Abstract
We earlier identified a developmental series of seven isoforms/polymorphs of microparticulate inulin by comparing non-covalent bonding strengths. Their pharmaceutical utility lies in modulation of cellular immunity, exploited as vaccine adjuvants (Advax™) especially for delta inulin (DI). As such particles cannot be sterilized by filtration we explore the effect of 60Co gamma radiation (GR) on inulin isoforms, particularly DI. Its adjuvant activity and overt physical properties were unaffected by normal GR sterilizing doses (up to 25 kGy). Heating irradiated isoform suspensions near their critical dissolution temperature revealed increased solubility deduced to reflect a single lethal event in one component of a multi-component structure. Local oxidative effects of GR on DI were not found. The observed DI loss was almost halved by re-annealing at the critical temperature: surviving inulin chains apparently reassemble into smaller amounts of the original type of structure. Colorimetric tetrazolium assay revealed increases in reducing activity after GR of raw inulin powder, which yielded DI with normal physical properties but only 25% normal recovery yet 4× normal reducing ability, implying final retention of some GR-changed inulin chains. These findings suggest minimal inulin chain cleavage and confirm that GR may be a viable strategy for terminal sterilization of microparticulate inulin adjuvants.
Keywords: adjuvant, immunity, inulin, polymorph, sterilization, vaccine
1. Introduction
Inulin's applications [1] include immune-active semi-crystalline isoforms (microparticulate inulin, MPI) acting as vaccine adjuvants [2 - 5]. Adjuvant technology based on delta inulin microparticles (Advax™) was successful in a Phase 2 human trial of a pandemic influenza vaccine [6], as well as enhancing humoral and cellular immunogenicity and improving protection across a broad range of preclinical vaccine models including human immunodeficiency virus-1 and malaria [7 - 15]. A key property especially for human vaccines is that Advax™ is non-inflammatory, with low reactogenicity and high human and animal safety, contrasting with more traditional inflammatory-pathway adjuvants [16, 17].
Inulin (α-D-[2→1] poly(fructo-furanosyl) β-D-glucose) comprises mostly linear polyfructose chains [18] with one terminal glucose. Our chicory-derived inulin (‘Raw Material’, RM) has chains of 15-100 fructose residues with a number-average degree of polymerization (DPn) ∼30. These spontaneously crystallize in seven MPI polymorphs [2], the most biologically useful being delta inulin [4]. Key to which form emerges is the temperature of formation or treatment. Preparation to Good Manufacturing Practice standard is straightforward and MPI is of clinical (BP/USP) purity and sterility. Such preparations can comprise true polymorphic forms of identical molecular content but distinguishable by strength of non-covalent bonding, defined by a critical dissolution temperature (Tc). The same seven phenotypes may also be assumed by MPI of analogous compositions but different DPn termed isoforms, which are inulin's usual presentation but carry a range of longer chains [2].
The particulate nature of inulin adjuvants rules out filtration to sterilize the final vaccine product. Gamma radiation (GR) sterilization is now in industrial use for pharmaceuticals [19] and may offer an alternative for this purpose. It may also throw light on MPI structure, with inulin particles conceived as layers of crystalline lamellae [2, 20 - 22] each comprising chains helically folded into rigid rods in parallel arrays. The arrays form broad sheets with the rods perpendicular to the lamellar plane, isoforms presumably reflecting variations in the rods’ makeup. Much is now known of low-dose ionizing radiation effects on biological materials [23]. We here analyze effects of calibrated GR of selected inulin isoforms and discuss relevancies to structural concepts of MPI.
2. Materials and methods
2.1. Inulin preparation and assay
Chicory inulin (Raftiline HP) was supplied as a single large batch in powder form by BENEO-Orafti, Tienen, Belgium. Isoform samples were purified to BP/USP compliance [2]. Sterilization of raw (dry) inulin samples with 25 kGy gamma irradiation or ethylene oxide was done by Steritech, Dandenong, Victoria, Australia.
