Background: CblC is a B12-processing enzyme that converts cyanocobalamin to cob(II)alamin.
Results: CblC catalyzes two previously unknown reactions: GSH-dependent reductive decyanation of CNCbl and reduction of OH2Cbl.
Conclusion: Worm and human CblC activities show different susceptibilities to oxygen.
Significance: The use of GSH for single-electron transfer is unexpected and expands the range of catalytic reactions supported by CblC.
Keywords: Adenosylcobalamin (AdoCbl), Enzyme Mechanism, Glutathione, Redox, Vitamin, Vitamin B12
Abstract
CblC is involved in an early step in cytoplasmic cobalamin processing following entry of the cofactor into the cytoplasm. CblC converts the cobalamin cargo arriving from the lysosome to a common cob(II)alamin intermediate, which can be subsequently converted to the biologically active forms. Human CblC exhibits glutathione (GSH)-dependent alkyltransferase activity and flavin-dependent reductive decyanation activity with cyanocobalamin (CNCbl). In this study, we discovered two new GSH-dependent activities associated with the Caenorhabditis elegans CblC for generating cob(II)alamin: decyanation of CNCbl and reduction of aquocobalamin (OH2Cbl). We subsequently found that human CblC also catalyzes GSH-dependent decyanation of CNCbl and reduction of OH2Cbl, albeit efficiently only under anaerobic conditions. The air sensitivity of the human enzyme suggests interception by oxygen during the single-electron transfer step from GSH to CNCbl. These newly discovered GSH-dependent single-electron transfer reactions expand the repertoire of catalytic activities supported by CblC, a versatile B12-processing enzyme.
Introduction
Vitamin B12 is a derivative of cobalamin, an essential cofactor involved in sulfur, branched-chain amino acid, odd-chain fatty acid, and cholesterol metabolism. Methylcobalamin (MeCbl)2 and 5′-deoxyadenosylcobalamin (AdoCbl) are the two active forms of the cofactor that are required by the B12-dependent enzymes, methionine synthase, and methylmalonyl-CoA mutase, respectively (1). Because mammals are unable to synthesize B12, they possess an elaborate pathway for converting dietary cobalamins to the active cofactor forms and delivering them to target proteins (2–4). Defects in the trafficking proteins result in a functional B12 deficiency and lead to cobalamin disorders that are classified into eight genetic complementation groups: cblA–F, cblJ, and cblX (5–7). Defects in the cobalamin-dependent enzymes, methionine synthase and methylmalonyl-CoA mutase, belong to the cblG (8–10) and mut (11) genetic complementation groups, respectively.
Defects in the cblC locus affect both B12-dependent enzymes and result in combined methylmalonic aciduria and homocystinuria (12). This clinical phenotype indicates that the gene product of cblC (hereafter referred to as CblC) functions in the early and common part of the B12 trafficking pathway before it bifurcates into the cytoplasmic (for methionine synthase) and mitochondrial (for methylmalonyl-CoA mutase) branches. The most common locus of mutations leading to cobalamin disorders is in the gene responsible for the cblC complementation group (13). Human CblC (also referred to as MMACHC or methylmalonic aciduria type C and homocystinuria) exhibits unusual and dual activities, catalyzing the flavin-dependent reductive decyanation of vitamin B12 (or cyanocobalamin (CNCbl)) (Reaction 1) (14) and GSH-dependent dealkylation of alkylcobalamins (e.g. MeCbl or AdoCbl) (Reaction 2) (15). The products of reductive decyanation are cyanide and cob(II)alamin, which is paramagnetic, whereas a glutathione thioether and cob(I)alamin are formed in the dealkylation reaction. The superreactivity of cob(I)alamin results in its rapid oxidation to either cob(II)alamin or aquocobalamin (OH2Cbl) under aerobic conditions.
 |
 |
Although CblC does not resemble other proteins at a sequence level, its crystal structures revealed that it houses a flavin-nitroreductase domain (16, 17). It can employ reduced flavin as an electron source in the reductive decyanation reaction (16). CblC binds alkylcobalamins in a “base-off” state in which the endogenous lower axial ligand of cobalamin, dimethylbenzimidazole, is not ligated to the cobalt. Base-off alkylcobalamins are readily distinguished by UV-visible absorption spectroscopy from the “base-on” form found in solution at physiological pH. The crystal structures of human CblC (hCblC) revealed that the tail leading to dimethylbenzimidazole resides in a crevice on the surface of the protein (16, 17).
Because the study of the cytoplasmic B12 trafficking pathway has been hampered by the inability to express human methionine synthase, we have cloned the corresponding CblC–E- and CblG-encoding genes from Caenorhabditis elegans as a first step toward elucidating the interactions of the encoded proteins. Our characterization of the C. elegans CblC (ceCblC), which shares 36% identity and 53% similarity with hCblC, led to the unexpected finding that ceCblC catalyzes the reductive decyanation of CNCbl in the presence of GSH or other thiols, an activity that had been missed previously with hCblC. The decyanation product, cob(II)alamin, is stabilized by ceCblC. The discovery of this new catalytic activity of ceCblC led us to determine its relevance to hCblC. We report that hCblC also harbors a latent GSH-dependent decyanation activity, which is only readily observed under anaerobic conditions, indicating efficient interception of the electron transfer step by oxygen in the human versus the C. elegans CblC. Furthermore, both human and C. elegans CblC exhibit GSH-dependent OH2Cbl reductase activity, which might be advantageous for maintaining the cofactor in the cob(II)alamin oxidation state for subsequent transfer to an acceptor protein in the trafficking pathway.
EXPERIMENTAL PROCEDURES
Materials
OH2Cbl, CNCbl, l-cysteine, dl-homocysteine, DTT, GSH, β-mercaptoethanol, FMN, and NADPH were purchased from Sigma-Aldrich.
Cloning of C. elegans CblC and MSR
Wild-type C. elegans (N2) worms (a generous gift from Dr. Ursula Jakob, University of Michigan) were homogenized in TRIzol (Invitrogen), and the total RNA was isolated using the RNeasy mini kit (Qiagen). cDNA was synthesized by SuperScript® II reverse transcriptase (Life Technologies) using the total RNA as the template. The C. elegans CblC and methionine synthase reductase (MSR) cDNAs were PCR-amplified by PfuUltra High-Fidelity DNA polymerase (Agilent Technologies) using the following primers: CblC (forward), 5′-TTTAAGAAGGAGATATAGATCATGGTAACTGAAATGAGCCATGCA-3′, and CblC (reverse), 5′-TTATGGAGTTGGGATCTTATTAGTCGATATTCTTTGCTCCTCCGT-3′; MSR (forward), 5′-TACTTCCAATCCAATGCAATGACAGATTTCCTCATTGCTTTTGG-3′, and MSR (reverse), 5′-TTATCCACTTCCAATGTTATTATCCCCACACATCCTCGA-3′.
The PCR products were subcloned using the ligation-independent cloning method into a ligation-independent cloning vector. A C-terminal His6 tag was introduced into the ceCblC construct using primers 5′-GAGCACCACCACCACCACCACTAAGATCCCAACTCCATAAGGA-3′ (forward) and 5′-GTGGTGGTGGTGGTGGTGCTCGAGGTCGATATTCTTTGCTCCTCC-3′ (reverse) to generate the expression construct, ceCblC-His6. MSR from C. elegans (ceMSR) was also subcloned into a ligation-independent cloning vector to generate the expression construct, His6-ceMSR.
