Background: Voltage-gated potassium channels are regulated by their lipid environment.
Results: The first lipids KvAP channels come in contact with can only be exchanged if the channel is in the open state.
Conclusion: Lipids bound to Kv channels are accessible only in a state-dependent manner.
Significance: The high affinity between lipids and integral membrane protein suggests that both should be treated as one complex.
Keywords: Electrophysiology, Gating, Ion Channel, Membrane Reconstitution, Potassium Channel, DOTAP, Lipid Regulation, Voltage-gated Potassium Channel
Abstract
As all integral membrane proteins, voltage-gated ion channels are embedded in a lipid matrix that regulates their channel behavior either by physicochemical properties or by direct binding. Because manipulation of the lipid composition in cells is difficult, we investigated the influence of different lipids on purified KvAP channels reconstituted in planar lipid bilayers of known composition. Lipids developed two distinct and independent effects on the KvAP channels; lipids interacting with the pore lowered the energy barriers for the final transitions, whereas voltage sensor-bound lipids shifted the midpoint of activation dependent on their electrostatic charge. Above all, the midpoint of activation was determined only by those lipids the channels came in contact with first after purification and can seemingly only be exchanged if the channel resides in the open state. The high affinity of the bound lipids to the binding site has implications not only on our understanding of the gating mechanism but also on the general experimental design of any lipid dependence study.
Introduction
Voltage-gated potassium (Kv) channels play an important role in the repolarization of excitable membranes in nerve and muscle cells. They sense the membrane potential by four peripheral voltage sensing domains (VSD)3 arranged around a single ion conducting pore (1, 2). Each VSD comprises four helical transmembrane segments (S1–S4), which undergo conformational changes upon membrane depolarization driven by the positively charged arginine-rich S4 helix. This movement is energetically coupled to the pore helices (S5–S6) and triggers pore opening (reviewed in Ref. 3).
Recent studies suggest that the surrounding lipid matrix strongly affects KV channel activity. Lipids with negatively charged headgroups in the vicinity of the channels shifted activation to lower potentials, whereas positively charged headgroups had the opposite effect (4–7). A similar effect was observed when enzymatically digesting the positively charged choline in sphingomyelin, leaving only the negatively charged phosphate behind (8–10).
The lipid dependence of channel function can be used to regulate the state of the channels in the absence of a membrane potential, making them available for spectroscopic measurements. It has been confirmed that voltage- and lipid-controlled open and closed states are equivalent (7, 11). The outermost arginines are suggested to interact with the headgroups of the surrounding phospholipids to stabilize themselves in the low dielectric membrane environment and facilitate the movement of the VSD from resting to the activated state (2, 4, 8, 9, 12, 13). Accordingly, a binding site for negatively charged polyunsaturated fatty acids has been identified in the vicinity of the outer arginines in the VSD of Shaker channels (14). Nevertheless, Zheng et al. (11) reported that the phosphate-arginine interaction is not required for the lipid-induced conformational changes of the VSD. Instead, lipids immediately around the VSD would contribute to the lipid-dependent gating by forming a functional unit with the ion channels.
Evidence for tight interactions between the annular lipids and the ion channels also derive from structural observations. X-ray structures of the paddle chimera Kv1.2/2.1 and the voltage-gated sodium channels NavAb resolved several lipid molecules bound between the VSDs and pore interface and between adjacent VSDs (2, 15). Solid state NMR studies have shown that the VSDs interact extensively with both the hydrophobic acyl chain and the headgroup region of phospholipids (16, 17), but also that the lipids influence pore structure (18, 19).
Here, we investigated the influence of different lipids as well as their affinity to KvAP channels. We found two distinct and independent effects on the voltage-dependent activation provoked by the annular or bound lipids interacting with the voltage sensor domain and cytosolic pore region, respectively. The lipids bound to the voltage sensor domain seem to influence the equilibrium between the closed and open state and, thus, vary the V½ of the conductance-voltage relationship, whereas lipids at the cytosolic side affect the energy barriers of pore opening and entry into the inactivated state. The shift of activation potential shows preferential affinity for the initial lipids and state-dependent exchange of the lipids.
EXPERIMENTAL PROCEDURES
KvAP Channel Purification and Reconstitution in Lipid Vesicles
KvAP coding sequence was inserted in the pQE70 vector (Qiagen) and expressed in Escherichia coli strain M15 cells (Qiagen). Cultures were grown in LB medium with 100 μg/ml of ampicillin and 25 μg/ml of kanamycin. At A600 = 0.6, channel expression was induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside (Biolynx). At the same time, 5% glycerol (Sigma) and 10 mm BaCl2 (Fisher Scientific) were added. Temperature was lowered to 25 °C during expression. After 3 to 5 h bacterial cells were harvested and lysed by pressure (15,000 p.s.i.) with an EmulsiFlex-C5 (Avestin). Membranes were isolated by centrifugation at 200,000 × g and solubilized with 2% decylmaltoside (Anatrace) for 1 h at 4 °C. The sample was bound to a cobalt affinity column (Talon Superflow, Clontech) and washed with 15 mm imidazole (Sigma) before elution with 400 mm imidazole. The sample was maintained in a 200 mm NaCl, 50 mm KCl, 50 mm Hepes, pH 7.4, buffer containing 0.25% decylmaltoside. Presence and purity of the channel were assessed by immunoblotting and Coomassie staining.
Small unilamellar vesicles were prepared by vigorous sonication of the respective lipids: DPhPC (1,2-diphytanoyl-glycero-3-phosphocholine), DOTAP (1,2-dioleoyl-3-trimethylammonium propane), POPS (1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine), POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine), POePC (1-palmitoyl-2-oleoyl-sn-glycero-3-ethylphosphocholine), and POPG (1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (Avanti Polar Lipids). The small unilamellar vesicles were mixed with the sample at a ratio of 1:5 protein:lipid (w/w) at a final concentration of 10 mg/ml, vortexed well, shortly sonicated, and rocked for 1 h at room temperature. This sample was loaded in a dialysis device to exchange the detergent (Slide-A-Lyzer MINI 20,000 MWCO, Pierce) and kept at 4 °C. Dialysis buffer (450 mm KCl, 10 mm Hepes, pH 7.4) was exchanged twice a day for 5 days.
Electrophysiology Recordings
Lipids were dissolved in 25 mg/ml of decane to form planar bilayers over an aperture of 250 μm in a polymer partition that separates two chambers with symmetric solutions (150 mm KCl, 10 mm Hepes, pH 7.4) (20). Vesicles containing the channels were shortly sonicated and fused to the voltage-clamped bilayer. 0.25 μm sorbitol was variably added to the vesicles before sonication to help the fusion. The elicited macroscopic currents were recorded with an Axiovert 1D amplifier (Axon instruments) and registered using “GPatch.” When sufficient current was observed, the bilayer chamber was carefully washed. Holding potential was −100 mV and the bilayer was depolarized to potentials up to 100 mV in 10-mV increments. Between depolarizing pulses, the channels were held at −100 mV for 100 s. For measurements at higher temperatures, a heating block with temperature feedback was used. Recordings were analyzed using the “Analysis” software, and activation curves were fit to a single Boltzmann distribution using the following equation.