2.2. Calibrated gamma irradiation
Samples were irradiated with measured doses at 50 mg inulin/ml in PBS pH 7.0 except where indicated, held in sterile, sealed polycarbonate tubes in holders ensuring uniform dosage. These were exposed to 60Co radiation from a Gammacell 220 irradiator at 4.2 kGy/h, monitored using ceric-cerous dosimetry [24]. The expanded uncertainty in the absorbed doses was calculated to be 3.5 % (k=2) [25]. The temperature during GR for all samples varied between 27 - 30 °C.
2.3. Adjuvant potency
Female BALB/c mice, 6-8 weeks old, were bred under specific pathogen-free conditions (Flinders University animal facility). All procedures were in accordance with the Animal Experimentation Guidelines of the National Health and Medical Research Council of Australia and approved by the Flinders Animal Welfare Committee. Mice (5 per group) were immunized twice intramuscularly at a 14-day interval with 1 μg hepatitis B surface antigen (HBsAg) alone or with 1 mg of un-irradiated or 10, 15, 20 or 25 kGy GR DI. Adjuvant potency of control and irradiated DI formulations was assessed by antigen-specific IgG1 measured in serum samples taken 14 days after the second immunization, and expressed as OD450nm read from direct ELISA assay [4].
2.4. Colorimetric assay of reducing activity
One g tetrazolium blue chloride (1.37 mmol) dissolved. (60 °C) in 500 ml of 0.1 M NaOH, and a 500 ml solution of 2M K/Na tartrate, both filtered through a Durapore (Millipore) filter, were combined in equal volumes [26]. Inulin solutions (10 mg/ml, dissolved at 85 °C in 1 mM Na bicarbonate) were diluted to 500 μl, mixed with 2 ml of combined reagent, re-heated (85 °C, 5 min) and rapidly cooled to 20 °C. Absorbencies at 660nm in the linear range (< 0.4) of triplicate tubes were read against a blank of parallel reagent alone.
3. Results
3.1. Lack of overt effects of GR
We used three separate GR runs, the first using metered doses of 0, 10, 15, 20 and 25 kGy (the range usual for microbial sterilization [27]). The isoforms selected were gamma, delta and epsilon inulins (GI, DI and EI, respectively), their sequence in the polymorph development series [2]; each may be derived from its precursor by simple heat treatment. GR produced no changes in these preparations measurable at room temperature (RT: ∼20 °C), monitoring: a) dissolved inulin (refractive index, RI, of supernatant), b) particle light scattering as measured by OD700nm at a concentration of 0.5 mg/ml, a sensitive parameter for monitoring inulin suspensions [2, 4, 28, 29], c) particle size (centrifugation behaviour and optical microscopy), and d) physical appearance. An exception at 25 kGy was a faint yellowish tinge in the GR material. Notably, adjuvant activity of DI in vivo (ability to enhance immunogenicity of hepatitis B surface antigen in Balb/c mice) was not affected by GR (Fig. 1).
Fig. 1.
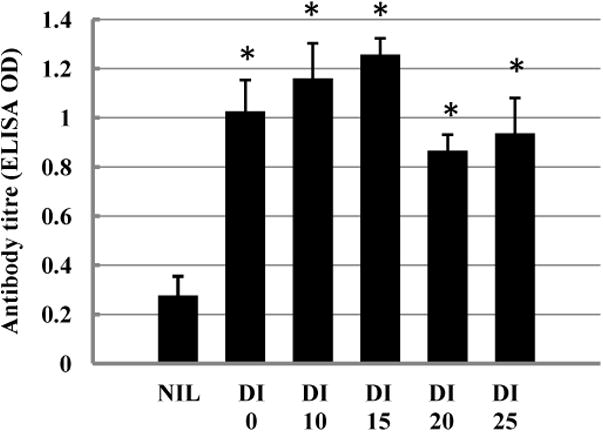
Lack of effect of GR on adjuvant activity of delta inulin. The DI samples were those described in Fig. 2C. Balb/c mice (5 per group) were immunized with 1 μg recombinant hepatitis B surface antigen (HBsAg) alone or in combination with 1 mg of DI either unirradiated or irradiated as indicated (kGy), and boosted with the same inocula at 14 days. The 28 day sera were assayed for HBsAg-specific IgG1 using ELISA colorimetric assay (Methods). Error bars are SD; * = significantly different from Nil dose (p < 0.001, t test); the irradiated doses did not significantly differ from each other.