Expression and Purification of ceCblC and ceMSR
Escherichia coli BL21 (DE3) was transformed with ceCblC-His6 and grown overnight at 37 °C in 100 ml of Luria Bertani medium containing ampicillin (100 μg ml−1). Then, 6 × 1 liter of the same medium containing ampicillin was inoculated with the starter culture and grown at 37 °C. After 4 h, when the A600 had reached 0.5–0.6, the temperature was reduced to 15 °C. The cultures were induced with 100 μm isopropyl β-d-1-thiogalactopyranoside, and the cells were harvested 16 h later. The cells pellets were stored at −80 °C until use.
For purification of ceCblC, the cell pellets were suspended in 200 ml of buffer containing 100 mm Tris-HCl, pH 8.0, 150 mm KCl, 0.15 mg ml−1 lysozyme, 5% (v/v) glycerol, and two protease inhibitor cocktail tablets (Roche Applied Science). The cell suspension was stirred at 4 °C for 20 min and then sonicated (power setting = 6.5) on ice for 8 min at 15-s intervals separated by 45-s cooling periods. The sonicate was centrifuged at 38,000 × g for 30 min, and the supernatant was loaded on to a Ni-NTA-agarose column (2.5 × 5 cm, Qiagen) pre-equilibrated with Buffer A (100 mm Tris-HCl, pH 8.0, 150 mm KCl, and 5% (v/v) glycerol). The column was washed with 200 ml of a buffer containing 30 mm imidazole in Buffer A and eluted with 300 ml of a linear gradient ranging from 30 to 300 mm imidazole in Buffer A. The fractions containing ceCblC were identified by SDS-PAGE analysis, pooled and concentrated to 5 ml, and dialyzed overnight against 1 liter of dialysis buffer containing 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol. The dialyzed protein was loaded on to a Superdex 200 column (120 ml, GE Healthcare) pre-equilibrated with dialysis buffer. The fractions of interest were collected and stored at −80 °C.
To purify His6-ceMSR, E. coli BL21 (DE3) was transformed with the His6-ceMSR expression vector and grown overnight at 37 °C in 100 ml of Luria Bertani medium containing ampicillin (100 μg ml−1). The following day, 6 × 1 liter of Luria Bertani medium containing ampicillin was inoculated with the starter culture and grown at 37 °C with shaking (220 rpm). After 4 h, when the A600 was 0.5–0.6, the temperature was reduced to 18 °C. The cultures were induced with 100 μm isopropyl β-d-1-thiogalactopyranoside, and the cells were harvested 16 h later. The cell pellets were stored at −80 °C until use. His6-ceMSR was purified from cell lysate by Ni-NTA-agarose column using the same protocol as described above for ceCblC purification. The fractions containing ceMSR were identified using SDS-PAGE analysis, pooled, concentrated, dialyzed to remove imidazole, and stored at −80 °C. Before use, ceMSR was thawed on ice and mixed with 1 eq of FMN. Excess FMN was removed by centrifugation using an Amicon Ultra-15 centrifugal filter (50-kDa cutoff).
Decyanation of CNCbl by ceCblC
The reactions were carried out with 50 μm ceCblC and 20 μm CNCbl in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol in a total volume of 200 μl. The reactions were conducted at 20 °C because ceCblC tended to precipitate at higher temperatures. Reactions were initiated by the addition of GSH (0.10–2.0 mm) or other thiols (8 mm) or ceMSR (4–10 μm) in the presence of 0.2 mm NADPH and followed by the disappearance of CNCbl (decrease in absorbance at 530 nm, Δϵ = 3.7 mm−1 cm−1). To determine the kinetic parameters of GSH-dependent decyanation, the initial rate of the reaction was plotted against GSH concentration and fitted to the Hill equation below.
Decyanation of CNCbl by hCblC
hCblC (extending from residues 1–244) was purified as described previously (16). The reaction mixtures contained 50 μm hCblC and 20 μm CNCbl in 100 mm HEPES, pH 7.0 or 8.0, 150 mm KCl, and 10% (v/v) glycerol in a total volume of 200 μl. Reactions were initiated by the addition of various thiols (8 mm) and carried out at 37 °C under either anaerobic or aerobic conditions.
Reduction of OH2Cbl by ceCblC
OH2Cbl bound to ceCblC was generated by mixing 40 μm ceCblC with 20 μm OH2Cbl in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol. Unbound OH2Cbl was removed using an Amicon Ultra-15 centrifugal filter (30-kDa cut off). The ceCblC·OH2Cbl complex was diluted in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol to obtain a final concentration of 10–20 μm cobalamin. The reactions were initiated by the addition of various thiols (2–8 mm) or ceMSR (600 nm) with 0.2 mm NADPH in a total volume of 200 μl and carried out at 20 °C under aerobic conditions. The reaction was followed by the disappearance of OH2Cbl at 525 nm (Δϵ = 5.5 mm−1 cm−1).
Reduction of OH2Cbl by hCblC
The reaction mixtures contained 50 μm hCblC and 20 μm OH2Cbl in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol in a total volume of 200 μl. Reactions were initiated by the addition of 8 mm GSH and carried out at 37 °C under either anaerobic or aerobic conditions.
MSR Activity Assay
Reduction of free OH2Cbl (35 μm) by ceMSR (100–300 nm) was carried out in 50 mm potassium phosphate buffer, pH 7.2, and 0.2 mm NADPH at 37 °C, as described previously (18). Reduction of cytochrome c (60 μm) was performed with 50–150 nm ceMSR in 50 mm Tris-HCl (pH 7.5) and 0.2 mm NADPH at 37 °C. Reactions were monitored by the increase in absorbance at 550 nm (Δϵ = 21 mm−1 cm−1), as described previously (19).
HPLC Analysis of Cobalamins
Decyanation of CNCbl (50 μm) by hCblC (20 μm) in the presence of 8 mm GSH was carried out in 100 mm HEPES, pH 8.0, 150 mm KCl, and 10% (v/v) glycerol under aerobic conditions. After a 30-min reaction at 37 °C, the protein was inactivated by heating (70 °C, 10 min), and the cofactors were released into solution. The supernatant was analyzed by HPLC as described previously (20).
HPLC Analysis of GSH and GSSG
Reduction of CNCbl or OH2Cbl by ceCblC in the presence of 4 mm GSH was carried out in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol. The amount of GSH and GSSG in the reaction mixtures was analyzed by HPLC using a modification of a previously described method (21). Briefly, 100 μl of reaction mixture was mixed with an equal volume of metaphosphoric acid solution (16.8 mg ml−1 metaphosphoric acid, 2 mg ml−1 EDTA, and 9 mg ml−1 NaCl) to precipitate proteins. Thiols in the supernatant were alkylated with monoiodoacetic acid at a final concentration of 7 mg ml−1, with the pH adjusted to 7–8 by saturated K2CO3. After incubation for 1 h in the dark at room temperature, an equal volume of a 2,4-dinitrofluorobenzene solution (1.5% v/v in ethanol) was added. The derivatization reaction was performed in the dark at room temperature for 4 h. The N-dinitrophenyl derivatives of GSH and GSSG were separated by HPLC on a μBondapak NH2 column (Waters, 300 × 3.9 mm, 10 μm) at a flow rate of 1 ml min−1. The mobile phase consists of solvent A (4:1 methanol/water mixture) and solvent B, which was prepared by mixing 154 g of ammonium acetate in 100 ml of water and 400 ml of acetic acid and adding 500 ml of the resulting solution to 1000 ml of solvent A. A gradient from 18 to 60% (v/v) solvent B was used to elute GSH and GSSG and the absorbance was monitored at 355 nm.