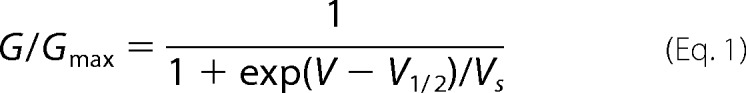 |
RESULTS
Limited exchange of Annular Lipids around KvAP
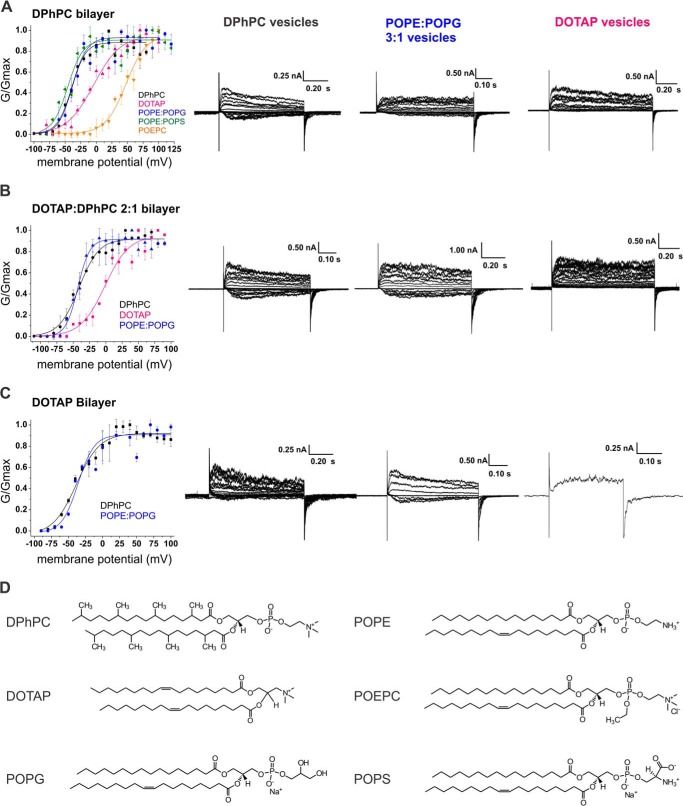
Purified KvAP were reconstituted into small unilamellar lipid vesicles composed of different common phospholipids. Both POPS (phosphoserine) and POPG (phosphoglycerol) bear one net negative charge in their headgroup, whereas POPE (phosphoethanolamine) and DPhPC (methylated tail phosphocholine) are zwitterionic and carry no net charge (Fig. 1d). Although not found in nature, DPhPC is widely used to form bilayers and has a high mechanical and chemical stability. POePC and DOTAP both carry a net positive charge in their headgroups. In POePC, the phosphate group is ethylated, whereas the phosphate has been removed in DOTAP. Primarily used for cell transfection, DOTAP has been shown to shift the voltage dependence of KvAP to more positive potentials, most likely by favoring the resting state of the VSD (4, 7, 11). Although DOTAP is not a phospholipid, KvAP reconstituted in pure DOTAP vesicles has been shown to adopt the native closed state conformation of the protein (7, 11, 16).
FIGURE 1.
Conductance-voltage relationship of KvAP in variable lipid conditions. KvAP channels were reconstituted in small unilamellar vesicles of DPhPC (black), POPE:POPG, 3:1 (blue), DOTAP (pink), POPC:POPE:POPS, 5:3:2 (green), and POePC (orange) and fused to a pure DPhPC planar lipid bilayer (A), a DOTAP:DPhPC, 2:1, bilayer (B), and a pure DOTAP bilayer (C). Voltage ranged from −100 to + 100 mV with 10 mV increments. Holding potential was kept at −100 mV. A single Boltzmann function was fit to the data (solid lines) and the activation parameters V½ and VS are presented in Table 1. The number of assays for each condition ranged from n = 2 to 7, error bars are S.D. For each bilayer condition, current traces are displayed for vesicles of DPhPC, POPE:POPG, 3:1, and DOTAP (from left to right). D, structures of DPhPC, DOTAP, POPG, POPE, POePC, and POPS.
In previous work (7), we noticed that the lipid composition of the proteoliposomes seems to play a more important role than that of the bilayer. This led us to the question whether interaction between voltage-gated ion channels and lipids is mediated via a high affinity binding site or whether it is a more indirect influence via the lipid environment. To investigate this question in more detail, we fused KvAP-proteoliposomes formed of DPhPC, POPE:POPG (3:1), and DOTAP into painted lipid bilayer of variable lipid composition and obtained the conductance-voltage relationships (GV; Fig. 1). A striking observation when comparing the voltage dependences of pore opening was that the GVs shifted according to the lipids in the vesicles not in the bilayer (Table 1). For instance, DOTAP vesicles evidently shifted the GV to more positive potentials and resulted in a V½ = (−4.3 ± 1.9) mV even in pure DPhPC bilayers. Vice versa, the GV was found at V½ ≈ −40 mV for vesicles formed of POPE:POPG or DPhPC irrespective of the bilayer composition. Unfortunately, no values are available for DOTAP vesicles fused to a DOTAP bilayer. Although the channels remained functional and yielded macroscopic current, fusion was very poor. Additionally, pure DOTAP bilayers did not remain stable long enough to achieve sufficient fusion and then record a complete voltage dependence protocol. We therefore used the mixture DOTAP:DPhPC, 2:1, which allowed us to gain reasonable bilayer stability to support insertion of sufficient channels to obtain a conductance-voltage relationship.
TABLE 1.
Midpoint of activation (V1/2) and steepness degree (Vs) of KvAP conductance-voltage relations (mV)
V1/2 (top) and Vs (bottom, italic) are presented for each bilayer and vesicle conditions were assessed. Parameters in light grey boxes correspond to the single Boltzmann fits of the data presented in Fig. 1 and show that the V1/2 values are dependent on the vesicles lipid content. (n = 2–7) V1/2 values in the light blue boxes show a clear affinity for DPhPC in pre-mixed vesicles of DOTAP and DPhPC (n = 2). V1/2 and Vs values in light green boxes show titration of DOTAP effects by addition of POPG in the bilayer. (n = 3) Error on values are S.D.