3.2. GR damage revealed by isoform analysis
More informative was analysis with the sensitive OD700nm assay at 0.5 mg/ml and higher temperatures around the Tc, as previously used to differentiate isoforms. The second and third GR runs included doses up to 120 kGy. Fig. 2A compares the Tc as 50% OD700nm thermal transition points for the three forms used here, and for alpha-2 inulin (AI-2) from which they were originally derived. The Tc is the critical dissolution temperature at which particle disassembly begins, analogous to a melting point. Such OD700nm thermal transition curves were repeated on every irradiated sample of each isoform, re-expressing data in terms of GR dose (Figs. 2B, C, D) at particularly informative temperatures. Each point is referenced to the starting OD (OD700nm at 20 °C and zero dose). Particle structures appeared largely unaffected up to 25 kGy when tested for solubility at 20 °C and were only detectably affected by GR when testing solubility near each variant's Tc (GI: 45 °C; DI: 53 °C; EI: 64 °C). There GR particles were more prone to dissolution (compare especially the irradiated DI solubilities at 50 °C and 51 °C). Thus despite internal GR–induced changes MPI structure remained intact until tested at higher temperatures, revealing covert differences from the un-irradiated (control) particles near the Tc. This suggested multiple structures in MPI where some component(s) could maintain overall particle integrity at 20 °C even though another component was affected by GR.
Fig. 2.
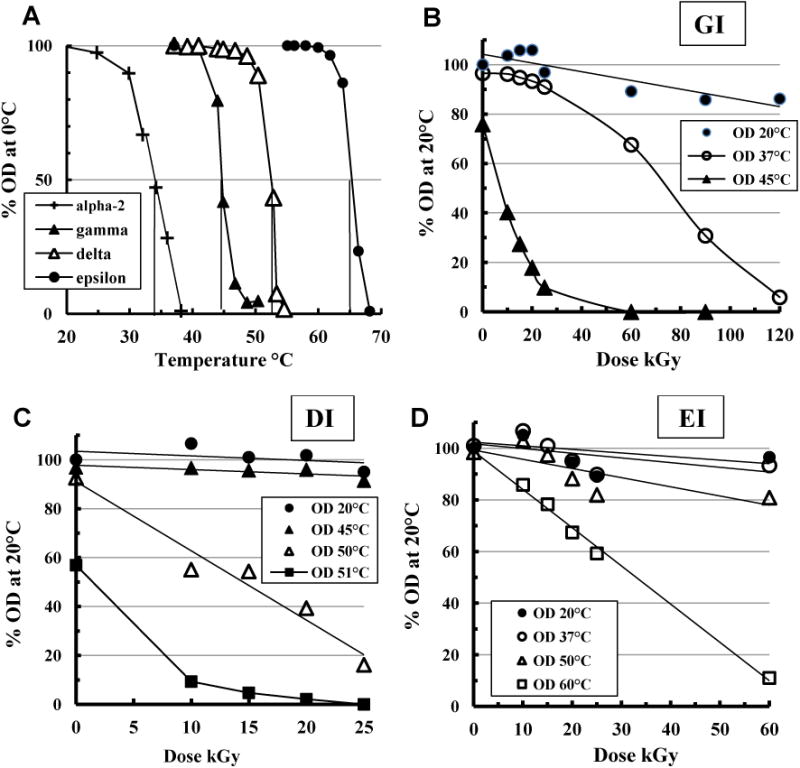
GR changes to inulin isoforms revealed at higher temperatures. A. Typical OD700nm temperature curves of un-irradiated AI-2, GI, DI and EI samples. Glass tubes containing isoform dilutions (0.5 mg/ml PBS) were progressively heated and the OD700nm measured after 10 min equilibration at the indicated temperatures. B, C, D. Similar dilutions of the isoform samples irradiated in PBS alone were heated (10 min) at the indicated temperatures and their OD700nm expressed as % of the OD700nm of the control (un-irradiated sample measured at 20 °C).