RESULTS
Expression and Purification of ceCblC
ceCblC was obtained in a yield of ∼10 mg/liter of culture, and the protein was judged to be >90% pure by SDS-PAGE analysis following a single Ni-NTA purification step. The purified protein migrated as a single peak by gel filtration chromatography on a Superdex 200 column and eluted with an apparent molecular mass of ∼30 kDa. This is consistent with ceCblC being a monomer (predicted mass ∼32 kDa). AdoCbl and MeCbl bind to ceCblC in a base-off state as evidenced by the blue shift in the α/β bands from ∼523 nm for the free cofactor to 453 nm for the ceCblC-bound forms (Fig. 1).
FIGURE 1.

ceCblC binds to AdoCbl and MeCbl in a base-off state. A and B, the spectra were generated in the presence of 20 μm AdoCbl (A) or 15 μm MeCbl (B) and 30 μm ceCblC in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol at 20 °C. The UV-visible spectra before and after the addition of ceCblC to the cobalamins are shown.
Thiol-dependent Decyanation of CNCbl by ceCblC
CNCbl bound to ceCblC appears to exist as a mixture of the base-on (λmax = 548 nm) and base-off (λmax = 527 nm) species as suggested by the broad peak between 520 and 550 nm, which is intermediate between the two species free in solution (Fig. 2). As reported for bovine (22) and human CblC (16), the addition of GSH shifted the equilibrium to the base-off species, as revealed by the blue shift in the absorption maximum to 527 nm.
FIGURE 2.
GSH shifts the equilibrium of ceCblC-bound CNCbl to the base-off state. The sample contained 20 μm CNCbl and 40 μm ceCblC in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol and was preincubated for 10 min at 20 °C in the absence of GSH. The UV-visible spectra recorded before (solid gray line) and immediately after (solid black line) the addition of GSH are shown. The base-on free CNCbl sample contained CNCbl (20 μm) in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol (short dashed line). The base-off CNCbl sample contained CNCbl in 6% (v/v) HClO4 (short dashed-dot line). The absorption maxima of CNCbl are indicated by arrows, which were 548 nm for base-on CNCbl, a broad absorption between 520 and 550 nm for CNCbl bound to ceCblC, 527 nm for CNCbl bound to ceCblC in the presence of GSH, and 527 nm for base-off CNCbl. a.u., absorbance units.
Unexpectedly, continued incubation of the ceCblC·CNCbl complex with GSH resulted in decyanation of CNCbl and formation of cob(II)alamin under aerobic conditions, as indicated by the increase in absorption at 473 nm (Fig. 3A). Isosbestic points for the conversion of CNCbl to cob(II)alamin were at 339, 370, and 491 nm. Cob(II)alamin production was also observed under anaerobic conditions (not shown). Formation of cob(II)alamin is consistent with a one-electron reductive elimination of the cyanide group, as previously observed with hCblC and reduced flavin or a flavoprotein (14, 16). The initial rates of cob(II)alamin formation under aerobic and anaerobic conditions were virtually superimposable in the presence of 8 mm GSH (Fig. 3A, inset). The cob(II)alamin formed was stable for at least 2 h under aerobic conditions. The dependence of the kobs for cob(II)alamin formation on GSH concentration was determined under aerobic conditions by following the decrease in absorbance at 530 nm (Fig. 3B). From a Hill plot analysis of the data, the Kact for GSH was estimated to be 291 ± 24 μm; kcat for decyanation was 0.046 ± 0.003 min−1, and the Hill coefficient, n, was 2.1 ± 0.3. The reaction rates increased with pH (Fig. 3B, inset). Other thiols, such as DTT, β-mercaptoethanol, and homocysteine, could substitute for GSH and convert ceCblC-bound CNCbl to cob(II)alamin (Fig. 3C), albeit with widely differing rates (Table 1) at a fixed 8 mm concentration of each thiol. The order of the reaction rates was β-mercaptoethanol > DTT > GSH > homocysteine. Neither cysteine nor tris(2-carboxyethyl)phosphine supported the reductive decyanation of ceCblC-bound CNCbl under the same conditions. Because the other biologically relevant thiols (cysteine and homocysteine) are present at 100–1000-fold lower concentrations, respectively, in cells, the data obtained at 8 mm concentrations of each thiol yield a qualitative comparison, with the differences likely to be even greater at their physiologically relevant concentrations. Reductive decyanation of ceCblC-bound CNCbl was not observed in the absence of thiols (not shown). Unlike GSH, the other thiols did not shift the equilibrium of ceCblC-bound CNCbl to the base-off conformation.
FIGURE 3.
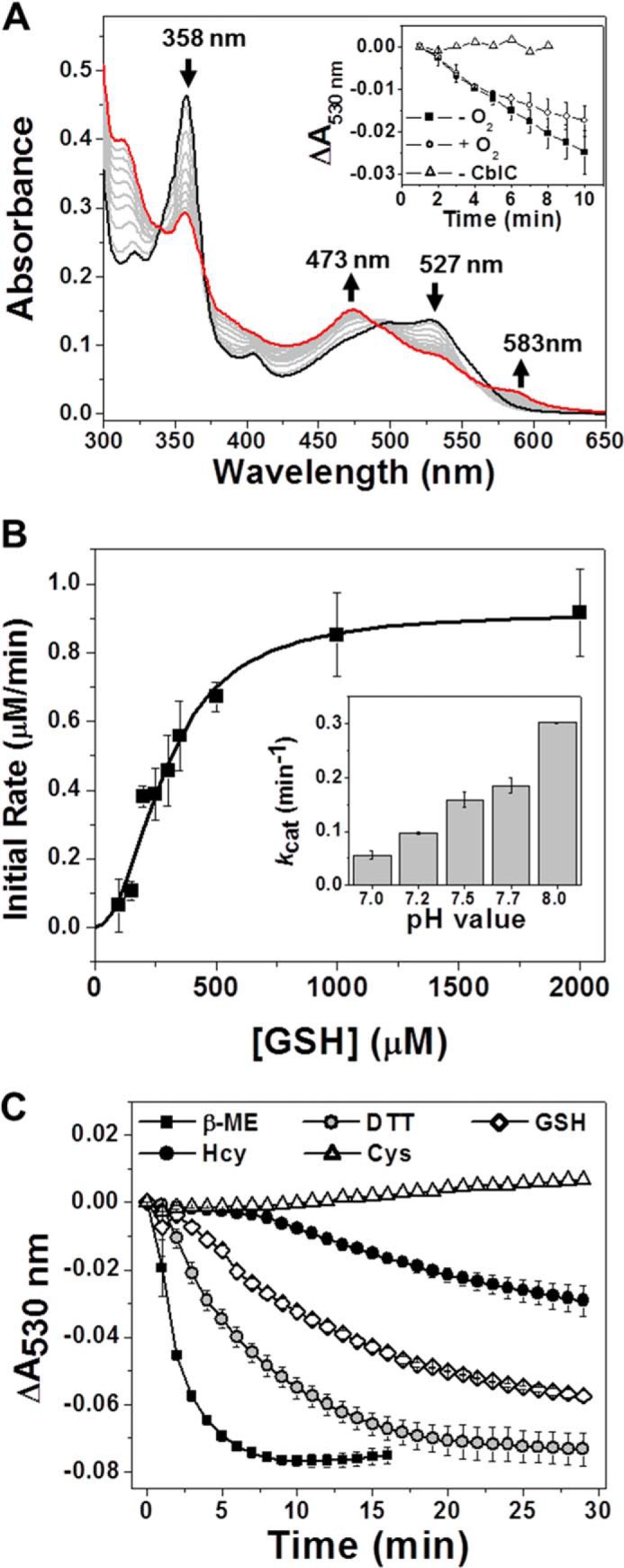
Decyanation of CNCbl by ceCblC in the presence of thiols. A, time-dependent changes in the UV-visible absorption spectra of CNCbl (20 μm) bound to ceCblC (40 μm) in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol following the addition of 8 mm GSH under aerobic conditions. Black trace, 0 min, the absorption maxima are 358 and 527 nm; red trace, 10 min, the new absorption maxima are 473 and 583 nm; gray trace, intermediate time points. Inset, changes in absorbance at 530 nm over time when the reactions were run at 20 °C anaerobically (solid squares), aerobically (empty circles), and in the absence of CblC (empty triangles). Error bars indicate ± S.D. B, dependence of the initial rate of decyanation on GSH concentration. Reaction mixtures contained 20 μm CNCbl, 40 μm ceCblC, and 0–2 mm GSH in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol. The reactions were performed aerobically at 20 °C and monitored at 530 nm; inset, pH dependence of the decyanation rate in the presence of 8 mm GSH. Error bars indicate ± S.D. C, comparison of reaction rates of ceCblC-catalyzed decyanation of CNCbl by various thiols (each at 8 mm). Error bars indicate ± S.D.