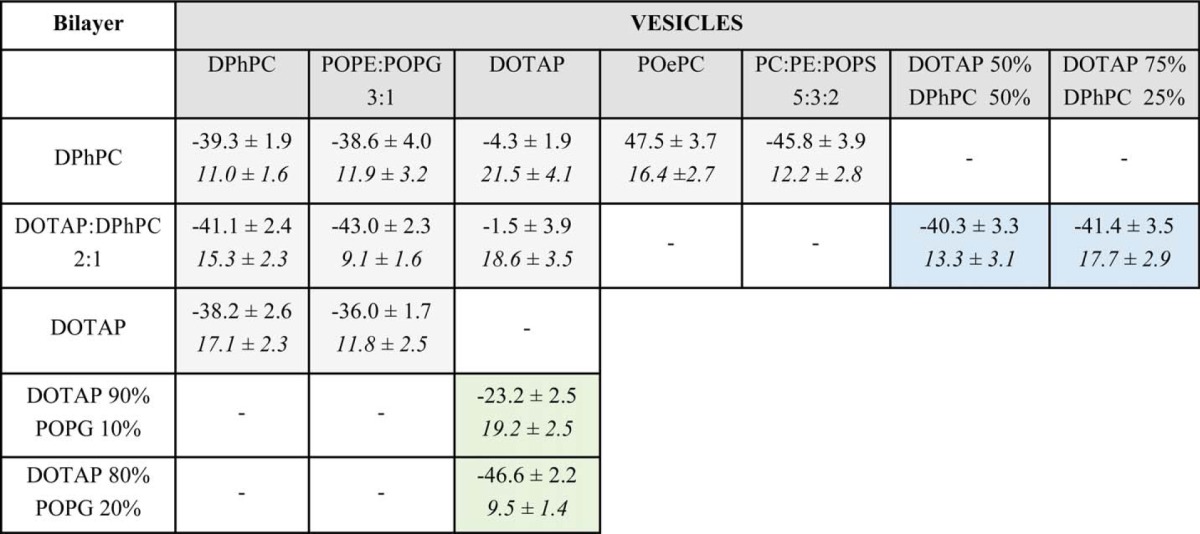
The positive shift of the conductance-voltage relationship was not specific for DOTAP. When we fused vesicles formed of POPS (negatively charged) and POePC (positively charged) into bilayers formed of DPhPC, the conductance-voltage relationships were shifted to more negative and positive potentials, respectively, indicating that the electrostatic charge of the lipid headgroups plays a significant role, as proposed previously (Fig. 1A, Table 1) (4, 8). However, the differences between PG and PS and between DOTAP and POePC suggest that the chemical structure and charge distribution also influence the voltage dependence of the channels.
In all cases, the bilayer composition had very little to no effect on the midpoint of activation. This would suggest very limited exchange between the annular lipids immediately surrounding the channels and the “bulk” lipids in the bilayer. To ensure that vesicle fusion had no substantial influence on bulk lipid composition, we estimated the surface area of bilayer and fused vesicles. If we consider an exaggerated number of 1000 fusion events to a bilayer with 250-μm diameter during a typical experiment, the surface ratio of vesicles versus bilayers would remain below 0.01%.4 At this low fraction, the influence of the vesicle lipids would be negligible with respect to the bulk lipids in a homogeneous mixture. The lipid “patch” created by a vesicle would have double the radius of the original vesicle. With diffusion constants in the range of 20 μm2/s, a complete exchange should occur within seconds (21). Both estimations indicate that the lipids surrounding the ion channels remain in close contact and do not diffuse into the main bilayer.
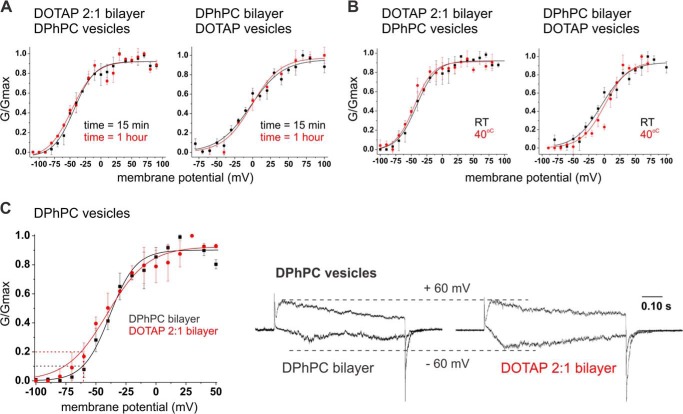
Despite the small surface area of the vesicle membrane, the differences in the physicochemical parameters of the lipids may slow down diffusion significantly. However, even 60 min after fusion of the vesicles, the conductance-voltage relationship was not altered independently of the lipid composition of bilayer and vesicles (Fig. 2A). We then verified whether the lack of mixing between DOTAP and DPhPC could be overcome by increasing the temperature and, thereby, the mobility of the lipids. Because neither of these two lipids have a phase transition in the physiological temperature range, we were able to perform bilayer measurements up to T = 40 °C without changing packing geometry. As shown in Fig. 2B, no temperature-related effect was noticed on either the midpoint or the steepness of the GV curves (Table 2). These results seem to indicate that the lipids that come into first contact with the protein have a high affinity to the protein and are thus not easily exchanged. Even at increased temperature and after longer time, the activation behavior persisted, suggesting either a strong channel-lipid interaction or a confinement of lipid molecules to a “microdomain” around the channel. Such microdomains have been suggested previously based on results obtained from channels expressed in Xenopus oocytes (9).
FIGURE 2.
Lack of exchange between vesicles and bilayer lipids and bulk effect of DOTAP on DPhPC vesicles. A, lipids from the bilayer do not over time mix with lipids from the vesicles. DPhPC and DOTAP vesicles containing KvAP fused to a DOTAP:DPhPC, 2:1, bilayer (left) and to a DPhPC bilayer (right), respectively, are incubated for 15 min (black) or 1 h (red) at a holding potential of −100 mV. In both cases, no significant change of the GV was observed. B, increase of the temperature does not promote mixing between the lipids of the bilayer and the vesicles. DPhPC and DOTAP vesicles containing KvAP were fused to a DOTAP:DPhPC, 2:1, bilayer (left) and a DPhPC bilayer, respectively (right). The bilayers were kept at room temperature (black) or between 35 and 40 °C (red). No significant changes indicated lipid mixing between DOTAP and DPhPC at higher temperature. V½ and VS values are presented in Table 2 (n = 2–4). C, the voltage dependence of KvAP reconstituted in DPhPC vesicles was shallower when fused to a DOTAP:DPhPC, 2:1, bilayer (red) than to a DPhPC bilayer (black). Current traces show one normalized pulse at a saturating voltage (+60 mV) and one less depolarizing pulse where the difference of steepness of the GV curves creates a difference in the open probability (−60 mV, dotted lines). The data are fit with a single Boltzmann function (solid lines, Table 1), n = 3 and 7, error bars are S.D.
TABLE 2.