3.3. Nature of the GR event
GR-induced solubility changes were explored using DI as example. Fig. 3 (logarithmic plot) shows that replicate GR runs were quantitatively reproducible (un-modified samples, circles). The rate of change closely approximated first-order (‘single-hit’) characteristics according to classical target theory when uncompromised by repair or bystander effects [30, 31]. Fig. 2 shows single-hit characteristics for all three inulin isoforms at doses up to 25 kGy. Although there could be oxidative effects by free radicals generated by ionization of water, inclusion in parallel samples of free radical scavengers (100 mg/ml glucose or fructose, or 5 mg/ml sodium ascorbate) had little effect on GR-induced solubility changes to DI. Oxidation might be catalytically enhanced by traces of metals e.g. Fe3+ [32] but inclusion during GR of chelating agents at pH 7 (10 mM EDTA or 10 mM sodium citrate) had no effect either (data not shown). GR using DI at 50 or 80 mg/ml, or formulated in PBS at pH 7 or pH 9, or water alone, or formulated as a hybrid particle with aluminium phosphate using GI or DI [29], similarly did not affect sensitivity to GR (data not shown).
Fig. 3.
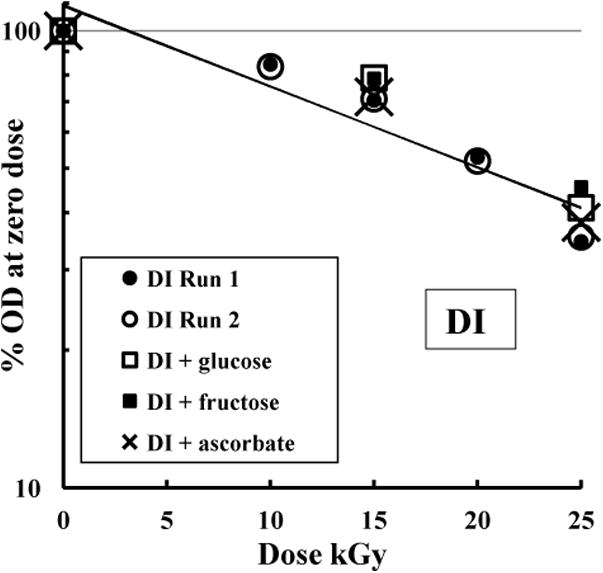
Analysis of the effect of GR on DI preparations. Samples of DI were irradiated in PBS alone (circles, GR runs 1 and 2) or in presence of glucose (100 mg/ml), fructose (100 mg/ml) or ascorbate (10 mg/ml) as indicated. Diluted samples (0.5 mg/ml) were then heated (10 min, 50 °C), and the OD700nm expressed as % of zero dose (logarithmic plot). The exponential trend-lines of DI Runs 1 and 2 are both shown and overlap identically.
3.4. Re-annealing repair of GR damage
Heat conversion of an inulin isoform/polymorph to the next higher in the series [2, 4, 28] resembles a snap re-crystallization in which some polymer chains are dissolved, re-forming on nearby chains a new structure more stable at the higher temperature. This has a higher Tc/aggregate strength of polymer-polymer non-covalent bonding, while lower DP chains are removed. We hypothesized that the complex of intact and GR-altered inulin chains could also be re-organised by heating to rescue undamaged chains, remove changed chains and reform the original isoform structure.
Fig. 4A, 4B (linear plots) confirms this with exemplar DI preparations. Portions of untreated and irradiated samples were heat-converted as if preparing DI from GI (2 h, 55 °C). Comparing OD700nm (0.5 mg/ml, 50 °C) at each GR dose point, the portion of 50 °C-soluble material generated after GR was approximately halved by re-annealing at the Tc of DI (Fig. 4A, the average amounts lost after GR and rescued by re-annealing of each of 15 replicate dose point DI pairs). The overall mean ratio lost: recovered was 2.24 ± 0.65 SD, representing 44.6% recovery. Fig. 4B compares the DI samples qualitatively, showing that although the quantity (Fig. 4A) of 50 °C-insoluble DI, both before and after repair, progressively drops with increasing GR dose, the quality (% DI content as measured by the ratio OD700nm 50 °C: 37 °C) of the rescued DI is essentially 100% DI. That is, half the DI lost is recovered and the recovered material is in as-new condition. A single series of dose comparisons gave similar results for GI using the Tc of GI (45 °C) for annealing repair (Figs. 4C, 4D), and so re-annealing repair was not restricted to the DI isoform. The quality of the rescued GI (OD700nm ratios 37 °C: 20°C: Fig. 4D) was poor at high doses and inactivation departed from exponential.