TABLE 1.
Summary of the initial rates for decyanation of CNCbl by ceCblC and hCblC
| Thiols | ceCblCa | hCblCb |
|---|---|---|
| min−1 | min−1 | |
| Glutathione | 0.046 ± 0.03 | 0.021 ± 0.001 |
| Dithiothreitol | 0.13 ± 0.01 | 0.44 ± 0.01 |
| β-Mercaptoethanol | 0.35 ± 0.01 | 0.21 ± 0.01 |
| l-Cysteine | NDc | 0.060 ± 0.006 |
| dl-Homocysteine | 0.020 ± 0.002 | 0.13 ± 0.01 |
| ceMSR | 0.085 ± 0.008 | 0.068 ± 0.007d |
a Reaction rates for ceCblC were monitored under aerobic conditions at 20 °C in the presence of 8 mm thiols in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol. Reaction rates were estimated based on the decrease in absorption at 530 nm.
b Reaction rates for hCblC were determined at 37 °C in the presence of 8 mm thiols, 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol under anaerobic conditions. Reaction rates were estimated based on the decrease in absorption at 530 nm. Reduction by DTT and β-mercaptoethanol can also be observed aerobically with initial rates of 0.42 ± 0.03 and 0.20 ± 0.01 min−1, respectively.
c ND denotes not detectable.
d Obtained from Ref. 14.
Curiously, a long wavelength band with an absorption maximum at 583 nm was observed under aerobic (Fig. 3A) and anaerobic conditions (not shown) during the decyanation reaction with GSH but not with the other thiols. The origin of this band is not known. We speculate that it might represent a charge transfer band between the glutathionyl radical (GS•) and cob(II)alamin.
Thiol-dependent Decyanation of CNCbl by hCblC
Under anaerobic conditions, incubation of the hCblC·CNCbl complex with 8 mm GSH at 37 °C resulted in decyanation of CNCbl and generation of cob(II)alamin as indicated by the absorption maximum at 476 nm (Fig. 4A). The isosbestic points for the conversion of CNCbl to cob(II)alamin were at 336, 367, and 484 nm. A long wavelength band with an absorption maximum at 581 nm was observed with hCblC as with ceCblC, and in fact, was more pronounced with the human enzyme (Fig. 4A). Cysteine, homocysteine, β-mercaptoethanol, and DTT also supported the reductive decyanation under anaerobic conditions, generating cob(II)alamin (Table 1).
FIGURE 4.

Decyanation of CNCbl bound to hCblC. A and B, changes in the UV-visible absorption spectrum of hCblC (50 μm)-bound CNCbl (20 μm) after the addition of 8 mm GSH in 100 mm HEPES, pH 8.0, 150 mm KCl, and 10% (v/v) glycerol at 37 °C under anaerobic (A) and aerobic conditions (B). A, black trace, 1 min, the absorption maxima are 358 and 528 nm; red trace, 20 min, the absorption maxima are 366, 476, and 581 nm; gray trace, intermediate time points. Inset, time-dependent changes in absorbance at 528 nm (circles), 476 nm (squares), and 581 nm (triangles). B, black trace, 0 min, the absorption maxima are at 361 and 545 nm; red trace, 3 min, the absorption maxima are 358, 500, and 530 nm; the spectrum was unchanged over the next 30 min; gray trace, intermediate time points. Inset, determination of cobalamins by HPLC. Gray trace, free OH2Cbl, GSCbl (glutathionylcobalamin), and CNCbl (each at 20 μm); red trace, analysis of a reaction mixture containing 20 μm CNCbl, 50 μm hCblC and 8 mm GSH in 100 mm HEPES, pH 8.0, 150 mm KCl, and 10% (v/v) glycerol incubated at 37 °C for 30 min under aerobic condition.
As noted previously (14), and in contrast to the result with ceCblC, GSH-dependent decyanation of CNCbl bound to hCblC was not observed spectrally under aerobic conditions (Fig. 4B). Instead, the addition of GSH resulted in a blue shift of the CNCbl spectrum due to a shift in the equilibrium to the base-off state (Fig. 4B), as also seen with ceCblC (Fig. 2). Because the apparent lack of reaction with GSH under aerobic conditions with hCblC but not ceCblC was surprising, we sought to verify this result by HPLC analysis of the reaction mixture (Fig. 4B, inset). The data revealed that although the majority of CNCbl (retention time = 19 min) remained intact after 30 min of incubation, a small amount (∼15%) was converted to glutathionylcobalamin (retention time = 14.5 min) resulting from the reaction between OH2Cbl (retention time = 12 min), the oxidation product of cob(II)alamin released from hCblC during sample preparation, and GSH present in the reaction mixture.
ceMSR Serves as an Electron Donor to ceCblC
We have previously reported that the flavoprotein, MSR, in the presence of NADPH can provide electrons to hCblC for the reductive decyanation of CNCbl (16). To assess whether the orthologous diflavin oxidoreductase can serve an equivalent function for ceCblC, the gene encoding ceMSR was cloned, and the protein was purified. ceMSR was judged to be active using the cytochrome c or OH2Cbl reduction assay in the presence of 0.2 mm NADPH as described previously (18, 19). ceMSR was more prone to losing activity following dilution than hMSR, presumably due to loss of the flavin cofactor. The kcat values for the ceMSR-catalyzed reduction of cytochrome c and free OH2Cbl at 37 °C were estimated to be 1.15 ± 0.07 s−1 and 40 ± 4 min−1, respectively. These activities are 3–5-fold lower than the values reported for hMSR (18, 19).