Midpoint of activation (V1/2) and steepness degree (VS) of conductance-voltage relationship of KvAP in function of time, temperature, and state (mV)
V1/2 (top, normal) and VS (bottom, italic) are presented for different conditions of time, temperature, or holding potential for the lipid conditions indicated. Unless specified, the voltage was held at −100 mV during the experiments. The number of experiments ranged from n = 2 to 5, errors are S.D.
| DPhPC vesicles in DOTAP, 2:1, bilayer |
DOTAP vesicles in DPhPC bilayer |
|||||||
|---|---|---|---|---|---|---|---|---|
| 15 min | 1 h | 22 °C | 40 °C | 15 min | 1 h | 22 °C | 40 °C | 30 min (+80 mV) |
| −40.6 ± 2.6 | −41.8 ± 2.3 | −41.1 ± 2.4 | −45.2 ± 2.9 | −2.8 ± 2.2 | −1.3 ± 3.6 | −4.3 ± 1.9 | 1.0 ± 2.7 | −16.5 ± 2.6 |
| 16.7 ± 2.4 | 16.8 ± 2.9 | 15.3 ± 2.3 | 15.2 ± 2.7 | 22.5 ± 2.0 | 19.7 ± 3.0 | 21.5 ± 4.1 | 19.0 ± 2.0 | 19.6 ± 2.2 |
KvAP in the cationic DOTAP or POePC had a significantly shallower GV than in DPhPC or POPE:POPG. Although both effects, shift of the midpoint of activation and the change in steepness, might have been caused by the same mechanism, it seems that they occur independently of one another. When DPhPC vesicles were fused to DOTAP bilayers, we noted a small but clear steepness decrease in the activation curve despite not having any effect on the V½ (Fig. 2c). The VS value increased by 6 mV to 17.1 ± 2.3 mV. Such a shallow voltage dependence was also observed when KvAP was reconstituted in DOTAP vesicles (VS > 18 mV). Although this was a subtle effect, it was emphasized in the onset of activation, which was shifted such that currents were observed at more negative potentials (Fig. 2C, right). Although the VS values for DPhPC vesicles increased with higher DOTAP content in the bilayer, the reverse effect was not observed, i.e. DPhPC in the membrane did not render the activation steeper for the DOTAP vesicles (Fig. 1, Table 1). Similarly, the presence of DOTAP in the bilayer had no effect on KvAP in the POPE:POPG mixture vesicles, which we will discuss later.
KvAP Shows Preferential Affinity to DPhPC
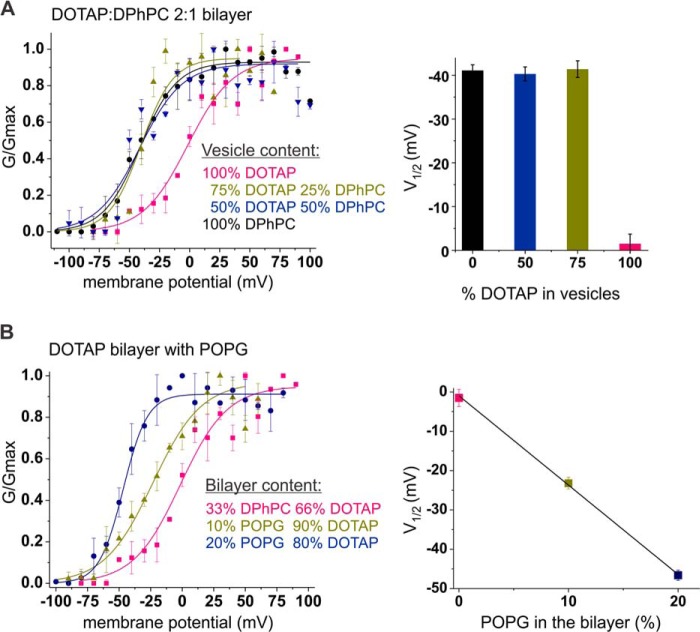
According to the above results, it seems that an interaction develops between the channel and the lipids during reconstitution of KvAP into the vesicles when detergent micelles are exchanged for lipid molecules. These lipids cannot be exchanged afterward, not by exposing the channels to high concentrations of a different lipid, and also not by increasing the incubation time or temperature. The most striking result, however, was that exchange occurs in neither direction, neither from DOTAP to DPhPC nor the other way around. To determine which lipid shows a higher affinity when exposing the isolated KvAP in detergent to pre-mixed lipid compositions, we reconstituted KvAP in vesicles of variable ratios of DOTAP and DPhPC. Up to a 3:1 ratio DOTAP:DPhPC in the vesicles, KvAP followed a GV with a negative V½ corresponding to DPhPC vesicles (Fig. 3A). Again, the presence of DOTAP was translated only by the shallower voltage dependence of the activation curves (Table 1). These results indicate that KvAP has a marked affinity for DPhPC over DOTAP. The higher affinity for DPhPC might be explained by the lack of the presumably stabilizing phosphate headgroup in the non-phospholipid DOTAP. However, despite this natural preference for DPhPC when simultaneously exposed, in the bilayer, it cannot displace DOTAP molecules originating from the vesicles, indicating a very low dissociation rate from the proposed binding site(s) at the VSD (Fig. 1A).
FIGURE 3.
KvAP lower affinity for DOTAP and titration of its effects by POPG. A, preferential affinity of KvAP for DPhPC over DOTAP within vesicles. KvAP channels were reconstituted in vesicles containing an increasing amount of DOTAP mixed with DPhPC: 0% (black), 50% (blue), 75% (green), and 100% (pink) DOTAP vesicles were fused to a DOTAP:DPhPC, 2:1, bilayer. No change in the midpoint of activation of the conductance-voltage relationship could be related to the increase of DOTAP concentration up to 75% in the pre-mixed vesicles. Data were fit with a single Boltzmann function (solid lines, Table 1) and V½ values are presented in the right panel. Number of assays are n = 2–7 and error bars are S.D. B, addition of negatively charged POPG in the bilayer titrates DOTAP effects. KvAP channels reconstituted in DOTAP vesicles were fused to a DOTAP bilayer containing 10 (green) or 20% (blue) POPG. For comparison, KvAP in DOTAP vesicles fused to DOTAP:DPhPC, 2:1, bilayer (pink) is shown. 10% of POPG in the bilayer is enough to shift the V½ value, but the VS value is not yet affected. With 20% of negative charges in the bilayer, the V½ and VS values are both restored to the POPE:POPG vesicles. The effect of DOTAP neutralization by POPG on the V½ is shown in the right panel. Values are shown in Table 1, n = 3–4, error bars are S.D.
