc-wt was noncooperative. Because P-Jak3 interacted with P-Shc-wt, we determined the structural determinants of Shc responsible for these interactions. Fig. 1G (fifth bar from the left) shows that the binding between P-Jak3-wt and P-Shc-wt was ∼3-fold higher as compared with binding between Jak3-wt and Shc-wt (third bar from the left) and that the binding increased by 2-fold when only Jak3 was autophosphorylated (fourth bar from the left). These results indicated that tyrosine phosphorylation of both the proteins in general and Jak3 in particular was important for the interactions. For these experiments the interactions between P-Jak3-wt and P-villin-wt was taken as positive control (second bar from the left), whereas interactions between Shc-wt and BSA (first bar from the left) were taken as negative control. Next, we determined whether truncation of Shc had an effect on the interactions between Shc and P-Jak3. As shown in Fig. 1H, deletion of SH2 domain of Shc had little effect on the interactions between P-Jak3 and Shc; however, deletion of either CH1 or CH1 plus PID domains resulted in significant decrease in the interactions between these two proteins. Next, we determined the structural determinants of Jak3 responsible for these interactions. Previously using truncation mutants of Jak3, we reported that FERM domain of Jak3 was sufficient for its interactions with cytoskeletal proteins (6). Using these mutants of Jak3, we determined their effects on interactions with Shc. As shown in Fig. 1I, truncation of either kinase or kinase along with pseudo-kinase domain (Jak3-T788* and Jak3-V484*, respectively) resulted in decreased binding between Jak3 and P-Shc-wt, which was comparable with Jak3-wt (Fig. 1G). However, truncation of kinase, pseudo-kinase, and SH2 domains altogether (Jak3-G257*) resulted in substantial increase in binding between Jak3 and P-Shc-wt, which was comparable with P-Jak3-wt. Next, we determined the binding kinetics of Jak3-G257* to P-Shc-wt, which showed that the FERM domain of Jak3 interacted with P-Shc-wt in a dose-dependent manner with a Kd of 2.7 nm, indicating that FERM domain by itself had higher affinity for P-Shc-wt as compared with P-Jak3-wt (supplemental Fig. S5). Taken together these data suggested that phosphorylation of both Jak3 and Shc was important for their interactions where CH1 and PID domains of Shc and FERM domain of Jak3 facilitated these interactions.
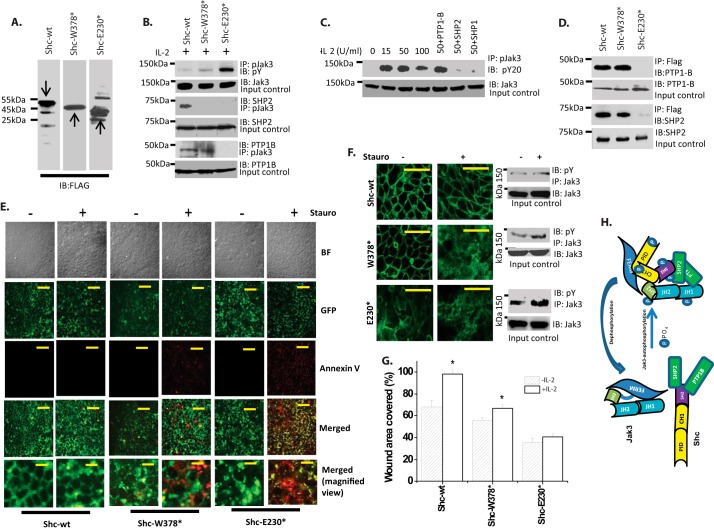
Jak3 Interactions with Shc Regulate Tyrosine Phosphorylation of Jak3 in Intestinal Epithelial Cells (IECs)
Because P-Shc interacted with P-Jak3, where deletion of CH1 and PID domains of Shc significantly decreased the interactions with P-Jak3-wt, we determined the physiological significance of these in IECs. Human colonic epithelial cells HT-29-Cl-19A were stably transfected with either pCDNA-FLAG-Shc-wt or pCDNA-FLAG-Shc-W378* or pCDNA-FLAG-Shc-E230*, and clones expressing comparable amounts of transfected proteins were selected (Fig. 2A). To determine whether Shc interactions with Jak3 had an effect on tyrosine phosphorylation of Jak3, these stably transfected cells were treated with IL-2, and cell lysates were immunoprecipitated with P-Jak3 and immunoblotted with phosphotyrosine antibody. Previously we reported that treatment with IL-2 led to increased tyrosine phosphorylation of Jak3 that facilitated Jak3 interactions with Shc (8). Fig. 