In the presence of NADPH, ceMSR supported the decyanation of ceCblC·CNCbl, generating cob(II)alamin as evidenced by an increase in absorbance at 473 nm and a decrease in absorbance between 520 and 550 nm with isosbestic points of 497 and 572 nm (Fig. 5A). The reaction required both ceCblC and ceMSR and was not observed in the absence of either enzyme (Fig. 5A, inset). The kobs for the MSR-dependent decyanation was 0.085 ± 0.008 min−1 for ceCblC and was comparable with that reported for hCblC (kobs = 0.068 ± 0.007 min−1) (14).
FIGURE 5.

Reduction of CNCbl and OH2Cbl bound to ceCblC by ceMSR. A, UV-visible absorption changes following the addition of 4 μm ceMSR and 0.2 mm NADPH to a mixture of ceCblC (40 μm) and CNCbl (20 μm) in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol at 20 °C under aerobic conditions. Black trace, 0 min, the absorption maximum is 529 nm; red trace, 15 min, the absorption maximum is 473 nm; gray trace, intermediate time points. Inset, the decyanation reaction is dependent on both ceCblC and ceMSR. Time-dependent changes in absorbance at 530 nm are shown for reactions containing ceCblC and ceMSR (squares), ceCblC (triangles) only, or ceMSR only (circles). B, changes in the UV-visible absorption spectra of OH2Cbl (10 μm) in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol following the addition of 1.2 μm ceMSR and 0.2 mm NADPH at 20 °C under aerobic conditions. Black trace, 0 min, the absorption maximum is 526 nm; red trace, 5 min, the absorption maximum is 473 nm; gray trace, intermediate time points.
Like hMSR, ceMSR reduced ceCblC-bound OH2Cbl to cob(II)alamin (Fig. 5B). The kcat values for the reduction of ceCblC-bound versus free OH2Cbl were 13.6 ± 0.4 min−1 versus 29 ± 2 min−1at 20 °C, indicating that the presence of ceCblC inhibited reduction ∼2-fold. The decyanation (Fig. 5A) and reduction (Fig. 5B) reactions were both incomplete due to the rapid inactivation of ceMSR.
Reduction of CblC-bound OH2Cbl by Thiols
We tested whether OH2Cbl bound to ceCblC can be reduced by thiols (Fig. 6A). The addition of GSH to the mixture resulted in the conversion of OH2Cbl to cob(II)alamin (kobs = 1.27 ± 0.04 min−1, Fig. 6A). Other thiols, such as DTT, β-mercaptoethanol, cysteine, and homocysteine, also reduced ceCblC-bound OH2Cbl to cob(II)alamin under aerobic conditions with rates that were either comparable with or slower than that obtained with GSH (GSH ≈ β-mercaptoethanol ≈ DTT > cysteine > homocysteine). In contrast, in the absence of CblC, glutathionylcobalamin was formed as evidenced by a red shift in the cobalamin spectrum to 535 and 553 nm (Fig. 6B).
FIGURE 6.

Reduction of OH2Cbl by CblC in the presence of GSH. A, changes in the UV-visible absorption spectra of ceCblC-bound OH2Cbl (15 μm) in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol following the addition of 8 mm GSH at 20 °C under aerobic conditions. Black trace, 0 min, the absorption maxima are 351 and 527 nm; red trace, 5 min, the absorption maximum is 473 nm; gray trace, intermediate time points. Inset, changes in absorbance at 525 nm (circles) and 473 nm (squares) over time. B, UV-visible absorption changes of free OH2Cbl following the addition of 8 mm GSH at 20 °C under aerobic conditions. Black trace, 0 min; blue trace, 5 min; gray trace, intermediate time points. C, changes in the UV-visible absorption spectra of hCblC-bound OH2Cbl (20 μm) in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol following the addition of 8 mm GSH at 37 °C under aerobic condition. Black trace, 0 min, the absorption maxima are 356 and 526 nm; gray trace, 1 min; red trace, 2 min, the absorption maxima are 355, 497, and 523 nm; the spectrum was unchanged over the next 30 min. The base-off OH2Cbl spectrum is of OH2Cbl in 6% (v/v) HClO4 (short dashed-dot line). Inset, reduction of hCblC-bound OH2Cbl (20 μm) in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol in the presence of 8 mm GSH at 37 °C under anaerobic conditions. Black trace, 0 min, the absorption maxima are 356 and 523 nm; red trace, 10 min, the absorption maximum is 473 nm; gray trace, intermediate time points.
The addition of GSH to hCblC-bound OH2Cbl under aerobic conditions resulted in a blue shift in the OH2Cbl spectrum to 497 and 523 nm (from an initial broad peak between 500 and 530 nm) within the first minute, but no further change was observed over a 30-min period (Fig. 6C). The blue shift in the spectrum is likely to result from a conformational change perhaps reflecting a switch to the base-off state and does not correspond to the stable production of cob(II)alamin. Reduction of OH2Cbl bound to hCblC to cob(II)alamin could be observed under anaerobic conditions (kobs = 0.36 ± 0.02 min−1, Fig. 6C, inset) consistent with the greater oxygen sensitivity of the electron transfer step from GSH to cobalamin in human versus the C. elegans CblC.
GSSG Is Produced during GSH-dependent Reduction of CNCbl and OH2Cbl by ceCblC
We reasoned that GS• generated via one-electron oxidation of GSH during decyanation of CNCbl or reduction of OH2Cbl might result in the formation of GSSG. To test this hypothesis, we used HPLC to monitor the concentrations of GSH and GSSG. We observed conversion of 2 mm GSH to 1 mm GSSG in 15 min during aerobic decyanation of CNCbl in a reaction mixture originally containing 50 μm ceCblC, 25 μm CNCbl, and 4 mm GSH (Fig. 7A). In contrast, very little oxidation of GSH was observed during the same time period in the absence of either ceCblC or CNCbl. A similar concentration of GSSG formation was observed during reduction of ceCblC-bound OH2Cbl by GSH (Fig. 7B). The stoichiometry of GSSG formed versus cobalamin bound to the ceCblC active site suggests that cob(II)alamin generated during decyanation or reduction is prone to oxidation and forms OH2Cbl. The latter, once formed, is rapidly reduced to cob(II)alamin by GSH. The oxidation-reduction cycle is predicted to result in a futile cycle consuming GSH (Fig. 7C). This redox cycle could be limited by protecting cob(II)alamin from oxidation. This prediction was confirmed by HPLC analysis of the reactions conducted under anaerobic conditions where GSSG production was significantly diminished (Fig. 7B).
FIGURE 7.