DOTAP Effect Is Titrated by Negative Charged Phospholipids (POPG)
In order to modulate the electric field around KvAP (7), DOTAP, with its very high positive surface potential (22), has to enter into very close proximity of the channels. From here, it seems, they cannot be displaced once bound. The low dissociation rate and the fact that the bulk lipids influence the GV steepness indicate that a binding site rather than a microdomain was responsible for the shift of the GV. But can we also influence the V½ directly from the bulk lipids? To answer this, we tested whether the positive charges of DOTAP may be titrated by addition of negative charges into the bulk bilayer. The positive charge in DOTAP is located at the position of the negatively charged phosphate groups of the phospholipids. If the lipids can freely diffuse also around the channel, the anionic POPG (Fig. 1D) should be able to neutralize one or both effects of the cationic DOTAP. We added increasing percentages (10 and 20%) of POPG directly in a bilayer formed otherwise only of DOTAP and fused pure DOTAP vesicles into these bilayers (Fig. 3B). The V½ shifted linearly with increasing POPG content until, at 20% POPG in the bilayer, the effects of DOTAP on KvAP were neutralized. V1/2 and VS values corresponded to the POPE:POPG vesicles fused to a DOTAP bilayer (Table 1). At 10% POPG, the V½ adopted an intermediate value and the slope was not yet affected.
The fact that we were able to neutralize the effect of DOTAP with POPG confirms that the lipids can diffuse, at least to a certain extent, between the bulk bilayer and the vesicle lipids. It also confirms the hypothesis that the positive charge plays the decisive role, as titration with a counter charge neutralized the DOTAP effects. We cannot be sure whether POPG displaces DOTAP from the presumed binding site or whether it occupies a site as the closest neighbor and thus neutralizes the electrostatic charge of a “bound” DOTAP. The functional effects would be alike. The titration explains, however, why no increase in the slope upon addition of DOTAP was observed in POPE:POPG bilayers but in DPhPC bilayers (Fig. 1). The DOTAP in the bulk lipids would be neutralized by the POPG in the microenvironment.
Access to the Lipid Binding Site in Kv Channels Seems to be State-dependent
The preferential affinity of KvAP to the initial lipids may be explained if the presumed binding site was only accessible when the channels are in detergent micelles. The binding site might have a higher accessibility or a lower affinity in the open than in the closed state. In detergent micelles, the channels would not be exposed to any membrane potential and would, thus, be in the open state, whereas they would be closed once a negative membrane potential is applied. Jensen et al. (23), for instance, suggested that the entire VSD turns and separates from the pore during gating. It is also known that the VSD undergoes major internal conformational changes upon gating, opening the possibility that the binding site or access to it is altered.
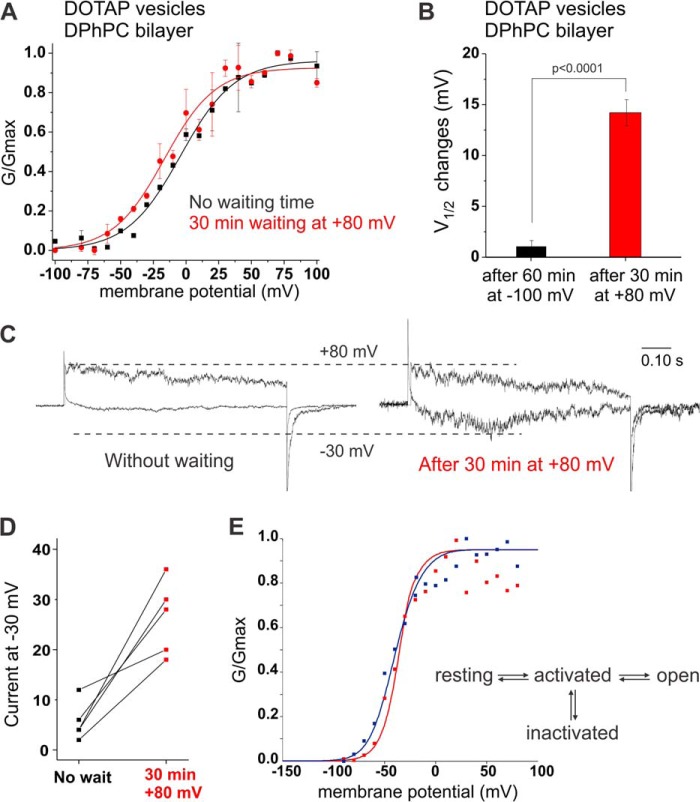
In a typical experiment, we hold the membranes at a potential of −100 mV and the channels in the closed state during the waiting or incubation periods. If the affinity for the initial lipids is indeed lower in the open state, we might be able to prompt their exchange when holding at depolarized potentials. We, thus, held the membrane potential at +80 mV after fusing DOTAP-KvAP proteoliposomes into DPhPC bilayers and determined the conductance-voltage relationship. Under these conditions, the midpoint of activation was shifted to more hyperpolarized potentials (Fig. 4A).
FIGURE 4.
State-dependent accessibility of lipid binding site. A, KvAP reconstituted in DOTAP vesicles were fused to a DPhPC bilayer. Voltage pulses ranging from −100 to +100 mV were applied immediately after fusion (black) or after waiting 30 min at +80 mV (red). Holding at this depolarizing pulse promoted a shift of the midpoint of activation by −12.6 mV. V½ and VS values are presented in Table 2, n = 5, error bars are S.D. B, changes observed in the V½ values when KvAP channels reconstituted in DOTAP vesicles and fused to a DPhPC bilayer are held 60 min at −100 mV (black) and 30 min at +80 mV (red, p < 0.0001). C, example of current traces at −30 mV compared with +60 mV before and after 30 min waiting time at +80 mV. D, normalized currents at −30 mV for each bilayer experiment show an increase in the open probability after waiting 30 min at +80 mV. E, kinetic model for the gating behavior of KvAP (27). The transition from the resting to the activated state is the major charge carrying step. In the graph, the GV for KvAP reconstituted in DPhPC vesicles and fused to DPhPC (red) and DOTAP:DPhPC, 2:1 (blue), are shown. The data has been fitted to the kinetic model, where the blue fit has a 2.3-fold lower energy barrier for transitions from the activated to the open and inactivated state.
Assuming a symmetric fusion of the vesicles into the bulk bilayer, bilayer and solutions in our system are fully symmetric and should behave identically at symmetric potentials. The difference in the lipid effect at reversed polarity must therefore be caused by the channel proteins, suggesting that either affinity or accessibility of the lipid binding site(s) is increased in the open state.
Despite the large shift to V½ = −16.5 mV from initially V½ = −3.9 mV (p < 0.00005), the effect is somewhat less obvious due to the shallow GVs. However, in comparison, the V½ shifted by +1.4 mV over a period of 60 min when held at −100 mV (p < 0.1)5 (Fig. 4b). Also inward currents only developed after holding the membrane at depolarized potentials (Fig. 4, C and D). We were not able to reach as low as −40 mV, indicating that the local concentrations did not allow a complete exchange.