2B, first panel, shows that Shc-wt-overexpressing cells had decreased tyrosine phosphorylation of Jak3; however, deletion of either SH2 domain (Shc-W378*) or SH2 plus CH1 domain (Shc-E230*) increased the tyrosine phosphorylation of Jak3 in a domain-dependent manner. Jak3 remained phosphorylated for over 6 h after IL-2 treatment in these cells (data not shown). To determine the reason for increased and sustained phosphorylation of Jak3, we investigated whether truncation of Shc had an effect on P-Jak3 interactions with phosphatases; SHP2 and PTP1B. Although the data in the third panel indicate that SH2 domain of Shc was required for P-Jak3 interactions with SHP2 and thereby to dephosphorylate Jak3, the data in the fifth panel show that both SH2 and CH1 domains were required for P-Jak3 interactions with PTP1B and thereby to dephosphorylate Jak3. These results were further confirmed by co-localization studies, which showed that endogenous proteins of Shc interact with SHP2 (supplemental Fig. S6) and PTP1B (supplemental Fig. S7) and deletion of SH2 or SH2 plus CH1 leads to disruption of these interactions (Merged panels). Together these results show that Shc facilitated P-Jak3 interactions with SHP2 and PTP1B where SH2 domain of Shc was required for P-Jak3 interactions with SHP2 and CH1 domain of Shc was required for P-Jak3 interactions with PTP1B, and disruption of P-Jak3 interactions with SHP2 and PTP1B resulted in an increase of tyrosine phosphorylation of Jak3 that was dependent on the disruption of the number of phosphatases that interacted with Jak3 (Fig. 2B, first panel). To further confirm these observations, using a previously reported in vitro phosphatase assay (7), we determined whether recombinant purified phosphatases could dephosphorylate tyrosine-phosphorylated Jak3 that was immunoprecipitated from the cell lysates of IL-2-induced untransfected HT-29 Cl-19A cells. Previously we reported that IL-2 induced a dose-dependent increase in tyrosine phosphorylation of Jak3 up to 50 units/ml, whereas a higher concentration decreased it (6, 7). Fig. 2C shows that although IL-2 had similar effects on Jak3 phosphorylation, SHP1 did not have any effect on tyrosine-phosphorylated Jak3. However, incubation of immunoprecipitated P-Jak3 with SHP2 or PTP1B resulted in a substantial decrease in tyrosine phosphorylation of Jak3. To determine how Shc facilitated P-Jak3 interactions with phosphatases, we determined the structural determinants of Shc responsible for interactions with phosphatases. Pairwise binding using recombinant and purified proteins of SHP2 and PTP1B and truncation mutant proteins of FLAG-tagged-Shc showed that both SH2 and CH1 domains of Shc were essential for the direct interactions between Shc and SHP2 and PTP1B (Fig. 2D). To further confirm that Jak3 interactions with its phosphatases rely on the presence of Shc, Jak3 was allowed to interact with SHP2 and PTP1B in the presence or absence of purified Shc proteins (supplemental Fig. S8), which showed that Jak3 failed to interact with these phosphatases in the absence of Shc. Taken together these results showed that SHP2 and PTP1B dephosphorylated IL-2-induced tyrosine-phosphorylated Jak3 through Jak3 interactions with Shc where deletion of SH2 and CH1 domains of Shc led to disruption of Jak3 interactions with SHP2 and PTP1B, respectively, that resulted in increased tyrosine phosphorylation of Jak3.
FIGURE 2.