GSH-dependent reduction of CNCbl and OH2Cbl by ceCblC generates GSSG. A, HPLC analysis of GSH and GSSG after a 15-min incubation of 50 μm ceCblC + 25 μm CNCbl + 4 mm GSH (solid line), 40 μm ceCblC + 4 mm GSH (black dotted line), 25 μm CNCbl + 4 mm GSH (black dashed line), or 4 mm GSH (gray dotted line) in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol at 20 °C under aerobic conditions. GSH and GSSG eluted at 17 and 21 min, respectively. The identity of the peak eluting at ∼18 min is not known. mAU, milliabsorbance units. B, oxygen dependence of GSSG production during reduction of CNCbl and OH2Cbl by ceCblC. The GSSG concentration was measured after 15 min of incubation of 25 μm CNCbl or OH2Cbl with 50 μm ceCblC and 4 mm GSH in 100 mm HEPES, pH 7.0, 150 mm KCl, and 10% (v/v) glycerol at 20 °C under either aerobic or anaerobic conditions. Error bars indicate ± S.D. C, oxidation/reduction of cob(II)alamin formed during decyanation of CNCbl could set up a futile redox cycle consuming GSH and producing ROS and reactive sulfur species.
DISCUSSION
CblC is proposed to be the first processing enzyme that B12 encounters in the cytoplasm as it exits the lysosomal compartment (3, 4). The role of CblC in the trafficking pathway is to convert dietary cobalamins to a common intermediate, cob(II)alamin, which can then be partitioned to AdoCbl and MeCbl synthesis to meet cellular needs for supporting methylmalonyl-CoA mutase and methionine synthase, respectively. hCblC exhibits remarkable chemical versatility, albeit at the cost of efficiency, and catalyzes a range of chemically distinct reactions. Thus, hCblC catalyzes the cleavage of the cobalt-carbon bond of alkylcobalamins via a heterolytic mechanism, transferring the alkyl group to the thiolate of GSH (15, 23). With CNCbl, CblC exhibits an entirely different activity using an electron donor for the reductive elimination of cyanide and generating cob(II)alamin in the process (14). Both free and MSR-bound reduced flavin are able to serve as an electron donor to CblC during the decyanation reaction (16). GSH, which is a co-substrate in the dealkylation reaction, increases the binding affinity for CNCbl and shifts the equilibrium of CNCbl bound to hCblC to the base-off state (16, 22). However, GSH was not observed to support decyanation under the aerobic conditions used to monitor this reaction (14). The physiological relevance of the slow decyanation and alkyltransferase reactions has been established by examining the fate of [57Co]CNCbl or [57Co]alkylcobalamins fed to fibroblasts from CblC patients versus normal individuals. Our study demonstrated that in the absence of functional CblC, the cobalamin precursors were not processed into the active cofactor forms (23).
As part of our long term goal of studying the interactions between the C. elegans cytoplasmic B12 trafficking proteins and methionine synthase, we made the unexpected observation that ceCblC displays a heretofore unobserved activity, i.e. decyanation of CNCbl in the presence of GSH (Fig. 8). This activity was surprising for two reasons. First, as noted above, it had not been observed with hCblC under aerobic conditions (14), and second, the use of GSH as a single-electron donor was unexpected. Although the decyanation activity was also supported by nonphysiological reductants, e.g. DTT and β-mercaptoethanol (Table 1), it was considerably slower with homocysteine, which unlike GSH is present at low micromolar versus 1–10 mm concentrations in the cell. Hence, GSH, which binds with a relatively high affinity to ceCblC (Kact = 291 ± 24 μm), is likely to be the only physiologically relevant thiol that supports its decyanation activity.
FIGURE 8.

Thiol-dependent reactions catalyzed by CblC. X = CN or OH2; R = Me or Ado.
The GSH-dependent decyanation activity of ceCblC under aerobic and anaerobic conditions (Fig. 3A, inset) led us to reexamine the activity of hCblC under similar conditions. Under anaerobic conditions, the decyanation activity of hCblC could be monitored spectrally in the presence of GSH (Fig. 4A) and other natural and unnatural thiols (Table 1). In contrast, the major spectral change observed upon the addition of GSH to hCblC under aerobic conditions was a shift in the equilibrium of bound CNCbl to the base-off state (Fig. 4B) as seen previously (16), without detectable formation of cob(II)alamin. Because cob(II)alamin bound to hCblC is prone to oxidation and the spectrum of the oxidation product, base-off OH2Cbl, is very similar to that of base-off CNCbl, we used HPLC analysis to determine whether decyanation was occurring, albeit at a low level. Indeed, hCblC does catalyze inefficient GSH-dependent decyanation even under aerobic conditions as evidenced by the presence of glutathionylcobalamin in the HPLC trace (Fig. 4B, inset). This result indicates that the decyanation product, cob(II)alamin, is formed inefficiently under aerobic conditions and is rapidly oxidized to OH2Cbl. The latter is released from the protein during sample preparation and complexes with GSH, present in excess in the sample. The resulting product, glutathionylcobalamin, is detected by HPLC. Hence, oxygen appears to readily intercept the electron transfer step between GSH and CNCbl in the active site of hCblC but not ceCblC. Furthermore, ceCblC rapidly reduces OH2Cbl to cob(II)alamin in the presence of GSH (Fig. 6A) and does not form glutathionylcobalamin. Human CblC does not convert bound OH2Cbl to glutathionylcobalamin as reported previously for bovine CblC (24). In contrast to ceCblC (Fig. 4A), stable reduction of OH2Cbl to cob(II)alamin by hCblC is seen only under anaerobic but not aerobic conditions (Fig. 6C).
It is interesting that GSH can support the chemically distinct decyanation and dealkylation activities catalyzed by CblC. Although the dealkylation reaction has clear precedence both within the family of glutathione transferases as well as in MeCbl-dependent methionine synthase, which transfers a methyl group from MeCbl to homocysteine to generate methionine (25), the use of GSH as a single-electron donor is not common. Clearly, the choice between using GSH as a nucleophile versus an electron source is determined by the nature of the cofactor bound to CblC allowing dichotomous reaction mechanisms to be supported: heterolytic cleavage of the cobalt carbon bond resulting in alkyl group transfer versus reductive homolytic cleavage resulting in cyanide elimination (Fig. 8). A shorter and physiologically relevant thiol, such as homocysteine, supports the decyanation reaction (Table 1) but not the dealkylation reaction (15).
GSH is a major cellular antioxidant and is utilized to reduce H2O2 and other oxidized metabolites or protein residues and for conjugation to electrophilic xenobiotics. Formally, oxidation of GSH can occur via a one- or two-electron process. Single-electron oxidation of GSH, although not common, has been observed in reactions catalyzed by lactoperoxidase (26) and horseradish peroxidase (27). The redox potential and mechanism of reduction of CNCbl is very sensitive to the presence of a lower axial ligand (28). In the base-on state, reductive decyanation is a two-electron process yielding cob(I)alamin with a redox potential of ∼−0.86 V versus the standard hydrogen electrode (29). In the base-off state, reduction involves a one-electron process with a redox potential of −0.11 V (28). One-electron oxidation of GSH by CNCbl would generate the glutathionyl radical (GS•, Reaction 3), which can react with a second mole of GSH to give the radical anion, GSSG•− (Reaction 4).
 |
 |
 |
 |
A rate constant of 6.2 × 108 m−1 s−1 has been reported for formation of the GSH radical anion (Reaction 4) (30). Under aerobic conditions, GS• and GSSG•− can react with oxygen to form GSH peroxide (Reaction 5) or glutathione disulfide (GSSG) and superoxide (Reaction 6), respectively. In the ceCblC reactions, the 2:1 stoichiometry of GSH consumption to GSSG formation (Fig. 7A) is consistent with the reaction of GSSG•− rather than GS• with oxygen.