DISCUSSION
As integral membrane proteins, voltage-gated ion channels are naturally associated very closely with lipids of the surrounding membrane. It is, therefore, not unexpected that lipids have an influence on the channels' function and modulate their voltage dependence (4–9, 11, 16–19, 24–27). Here, we show that lipids affect Kv channels in two independent pathways that seem to be assigned to a state-dependent interaction of lipids bound to the voltage sensor domain, shifting the GVs, and an interaction with the lower pore region altering GV steepness.
Although we found a higher affinity for DPhPC than for DOTAP in the initial binding (Fig. 3A), bound DOTAP is not easily displaced by DPhPC in the bulk bilayer. Even with KvAP in the open state, no complete exchange was possible. The dissociation rate of the bound lipids, thus, has to be very low. As the binding can be mediated by lipids with such different headgroup characteristics including opposite charges and different steric geometry, the lipids are either indeed trapped at their position or the binding is mediated by the acyl chains, a notion supported by NMR studies of the isolated VSD of KvAP (16).
Decisive for the effect on the conductance-voltage relationship seems to be the charge distribution in the headgroups, with cationic headgroups (TAP, ePC) shifting it to depolarized potentials and the anionic headgroup (PS) shifting it to hyperpolarized potentials. Titration with the anionic POPG reversed the effect of the cationic DOTAP. This is consistent with previous findings that the negatively charged polyunsaturated fatty acids shift the V½ to hyperpolarized potentials, as does the cleavage of sphingomyelin by sphingomyelinase D leading to anionic ceramide-phosphate (8–10).
POePC had a more pronounced effect than DOTAP on the voltage dependence of KvAP. They vary slightly in their acyl chains (dioleoyl versus palmitoyloleoyl; Fig. 1D), leading to a difference in the bilayer thickness of 1–2 Å (28, 29). More importantly, the location of the positive charge in the headgroups differs: in POePC, the phosphate group is neutralized by ethylation, whereas in DOTAP the entire phosphate group has been removed (Fig. 1D). As a consequence, the cationic aminium takes the place of the phosphate group. This changes the location of the positive charge with respect to the arginines in the S4 helix. The location of the charge in DOTAP mimics the polyunsaturated fatty acids despite their opposite charge. It would thus destabilize the second gating charge of the S4 in the activated state (25). The charge of POePC is located such that the destabilizing effect of DOTAP is amplified.
We found that the bound lipids, having variable effects on the activation kinetics, seem to anneal to the channel in a state-dependent fashion. Once bound to the channel, we only observed lipid exchange if the channel was in the open state, not as long as it remained in the closed state. The lack of exchange in the closed state raises the question whether experimental design could possibly provoke this effect. We are fusing solvent-free small unilamellar vesicles in painted planar lipid bilayers that contain residues of solvent. It has also been shown that different lipids do not necessarily form a homogenous mixture but develop domains of different thickness or phases. It is, however, unlikely that these effects are responsible for the lack of exchange in the closed state. The thickness of the bilayers varies only by 1–2 Å among POPE, DOTAP, and DPhPC according to small angle neutron scattering measurements (28, 30). Against a physical separation by phase or domain borders between vesicle and bulk lipids also argues that the bulk lipids can come into close vicinity of the channels, as evidenced by the modulation of the GVs by DOTAP and POPG in the bulk bilayer (Figs. 2C and 3B). Finally, exchange is possible in a state-dependent manner in a fully symmetric system apart from channel orientation, indicating that not the lipid mixing in the bilayer but the exchange of the bound lipids is inhibited.
As mentioned above, the different lipids had a dual effect on the KvAP channels, both of which were dependent on the electrostatic charge of the headgroups: first the bound lipids shifted the GV curves, and second, the bulk lipids affected the steepness of the GV curves. The question remains as to what exactly defines “bound lipids”; are those the annular lipids immediately neighboring the channel protein or single lipid molecules linked to a specific binding pocket, as suggested earlier (19, 25)? In the crystal structure of the Kv1.2/2.1 chimera, a number of lipids surrounding the channel protein are resolved (2). Although all of them might be tightly bound to the channel, it is unlikely that all of them significantly influence channel gating. It would also be unlikely that all of the annular lipids would change their affinity in a state-dependent manner. A state-dependent “trapping” or affinity change is more feasible for one or a few lipid molecules. The most probable position for such a specific binding site is the N-terminal S4 near the first gating charges. This region has not only been shown to strongly interact with lipids (12, 16), but Börjesson and Elinder (25) also estimated that, in the Shaker potassium channel, the charge of a polyunsaturated fatty acids would be 6 and 16 Å away from the first gating charge in the open and closed states, respectively. This would place the bound lipid in the cleft between the S4 and the pore domain (Fig. 5). This location has also been identified as the sphingomyelin binding site based on competitive interaction with tarantula toxins (26). The state dependence of this binding site might be due to a general movement of the VSD with respect to the pore domain, trapping the lipids, or internal conformational changes of the VSD leading to different affinities in the closed and open states.
FIGURE 5.
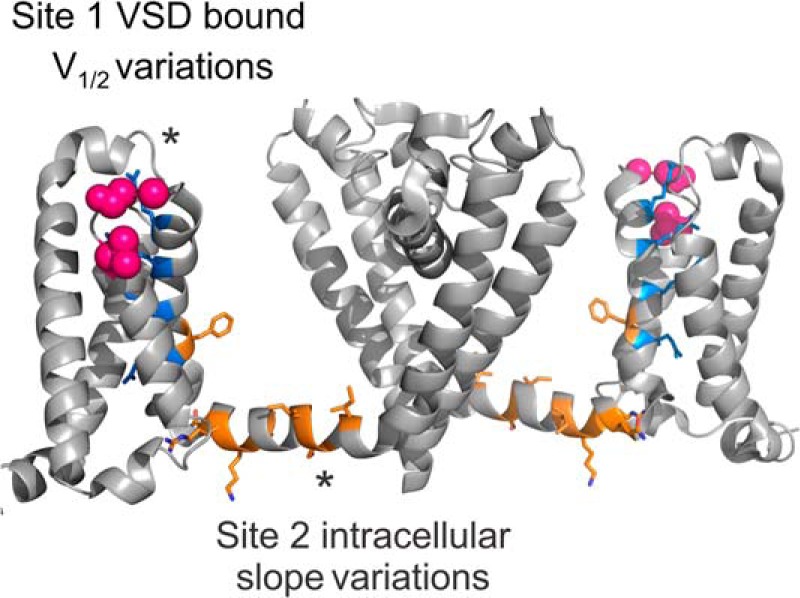
Model for lipid binding sites in KvAP. Side view of 2 subunits of the sodium channel NavAb (PDB 4EKW) (40) are shown, with gating arginines colored in blue. We located the site responsible for the V½ variations at the extracellular region of the voltage sensor, possibly at the S3–S4 loop, near the first gating charge. This region (pink spheres) has already been shown to strongly interact with lipids, and has also been identified for sphingomyelin based on competitive interaction with spider toxins (8–10, 14, 16, 17, 25, 26). We located the second site at the intracellular side of the channel. The residues in orange correspond to those shown to interact with lipids in the crystal structure of the Kv1.2/2.1 chimera (2). Lipids interacting in this region could affect the energy barriers of pore opening and entry into the inactivated state, possibly by interacting with the S4–S5 linker.