Adapter protein Shc regulates Jak3 activation through regulating Jak3 interactions with tyrosine phosphatases. A, Western analysis for the expression of FLAG-tagged ShcA-wt or its mutants was done using lysates from stably transfected HT-29 Cl-19A cells and FLAG antibody. B, Western analysis of co-immunoprecipitates from cell lysates of stably transfected cells from A treated with IL-2 (50 units/ml) were done using indicated antibodies for phosphotyrosine (pY), phospho-Jak3 (pJak3), and tyrosine phosphatase SHP2 and PTP1B using previously reported protocols (8). C, dephosphorylation of phospho-Jak3 was determined using in vitro phosphatase assay using P-Jak3-immunoprecipitated Jak3 from cell lysates of IL-2-treated HT-29 Cl-19A cells as described under “Experimental Procedures”. pY20, phosphotyrosine antibody from clone PY20. D, direct interactions between recombinant purified proteins of FLAG-tagged-ShcA-wt or mutants and indicated phosphatases were determined by pairwise binding assay as in Fig. 1, and protein complexes were immunoprecipitated using the indicated antibodies followed by IB by FLAG antibodies. For input controls IB was done using the indicated antibodies for phosphatases. E, susceptibility toward staurosporine-induced apoptosis was determined in stably transfected cells from A using a GFP-certified apoptosis assay kit as reported before (7). Yellow color in the Merged panels shows GFP and annexin V co-localized apoptotic cells, whereas green color indicates nonapoptotic healthy cells. F, immunofluorescence microscopy on control or staurosporine-treated cells (Stauro) from E was done using Alexa Fluor 488-conjugated phalloidin to stain F-actin. Right panels, P-Jak3 was assessed in cells from F and G using IP and IB with the indicated antibodies. G, wound closure was measured as a percentage of the original wound area as reported before (8). H, model for Shc-mediated Jak3 dephosphorylation. G, values are mean ± S.E. * indicates statistically significant differences from IL-2, p < 0.05, n = 3 experiments. E and F, images were stacked and processed using NIS Element software (Nikon). Representative blots (A–D and F) or images (E and F) are shown from n = 3 experiments. Scale bar, 14 μm.
Disruption of Jak3 Interactions with Shc Regulates IEC Apoptosis
Previously we reported that activated Jak3 tyrosine phosphorylated cytoskeletal protein villin (6, 8) and that tyrosine phosphorylation of villin promotes actin-severing activity of villin (13). Because increased actin severing promotes apoptosis (14), we determined the susceptibility toward staurosporine-induced apoptosis in cells where Jak3-Shc interactions were disrupted. Fig. 2E shows that HT-29 Cl-19A cells stably transfected with FLAG-ShcA-wt were resistant to staurosporine-induced apoptosis; however, deletion of either SH2 (W374*) or SH2 plus CH1 (E230*) domain resulted in increased susceptibility toward staurosporine-induced apoptosis in a domain-dependent manner. This increased susceptibility toward staurosporine coincided with corresponding decreased localization of F-actin toward the cell periphery in Shc-W378*-transfected control cells that showed punctate F-actin staining indicating partial severing of actin filaments, and there was complete severing of peripheral F-actin in Shc-E230*-transfected control cells (Fig. 2F, left panels). Moreover, treatment with staurosporine further severed the F-actin, making them more susceptible toward apoptosis in Shc mutant-transfected cells in a domain-dependent manner (Fig. 2F, middle panels). These results were further confirmed by immunoprecipitation studies (right panels), which showed strong correlation of decreased F-actin with increased P-Jak3. Together, these results indicated that Shc regulated epithelial apoptosis through regulating SHP2- and PTP1B-mediated dephosphorylation of Jak3 and that disruption of these resulted in increased P-Jak3 and increased F-actin severing that was associated with increased susceptibility toward staurosporine-induced apoptosis.
Disruption of Jak3 Interactions with Shc Decreases Wound Repair by IECs
Previously we reported that cyclic phosphorylation and dephosphorylation of Jak3 were important for cytoskeletal remodeling and wound repair (8). Because Shc regulated Jak3 phosphorylation, we investigated whether disruption of this regulation influenced the wound repair by IECs. Fig. 2G and supplemental Fig. S9 show that both autocrine-induced and IL-2-induced wound repair were compromised in Shc-W378*- and Shc-E230*-overexpressing IECs, which correlated with increased (and sustained) tyrosine phosphorylation of Jak3.