A complete understanding of the reaction mechanism of GSH-dependent decyanation awaits detailed kinetic studies on the formation of the GSH oxidation product(s) under aerobic and anaerobic conditions, and these studies are in progress. The oxygen-dependent reactions, subsequent to GSH-dependent dealkylation or decyanation (Fig. 7C), demonstrate that CblC can be a potential source of reactive oxygen species (ROS). It is likely that strategies exist to minimize futile redox cycling by CblC that would result in wasteful consumption of GSH and generation of noxious O2⨪, and subsequently, H2O2. Elevated ROS is a hallmark of many inborn errors of metabolism, and in fact, CblC patients show high levels of urinary oxidative damage markers (31). Higher ROS and increased susceptibility to apoptosis have also been reported in fibroblasts from CblC patients versus controls suggesting that ROS production could be a phenotypic modifier in CblC disorders (32). OH2Cbl had a protective effect in CblC patient fibroblasts, decreasing ROS production (32). It is possible that some pathogenic mutations promote futile cycling of cob(II)alamin bound to hCblC.
The oxygen sensitivity of the human versus the C. elegans CblC impedes GSH-dependent decyanation by hCblC in air. The stability of cob(II)alamin bound to ceCblC in the presence of 8 mm GSH, a physiologically relevant concentration (33), for >2 h is remarkable. Stabilization by ceCblC is aided by the ability of GSH to reduce the oxidation product OH2Cbl back to cob(II)alamin (kobs = 1.27 ± 0.04 min−1, Fig. 6A), which comes at the price of ROS production. The oxygen sensitivity of hCblC may have evolved to minimize ROS generation and GSH consumption. In contrast to ceCblC, GSH-dependent reduction of OH2Cbl by human CblC is barely detectable under aerobic conditions (Fig. 6B). It appears likely that ceCblC occludes oxygen from the GSH binding site more effectively than hCblC.
Cob(II)alamin is presumably the cofactor form that is further processed in the B12 trafficking pathway, and its stabilization would be advantageous for its subsequent transfer from CblC to downstream acceptors. The mechanisms by which ceCblC stabilizes cob(II)alamin and protects against oxygen interception during the decyanation reaction are not known. Sequence alignment reveals that ceCblC lacks the last ∼40 C-terminal residues present in mammalian CblCs (Fig. 9), which are predicted to be disordered (16). The C terminus of hCblC does not play an essential role in its catalytic function because deletion of the C-terminal 38 residues does not impair activity (16). A truncated form of CblC is observed in human fibroblasts and in murine tissue (16, 34), and the role of the C-terminal extension in mammalian CblCs is open to question. Although the residues lining the B12 binding pocket are largely conserved between the human and C. elegans active sites, there are a few differences (e.g. Ala-117, Ser-146, Cys-149, and Ile-160 in hCblC are substituted by methionine, isoleucine, serine, and phenylalanine, respectively, in ceCblC) (Fig. 9). The potential role of these residues in protecting against interception of the electron transfer step by oxygen will be interesting to elucidate.
FIGURE 9.
Sequence alignment of ceCblC and hCblC. Clustal Omega was used to generate the alignment. Identical residues are highlighted in black, and conserved residues are shaded in gray. Asterisks indicate the residues that interact with cobalamins as revealed by the hCblC structure (16).
Human CblC has been shown to interact with CblD, a protein involved in B12 trafficking, albeit its function is not understood. Stable interaction between CblC and CblD required the presence of alkylcobalamins and GSH (35). However, the chemical and oxidation state of the cobalamins in these experiments was not established and was likely to have been mixed due to the propensity of hCblC-bound B12 to undergo oxidation. In light of the newly discovered role of GSH in reduction of OH2Cbl to cob(II)alamin, reexamination of the cofactor form needed to promote complex formation between CblC and CblD is warranted. In summary, we have described two new activities associated with the human and C. elegans CblC, GSH-dependent decyanation and GSH-dependent reduction of OH2Cbl. Both activities could be physiologically relevant to B12 trafficking in the intracellular milieu where GSH concentrations are high and oxygen is limiting milieu.
Acknowledgment
We thank Dr. Ursula Jakob (University of Michigan) for providing wild-type C. elegans (N2) worms.
This work was supported, in whole or in part, by National Institutes of Health Grant DK45776 (to R. B.).
- MeCbl
- methylcobalamin
- AdoCbl
- 5′-deoxy-adenosylcobalamin
- ceCblC
- CblC from C. elegans
- CNCbl
- cyanocobalamin
- hCblC
- human CblC
- MSR
- methionine synthase reductase
- ceMSR
- MSR from C. elegans
- hMSR
- human MSR
- OH2Cbl
- aquocobalamin
- ROS
- reactive oxygen species
- Ni-NTA
- nickel-nitrilotriacetic acid
- GS
- glutathionyl.
REFERENCES
- 1. Banerjee R., Ragsdale S. W. (2003) The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Ann. Rev. Biochem. 72, 209–247 [DOI] [PubMed] [Google Scholar]
- 2. Banerjee R. (2006) B12 trafficking in mammals: a case for coenzyme escort service. ACS Chem. Biol. 1, 149–159 [DOI] [PubMed] [Google Scholar]
- 3. Banerjee R., Gherasim C., Padovani D. (2009) The tinker, tailor, soldier in intracellular B12 trafficking. Cur. Op. Chem. Biol. 13, 484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gherasim C., Lofgren M., Banerjee R. (2013) Navigating the B12 road: assimilation, delivery, and disorders of cobalamin. J. Biol. Chem. 288, 13186–13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shevell M. I., Rosenblatt D. S. (1992) The neurology of cobalamin. Can. J. Neurol. Sci. 19, 472–486 [PubMed] [Google Scholar]
- 6. Coelho D., Kim J. C., Miousse I. R., Fung S., du Moulin M., Buers I., Suormala T., Burda P., Frapolli M., Stucki M., Nürnberg P., Thiele H., Robenek H., Höhne W., Longo N., Pasquali M., Mengel E., Watkins D., Shoubridge E. A., Majewski J., Rosenblatt D. S., Fowler B., Rutsch F., Baumgartner M. R. (2012) Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat. Genet. 44, 1152–1155 [DOI] [PubMed] [Google Scholar]
- 7. Yu H. C., Sloan J. L., Scharer G., Brebner A., Quintana A. M., Achilly N. P., Manoli I., Coughlin C. R., 2nd, Geiger E. A., Schneck U., Watkins D., Suormala T., Van Hove J. L., Fowler B., Baumgartner M. R., Rosenblatt D. S., Venditti C. P., Shaikh T. H. (2013) An X-linked cobalamin disorder caused by mutations in transcriptional coregulator HCFC1. Am. J. Hum. Genet. 93, 506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gulati S., Baker P., Li Y. N., Fowler B., Kruger W., Brody L. C., Banerjee R. (1996) Defects in human methionine synthase in cblG patients. Hum. Mol. Genet. 5, 1859–1865 [DOI] [PubMed] [Google Scholar]