In addition to being shifted, the conductance-voltage relationship also displayed a lower slope in the presence of cationic lipids. A lower slope of the GV is typically associated with a reduction in the effective charge z of the voltage sensor movement. However, a reduction in z would lead to a shift to more hyperpolarized potentials, which had to be compensated by the destabilization of the activated state described above. The fact that the decreased steepness can be prompted by diffusion of DOTAP from the bulk membrane (Fig. 2C) suggests that steepness and midpoint are altered independently of one another at two different sites of interaction. Only one of the sites shows state-dependence, and they display different affinity. The fact that DOTAP can influence the GV of KvAP in DPhPC but not vice versa could be caused if the second interaction site has a higher affinity for DOTAP.
As the effect of DOTAP on KvAP in DPhPC is very subtle (Fig. 2C), the two effects might also be directly coupled. A lower steepness with a shift of the midpoint of activation to more depolarized potentials can be caused by shifting a second voltage-dependent step to more depolarized potentials (31). This would also act on the region around the cytosolic pore gate and have the same structural implications here but would exclude a direct interaction with the VSD at the outer surface. This possibility is therefore unlikely.
What can we say about the location of the second interaction site? As part of the Kv channel family, KvAP activates by movement of the four VSDs in a voltage-dependent manner; once all four S4 are in the activated conformation, concerted (cooperative) movements follow where all VSDs move together, leading to pore opening (32–38). In most Kv channels, pore opening is followed by fast (N-) and slow (C-type) inactivation. Although the activation pathway is thought to be identical for KvAP, inactivation has been proposed to be accessible directly from the activated, not open (pre-open) state (Fig. 4E) (27). This means that a fraction of the KvAP channels enter the inactivated instead of the open state once activated, similar to KV4 channels (39). In such a model, the observed lower steepness of the conductance-voltage relationship without changing the midpoint of activation can be achieved by lowering the energy barriers for both pore opening and inactivation (Fig. 4E).
What does this mean structurally? Considering that the channel has to expand to open the lower pore (7), it seems that DOTAP in the lipid matrix facilitates this opening by lowering the energy required for displacing the lipid matrix during a volume change. The strong inactivation that occurs prior to pore opening in KvAP has been proposed to be caused by a weak coupling efficiency between the VSD and pore domain (electromechanical coupling) (27). Electromechanical coupling is mediated by the S4–S5 linker and the C-terminal S6. During inactivation of KvAP, the link between the S4–S5 linker and the C-terminal S6 would slip (closed state inactivation, reviewed in Ref. 39). Thus both pore opening and inactivation occur at the helical bundle crossing and are related to the expansion of this region. DOTAP in the bulk bilayer influences mainly these final transitions of the gating process, whereas the electrostatic charge distribution of the bound lipids influences the gating charge movement.
We can therefore hypothesize that the second interaction site might be found close to the cytosolic pore gate and the region of electromechanical coupling. In Fig. 5, those residues in the region that have been identified by Long et al. (2) to interact with the surrounding lipids are marked in yellow.
CONCLUSIONS
According to the distinct effects on KvAP gating, we have to distinguish between the lipids bound to specific binding sites at the outer VSD and the lipids in the vicinity of the channel in the cytosolic membrane leaflet. Both influence KvAP gating. The effect of the bound lipids is mediated by the electrostatic charge of the headgroup, suggesting that charged lipid-like compounds offer an important pharmacological tool for the modulation of ion channels as drug targets, for instance, implicating epilepsy, cardiac arrhythmias, or ataxia. However, whereas the electrostatic charge distribution and affinity would allow easy tuning of the channels, the challenge would be to target specific cells or proteins.
The state-dependent affinity to the channel not only has implications for the structural changes during gating but should also be drawn into consideration when investigating protein lipid interactions. In this case, no effect would be observed when cells were held at resting potentials. If such a strong bond prevails also in the open state or for non-voltage-dependent proteins, protein and the bound lipids have to be considered as one macromolecular complex.
Acknowledgments
We thank Michel Brunette and Mireille Marsolais for technical assistance and Drs. James G. Omichinski and Nazzareno D'Avanzo for helpful discussions.
This work was supported in part by Canadian Institutes for Health Research Grant MOP-102689, Natural Sciences and Engineering Research Council Grant 327201-2012, and Canadian Foundation for Innovation Grant 950-225005. GÉPROM is a research group supported by the Fonds de recherche du Québec-Santé (FRQS).
The surface ratio between 1000 spheres (vesicles) of a typical radius r1 = 50 nm and a circle (bilayer) of radius r2 = 250 μm is 1.6 × 10−4 < 0.01%. If we calculate with an upper limit for the small unilamellar vesicle radius of r1 = 200 nm, the ratio would still remain below 0.3%.
Indicating that the shift when holding at −100 mV is not significant (p < 0.1), whereas the shift at +80 mV is significant (p < 0.00005).
- VSD
- voltage sensing domain
- DPhPC
- 1,2-diphytanoyl-glycero-3-phosphocholine
- DOTAP
- 1,2-dioleoyl-3-trimethylammonium propane
- POPS
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine
- POPE
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- POePC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-ethylphosphocholine
- POPG
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-1′-rac-glycerol
- GV
- conductance-voltage relationship.