DISCUSSION
Jak3 regulates different signaling pathways mainly through activation of the common γ chain of several cytokine receptors (1). Inactivating mutation of Jak3 leads to immunodeficiency (2, 15), and its abnormal activation is associated with different types of malignancies (2, 3, 16). Previously we reported the regulation of cytoskeletal remodeling and wound repair through Jak3 interactions with the actin-binding protein villin (6, 8). Jak3 also played an essential role during mucosal homeostasis and intestinal differentiation (4, 7, 8). Interestingly although Jak3 interactions with Shc were essential for mucosal homeostasis (7), the structural determinants and the molecular mechanism of Jak3 interactions with Shc were not known. To achieve this, we used previously reported wt and mutants of Jak3 (6) and wt and mutants of Shc to characterize Jak3-Shc interactions and determine the kinetics parameters of Shc trans-phosphorylation by Jak3. Our data showed that Jak3-wt trans-phosphorylated Shc-wt where the t½ of Shc trans-phosphorylation was lower than that of Jak3 autophosphorylation (Fig. 1B) (6), indicating that Jak3 autophosphorylation was rate-limiting during Jak3-Shc interactions. We further confirmed these interactions by inhibition studies where CP-690550 (17) inhibited Shc-wt trans-phosphorylation by Jak3. Shc is an adapter protein that relays extracellular signals downstream of tyrosine kinases. Activation of Shc signaling is associated with poor prognosis in cancer patients (9, 18). Therefore understanding of Shc-mediated signaling inside cell is essential. Our data showed that P-Jak3-wt interacted with P-Shc-wt in a dose-dependent manner with a Kd of 0.22 μm and a Hill coefficient of 1.08, indicating noncooperative binding (supplemental Figs. S3 and S4).
Understanding of structure-function relationship between Jaks and their interacting partners is limited. Available studies suggest that Jaks bind to their cytokine receptor through N-terminal FERM domain (19). In Jak3, FERM domain not only interacts with and activates kinase domain, but it also interacts with SH2 domain, thereby keeping it in a closed conformation in the nonphosphorylated Jak3 (6, 17). The present study showed that tyrosine phosphorylation of Jak3 was necessary for its interactions with Shc, and these interactions took place through direct contacts between FERM domain of Jak3 and PID and CH1 domains of Shc (Fig. 1). Isolated SH2 domain of Shc is reported to bind its ligand in solution; however, it loses binding ability in full-length Shc (20). Our data showed that the binding between isolated FERM domain of Jak3 and P-Shc was significantly higher than between P-Shc and full-length Jak3, indicating that the presence of other domains of Jak3 lowered the binding affinity. Tyrosine phosphorylation of Shc by cytosolic kinases shows that although v-Src phosphorylates Shc at both Tyr239/240 and Tyr317, c-Src phosphorylates only at Tyr239/240 (21). We showed that Jak3 phosphorylated four tyrosine residues in Shc. Although we confirmed Tyr420 and Tyr458 in SH2 domain as Jak3-mediated phosphorylation sites, it is yet unknown which tyrosine residues in CH1 and PID domains of Shc are phosphorylated by Jak3.
Adapter functions of Shc during intracellular signaling depend on various factors including phosphorylation/dephosphorylation of its residues (9). Our data suggested that in IECs, Shc was involved in the regulation of Jak3 phosphorylation/activation through regulating Jak3 interactions with phosphatases. Constitutive activation of Jak3 has been linked with various types of cancers where mutation in jak3 gene has been indicated as a cause of constitutive activation (22). However, it is not known whether the impairment of Jak3 interaction with other proteins could also lead to constitutive activation of Jak3. Our data suggested that inside epithelial cells, even when there was no mutation in jak3 gene, Jak3 was still constitutively active because the interactions between Jak3 and Shc were impaired. This was because phosphatases SHP2 and PTP1B dephosphorylated activated Jak3 and Shc facilitated Jak3 interactions with these phosphatases (Fig. 2). Protein phosphatase PP2A associates with Shc through PID domain, where phosphorylation of CH1 domain facilitates their dissociation (23). We showed that although CH1 domain of Shc was essential for its direct association with both the phosphatases PTP1B and SHP2 (Fig. 2B), it seems that the presence of both SH2 and CH1 domains of Shc was essential for Jak3 interactions with SHP2 and that the presence of only CH1 domain of Shc was essential for Jak3 interactions with PTP1B in a tetramolecular complex of Jak3-PTP1B-SHP2-Shc inside an IL-2-activated IEC. This may be because Jak3 could also be partially interacting with PTP1B through direct interactions in a tetramolecular complex (Fig. 2H).