- 9. Leclerc D., Campeau E., Goyette P., Adjalla C. E., Christensen B., Ross M., Eydoux P., Rosenblatt D. S., Rozen R., Gravel R. A. (1996) Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum. Mol. Genet. 5, 1867–1874 [DOI] [PubMed] [Google Scholar]
- 10. Chen H. P., Marsh E. N. (1997) Adenosylcobalamin-dependent glutamate mutase: examination of substrate and coenzyme binding in an engineered fusion protein possessing simplified subunit structure and kinetic properties. Biochemistry 36, 14939–14945 [DOI] [PubMed] [Google Scholar]
- 11. Ledley F. D., Lumetta M. R., Zoghbi H. Y., VanTuinen P., Ledbetter S. A., Ledbetter D. H. (1988) Mapping of human methylmalonyl CoA mutase (MUT) locus on chromosome 6. Am. J. Hum. Genet. 42, 839–846 [PMC free article] [PubMed] [Google Scholar]
- 12. Lerner-Ellis J. P., Tirone J. C., Pawelek P. D., Doré C., Atkinson J. L., Watkins D., Morel C. F., Fujiwara T. M., Moras E., Hosack A. R., Dunbar G. V., Antonicka H., Forgetta V., Dobson C. M., Leclerc D., Gravel R. A., Shoubridge E. A., Coulton J. W., Lepage P., Rommens J. M., Morgan K., Rosenblatt D. S. (2006) Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat. Genet. 38, 93–100 [DOI] [PubMed] [Google Scholar]
- 13. Lerner-Ellis J. P., Anastasio N., Liu J., Coelho D., Suormala T., Stucki M., Loewy A. D., Gurd S., Grundberg E., Morel C. F., Watkins D., Baumgartner M. R., Pastinen T., Rosenblatt D. S., Fowler B. (2009) Spectrum of mutations in MMACHC, allelic expression, and evidence for genotype-phenotype correlations. Hum. Mutat. 30, 1072–1081 [DOI] [PubMed] [Google Scholar]
- 14. Kim J., Gherasim C., Banerjee R. (2008) Decyanation of vitamin B12 by a trafficking chaperone. Proc. Natl. Acad. Sci. U.S.A. 105, 14551–14554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim J., Hannibal L., Gherasim C., Jacobsen D. W., Banerjee R. (2009) A human vitamin B12 trafficking protein uses glutathione transferase activity for processing alkylcobalamins. J. Biol. Chem. 284, 33418–33424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koutmos M., Gherasim C., Smith J. L., Banerjee R. (2011) Structural basis of multifunctionality in a vitamin B12-processing enzyme. J. Biol. Chem. 286, 29780–29787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Froese D. S., Krojer T., Wu X., Shrestha R., Kiyani W., von Delft F., Gravel R. A., Oppermann U., Yue W. W. (2012) Structure of MMACHC reveals an arginine-rich pocket and a domain-swapped dimer for its B12 processing function. Biochemistry 51, 5083–5090 [DOI] [PubMed] [Google Scholar]
- 18. Yamada K., Gravel R. A., Toraya T., Matthews R. G. (2006) Human methionine synthase reductase is a molecular chaperone for human methionine synthase. Proc. Natl. Acad. Sci. U.S.A. 103, 9476–9481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gherasim C. G., Zaman U., Raza A., Banerjee R. (2008) Impeded electron transfer from a pathogenic FMN domain mutant of methionine synthase reductase and its responsiveness to flavin supplementation. Biochemistry 47, 12515–12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hannibal L., Axhemi A., Glushchenko A. V., Moreira E. S., Brasch N. E., Jacobsen D. W. (2008) Accurate assessment and identification of naturally occurring cellular cobalamins. Clin. Chem. Lab Med. 46, 1739–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mosharov E., Cranford M. R., Banerjee R. (2000) The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39, 13005–13011 [DOI] [PubMed] [Google Scholar]
- 22. Jeong J., Kim J. (2011) Glutathione increases the binding affinity of a bovine B12 trafficking chaperone bCblC for vitamin B12. Biochem. Biophys. Res. Commun. 412, 360–365 [DOI] [PubMed] [Google Scholar]
- 23. Hannibal L., Kim J., Brasch N. E., Wang S., Rosenblatt D. S., Banerjee R., Jacobsen D. W. (2009) Processing of alkylcobalamins in mammalian cells: a role for the MMACHC (cblC) gene product. Mol. Genet. Metab. 97, 260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeong J., Ha T. S., Kim J. (2011) Protection of aquo/hydroxocobalamin from reduced glutathione by a B12 trafficking chaperone. BMB Rep. 44, 170–175 [DOI] [PubMed] [Google Scholar]
- 25. Banerjee R. V., Matthews R. G. (1990) Cobalamin-dependent methionine synthase. FASEB J. 4, 1450–1459 [DOI] [PubMed] [Google Scholar]
- 26. Nakamura M., Yamazaki I., Ohtaki S., Nakamura S. (1986) Characterization of one- and two-electron oxidations of glutathione coupled with lactoperoxidase and thyroid peroxidase reactions. J. Biol. Chem. 261, 13923–13927 [PubMed] [Google Scholar]
- 27. Harman L. S., Carver D. K., Schreiber J., Mason R. P. (1986) One- and two-electron oxidation of reduced glutathione by peroxidases. J. Biol. Chem. 261, 1642–1648 [PubMed] [Google Scholar]
- 28. Zheng D. H., Lu T. H. (1997) Electrochemical reactions of cyanocobalamin in acidic media. J. Electroanal. Chem. 429, 61–65 [Google Scholar]
- 29. Lexa D., Saveant J. M., Zickler J. (1980) Electrochemistry of vitamin-B12. 5. Cyanocobalamins. J. Am. Chem. Soc. 102, 2654–2663 [Google Scholar]
- 30. Hoffman M. Z., Hayon E. (1973) Pulse-radiolysis study of sulfhydryl compounds in aqueous-solution. J. Phys. Chem. 77, 990–996 [Google Scholar]
- 31. Mc Guire P. J., Parikh A., Diaz G. A. (2009) Profiling of oxidative stress in patients with inborn errors of metabolism. Mol. Genet. Metab. 98, 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richard E., Jorge-Finnigan A., Garcia-Villoria J., Merinero B., Desviat L. R., Gort L., Briones P., Leal F., Pérez-Cerdá C., Ribes A., Ugarte M., Pérez B., and MMACHC Working Group (2009) Genetic and cellular studies of oxidative stress in methylmalonic aciduria (MMA) cobalamin deficiency Type C (cblC) With homocystinuria (MMACHC). Hum. Mutat. 30, 1558–1566 [DOI] [PubMed] [Google Scholar]
- 33. Vitvitsky V., Dayal S., Stabler S., Zhou Y., Wang H., Lentz S. R., Banerjee R. (2004) Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R39–R46 [DOI] [PubMed] [Google Scholar]
- 34. Deme J. C., Miousse I. R., Plesa M., Kim J. C., Hancock M. A., Mah W., Rosenblatt D. S., Coulton J. W. (2012) Structural features of recombinant MMADHC isoforms and their interactions with MMACHC, proteins of mammalian vitamin B12 metabolism. Mol. Genet. Metab. 107, 352–362 [DOI] [PubMed] [Google Scholar]
- 35. Gherasim C., Hannibal L., Rajagopalan D., Jacobsen D. W., Banerjee R. (2013) The C-terminal domain of CblD interacts with CblC and influences intracellular cobalamin partitioning. Biochimie 95, 1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]