REFERENCES
- 1. Long S. B., Campbell E. B., Mackinnon R. (2005) Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 [DOI] [PubMed] [Google Scholar]
- 2. Long S. B., Tao X., Campbell E. B., MacKinnon R. (2007) Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 [DOI] [PubMed] [Google Scholar]
- 3. Blunck R., Batulan Z. (2012) Mechanism of electromechanical coupling in voltage-gated potassium channels. Front. Pharmacol. 3, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt D., Jiang Q. X., MacKinnon R. (2006) Phospholipids and the origin of cationic gating charges in voltage sensors. Nature 444, 775–779 [DOI] [PubMed] [Google Scholar]
- 5. Börjesson S. I., Hammarström S., Elinder F. (2008) Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids. Biophys. J. 95, 2242–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blunck R., Scheel O., Müller M., Brandenburg K., Seitzer U., Seydel U. (2001) New insights into endotoxin-induced activation of macrophages: involvement of a K+ channel in transmembrane signaling. J. Immunol. 166, 1009–1015 [DOI] [PubMed] [Google Scholar]
- 7. Faure É., Starek G., McGuire H., Bernèche S., Blunck R. (2012) A limited 4-Å radial displacement of the S4–S5 linker is sufficient for internal gate closing in Kv channels. J. Biol. Chem. 287, 40091–40098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramu Y., Xu Y., Lu Z. (2006) Enzymatic activation of voltage-gated potassium channels. Nature 442, 696–699 [DOI] [PubMed] [Google Scholar]
- 9. Xu Y., Ramu Y., Lu Z. (2008) Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature 451, 826–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Combs D. J., Shin H. G., Xu Y., Ramu Y., Lu Z. (2013) Tuning voltage-gated channel activity and cellular excitability with a sphingomyelinase. J. Gen. Physiol. 142, 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng H., Liu W., Anderson L. Y., Jiang Q. X. (2011) Lipid-dependent gating of a voltage-gated potassium channel. Nat. Commun. 2, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuello L. G., Cortes D. M., Perozo E. (2004) Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science 306, 491–495 [DOI] [PubMed] [Google Scholar]
- 13. Freites J. A., Tobias D. J., von Heijne G., White S. H. (2005) Interface connections of a transmembrane voltage sensor. Proc. Natl. Acad. Sci. U.S.A. 102, 15059–15064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Börjesson S. I., Parkkari T., Hammarström S., Elinder F. (2010) Electrostatic tuning of cellular excitability. Biophys. J. 98, 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Payandeh J., Scheuer T., Zheng N., Catterall W. A. (2011) The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krepkiy D., Gawrisch K., Swartz K. J. (2012) Structural interactions between lipids, water and S1–S4 voltage-sensing domains. J. Mol. Biol. 423, 632–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krepkiy D., Mihailescu M., Freites J. A., Schow E. V., Worcester D. L., Gawrisch K., Tobias D. J., White S. H., Swartz K. J. (2009) Structure and hydration of membranes embedded with voltage-sensing domains. Nature 462, 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Cruijsen E. A., Nand D., Weingarth M., Prokofyev A., Hornig S., Cukkemane A. A., Bonvin A. M., Becker S., Hulse R. E., Perozo E., Pongs O., Baldus M. (2013) Importance of lipid-pore loop interface for potassium channel structure and function. Proc. Natl. Acad. Sci. U.S.A. 110, 13008–13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weingarth M., Prokofyev A., van der Cruijsen E. A., Nand D., Bonvin A. M., Pongs O., Baldus M. (2013) Structural determinants of specific lipid binding to potassium channels. J. Am. Chem. Soc. 135, 3983–3988 [DOI] [PubMed] [Google Scholar]
- 20. Groulx N., Juteau M., Blunck R. (2010) Rapid topology probing using fluorescence spectroscopy in planar lipid bilayer: the pore-forming mechanism of the toxin Cry1Aa of Bacillus thuringiensis. J. Gen. Physiol. 136, 497–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaede H. C., Gawrisch K. (2003) Lateral diffusion rates of lipid, water, and a hydrophobic drug in a multilamellar liposome. Biophys. J. 85, 1734–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zuidam N. J., Barenholz Y. (1997) Electrostatic parameters of cationic liposomes commonly used for gene delivery as determined by 4-heptadecyl-7-hydroxycoumarin. Biochim. Biophys. Acta 1329, 211–222 [DOI] [PubMed] [Google Scholar]
- 23. Jensen M. Ø., Jogini V., Borhani D. W., Leffler A. E., Dror R. O., Shaw D. E. (2012) Mechanism of voltage gating in potassium channels. Science 336, 229–233 [DOI] [PubMed] [Google Scholar]
- 24. Abbott G. W. (2006) Molecular mechanisms of cardiac voltage-gated potassium channelopathies. Curr. Pharm. Des. 12, 3631–3644 [DOI] [PubMed] [Google Scholar]
- 25. Börjesson S. I., Elinder F. (2011) An electrostatic potassium channel opener targeting the final voltage sensor transition. J. Gen. Physiol. 137, 563–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milescu M., Bosmans F., Lee S., Alabi A. A., Kim J. I., Swartz K. J. (2009) Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nat. Struct. Mol. Biol. 16, 1080–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt D., Cross S. R., MacKinnon R. (2009) A gating model for the archeal voltage-dependent K+ channel KvAP in DPhPC and POPE:POPG decane lipid bilayers. J. Mol. Biol. 390, 902–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kučerka N., Nieh M. P., Katsaras J. (2011) Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta 1808, 2761–2771 [DOI] [PubMed] [Google Scholar]
- 29. Heberle F. A., Petruzielo R. S., Pan J., Drazba P., Kučerka N., Standaert R. F., Feigenson G. W., Katsaras J. (2013) Bilayer thickness mismatch controls domain size in model membranes. J. Am. Chem. Soc. 135, 6853–6859 [DOI] [PubMed] [Google Scholar]
- 30. Salvati A., Ristori S., Oberdisse J., Spalla O., Ricciardi G., Pietrangeli D., Giustini M., Martini G. (2007) Small angle scattering and ζ potential of liposomes loaded with octa(carboranyl)porphyrazine. J. Phys. Chem. B 111, 10357–10364 [DOI] [PubMed] [Google Scholar]
- 31. Batulan Z., Haddad G. A., Blunck R. (2010) An intersubunit interaction between S4–S5 linker and S6 is responsible for the slow off-gating component in Shaker K+ channels. J. Biol. Chem. 285, 14005–14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zagotta W. N., Hoshi T., Dittman J., Aldrich R. W. (1994) Shaker potassium channel gating: II: transitions in the activation pathway. J. Gen. Physiol. 103, 279–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoppa N. E., McCormack K., Tanouye M. A., Sigworth F. J. (1992) The size of gating charge in wild-type and mutant Shaker potassium channels. Science 255, 1712–1715 [DOI] [PubMed] [Google Scholar]
- 34. Schoppa N. E., Sigworth F. J. (1998) Activation of Shaker potassium channels. III. An activation gating model for wild-type and V2 mutant channels. J. Gen. Physiol. 111, 313–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalstrup T., Blunck R. (2013) Dynamics of internal pore opening in KV channels probed by a fluorescent unnatural amino acid. Proc. Natl. Acad. Sci. U.S.A. 110, 8272–8277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pathak M., Kurtz L., Tombola F., Isacoff E. (2005) The cooperative voltage sensor motion that gates a potassium channel. J. Gen. Physiol. 125, 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ledwell J. L., Aldrich R. W. (1999) Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J. Gen. Physiol. 113, 389–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith-Maxwell C. J., Ledwell J. L., Aldrich R. W. (1998) Role of the S4 in cooperativity of voltage-dependent potassium channel activation. J. Gen. Physiol. 111, 399–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bähring R., Covarrubias M. (2011) Mechanisms of closed-state inactivation in voltage-gated ion channels. J. Physiol. 589, 461–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Payandeh J., Gamal El-Din T. M., Scheuer T., Zheng N., Catterall W. A. (2012) Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 486, 135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]