Although mutation in jak3 gene is reported in different immunological disorders in humans, there is no report on the relevance of Shc mutations in these diseases. Knock-out of shcA gene results in embryonic lethality (12) in mice, and jak3 KO results in predisposition to colitis (4). Shc also regulated cell survival through expression of bcl-2/bcl-xl (24). Our data showed that disruption in Jak3 interactions with Shc resulted in increased sensitivity toward staurosporine-induced apoptosis and compromised wound repair in IECs in a Shc domain-dependent manner. This was because in these cells, increased tyrosine phosphorylation of Jak3 led to an increased amount of severed actin filaments, which could occur because activated Jak3-mediated sustained tyrosine phosphorylation-driven increased severing activities by cytoskeletal proteins (6, 8, 25).
Taken together these results showed for the first time the structural determinants of Jak3 and Shc responsible for their interactions (Fig. 2H) and the molecular mechanism and physiological relevance of the regulation of Jak3 activation by Shc.
Supplementary Material
This work was supported by Crohn's & Colitis Foundation of America (CCFA) Grant 2188 and National Institutes of Health Grant DK081661 (to N. K.).

This article contains supplemental Table ST1 and Figs. S1–S9.
- JH
- Jak homology
- SH
- Src homology
- Shc
- Src homology 2 domain (SH2)-containing protein-tyrosine phosphatase
- CH
- calponin homology
- PID
- phosphotyrosine interaction domain
- PTP1B
- protein-tyrosine phosphatase 1B
- P
- phosphorylated
- IEC
- intestinal epithelial cell
- IP
- immunoprecipitations
- IB
- immunoblotting.
REFERENCES
- 1. Safford M. G., Levenstein M., Tsifrina E., Amin S., Hawkins A. L., Griffin C. A., Civin C. I., Small D. (1997) JAK3: expression and mapping to chromosome 19p12-13.1. Exp. Hematol. 25, 374–386 [PubMed] [Google Scholar]
- 2. Cornejo M. G., Boggon T. J., Mercher T. (2009) JAK3: a two-faced player in hematological disorders. Int. J. Biochem. Cell Biol. 41, 2376–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin Q., Lai R., Chirieac L. R., Li C., Thomazy V. A., Grammatikakis I., Rassidakis G. Z., Zhang W., Fujio Y., Kunisada K., Hamilton S. R., Amin H. M. (2005) Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am. J. Pathol. 167, 969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mishra J., Verma R. K., Alpini G., Meng F., Kumar N. (2013) Role of Janus kinase 3 in mucosal differentiation and predisposition to colitis. J. Biol. Chem. 288, 31795–31806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang L. J., Constantinescu S. N., Lodish H. F. (2001) The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol. Cell 8, 1327–1338 [DOI] [PubMed] [Google Scholar]
- 6. Mishra J., Karanki S. S., Kumar N. (2012) Identification of molecular switch regulating interactions of Janus kinase 3 with cytoskeletal proteins. J. Biol. Chem. 287, 41386–41391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mishra J., Waters C. M., Kumar N. (2012) Molecular mechanism of interleukin-2-induced mucosal homeostasis. Am. J. Physiol. Cell Physiol. 302, C735–C747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar N., Mishra J., Narang V. S., Waters C. M. (2007) Janus kinase 3 regulates interleukin 2-induced mucosal wound repair through tyrosine phosphorylation of villin. J. Biol. Chem. 282, 30341–30345 [DOI] [PubMed] [Google Scholar]
- 9. Wills M. K., Jones N. (2012) Teaching an old dogma new tricks: twenty years of Shc adaptor signalling. Biochem. J. 447, 1–16 [DOI] [PubMed] [Google Scholar]
- 10. Sakai R., Henderson J. T., O'Bryan J. P., Elia A. J., Saxton T. M., Pawson T. (2000) The mammalian ShcB and ShcC phosphotyrosine docking proteins function in the maturation of sensory and sympathetic neurons. Neuron 28, 819–833 [DOI] [PubMed] [Google Scholar]
- 11. Bonfini L., Migliaccio E., Pelicci G., Lanfrancone L., Pelicci P. G. (1996) Not all Shc's roads lead to Ras. Trends Biochem. Sci. 21, 257–261 [PubMed] [Google Scholar]
- 12. Lai K. M., Pawson T. (2000) The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 14, 1132–1145 [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar N., Khurana S. (2004) Identification of a functional switch for actin severing by cytoskeletal proteins. J. Biol. Chem. 279, 24915–24918 [DOI] [PubMed] [Google Scholar]
- 14. Genescà M., Sola A., Hotter G. (2006) Actin cytoskeleton derangement induces apoptosis in renal ischemia/reperfusion. Apoptosis 11, 563–571 [DOI] [PubMed] [Google Scholar]
- 15. Macchi P., Villa A., Giliani S., Sacco M. G., Frattini A., Porta F., Ugazio A. G., Johnston J. A., Candotti F., O'Shea J. J., et al. (1995) Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 [DOI] [PubMed] [Google Scholar]
- 16. Chen E., Staudt L. M., Green A. R. (2012) Janus kinase deregulation in leukemia and lymphoma. Immunity 36, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chrencik J. E., Patny A., Leung I. K., Korniski B., Emmons T. L., Hall T., Weinberg R. A., Gormley J. A., Williams J. M., Day J. E., Hirsch J. L., Kiefer J. R., Leone J. W., Fischer H. D., Sommers C. D., Huang H. C., Jacobsen E. J., Tenbrink R. E., Tomasselli A. G., Benson T. E. (2010) Structural and thermodynamic characterization of the TYK2 and JAK3 kinase domains in complex with CP-690550 and CMP-6. J. Mol. Biol. 400, 413–433 [DOI] [PubMed] [Google Scholar]
- 18. Ursini-Siegel J., Muller W. J. (2008) The ShcA adaptor protein is a critical regulator of breast cancer progression. Cell Cycle 7, 1936–1943 [DOI] [PubMed] [Google Scholar]
- 19. Haan C., Kreis S., Margue C., Behrmann I. (2006) Jaks and cytokine receptors: an intimate relationship. Biochem. Pharmacol. 72, 1538–1546 [DOI] [PubMed] [Google Scholar]
- 20. George R., Schuller A. C., Harris R., Ladbury J. E. (2008) A phosphorylation-dependent gating mechanism controls the SH2 domain interactions of the Shc adaptor protein. J. Mol. Biol. 377, 740–747 [DOI] [PubMed] [Google Scholar]
- 21. Sato K., Otsuki T., Kimoto M., Kakumoto M., Tokmakov A. A., Watanabe Y., Fukami Y. (1998) c-Src and phosphatidylinositol 3-kinase are involved in NGF-dependent tyrosine phosphorylation of Shc in PC12 cells. Biochem. Biophys. Res. Commun. 250, 223–228 [DOI] [PubMed] [Google Scholar]
- 22. Koo G. C., Tan S. Y., Tang T., Poon S. L., Allen G. E., Tan L., Chong S. C., Ong W. S., Tay K., Tao M., Quek R., Loong S., Yeoh K. W., Yap S. P., Lee K. A., Lim L. C., Tan D., Goh C., Cutcutache I., Yu W., Ng C. C., Rajasegaran V., Heng H. L., Gan A., Ong C. K., Rozen S., Tan P., Teh B. T., Lim S. T. (2012) Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2, 591–597 [DOI] [PubMed] [Google Scholar]
- 23. Ugi S., Imamura T., Ricketts W., Olefsky J. M. (2002) Protein phosphatase 2A forms a molecular complex with Shc and regulates Shc tyrosine phosphorylation and downstream mitogenic signaling. Mol. Cell. Biol. 22, 2375–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lord J. D., McIntosh B. C., Greenberg P. D., Nelson B. H. (1998) The IL-2 receptor promotes proliferation, bcl-2 and bcl-x induction, but not cell viability through the adapter molecule Shc. J. Immunol. 161, 4627–4633 [PubMed] [Google Scholar]
- 25. Kumar N., Tomar A., Parrill A. L., Khurana S. (2004) Functional dissection and molecular characterization of calcium-sensitive actin-capping and actin-depolymerizing sites in villin. J. Biol. Chem. 279, 45036–45046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.