Fig. 4.
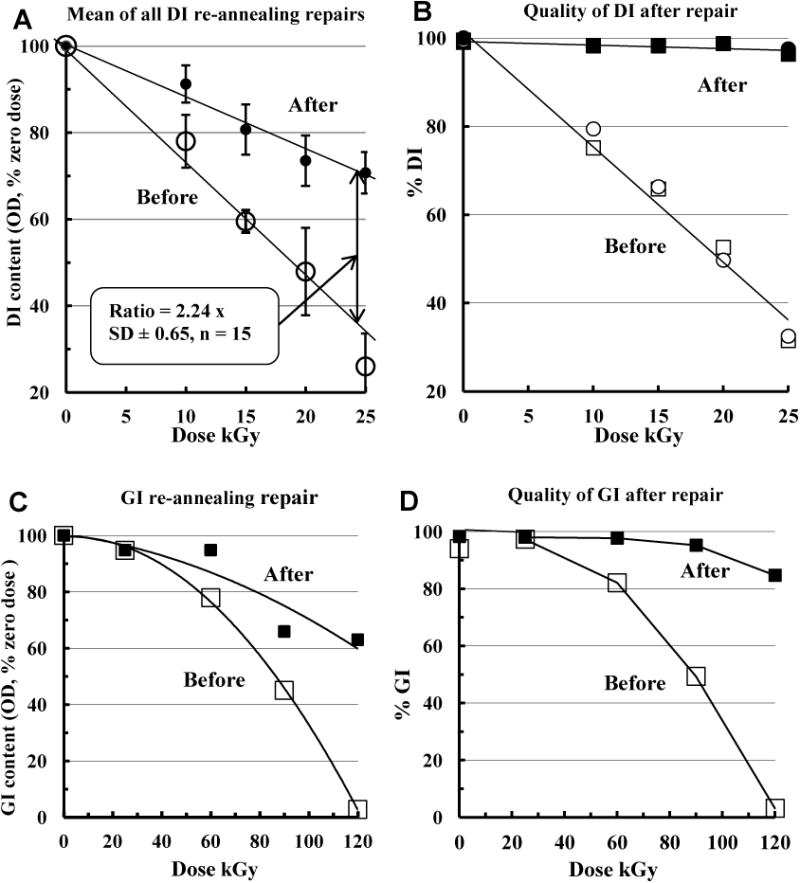
Re-annealing repair of irradiated DI and GI. A. Data presentation similar to Fig. 3 but linear plot. Means of individual dose points of four replicate GR runs comparing DI before (open circles) and after (filled circles) re-annealing repair at 55 °C (50 mg/ml, 2 h). Diluted samples (0.5 mg/ml in PBS) were then heated (10 min, 50 °C) and their OD700nm expressed as % of zero dose. The ratio given is the mean of ratios of the 15 before-and-after dose point pairs in these repeats. B. The data of Fig. 4A re-expressed as % DI content (ratio of OD700nm at 50 °C: OD700nm at 20 °C) of each irradiated and irradiated/repaired sample. C. Effect of GR on GI before (□) and after (■) repair at 45 °C (50 mg/ml, 2 h). Control and irradiated samples before and after repair were diluted (0.5 mg/ml PBS) and heated (10 min, 37 °C). Their OD700nm was expressed as % of zero doses (logarithmic plot). D. The data of Fig. 4C re-expressed as % GI content (OD700nm ratio 37 °C: 20 °C) of each irradiated and irradiated/repaired sample.
3.5. Effect of GR on ‘DI potential’
The amount of e.g. DI that can be obtained by heat conversion (2 h, 53 - 55 °C) from its precursor preparation (GI) is termed its DI potential, which in turn depends on how the GI was made. All four preparations in GI format were re-precipitated at 5 °C in high yield from one lot of DI dissolved at 80 °C so that the GI and DI inulin chain contents were identical. That is, these GI format preparations were true polymorphic forms of their parent DI format inulin [2]. Their content of chains in the DI format (OD700nm ratio 50 °C: 20 °C) was 1 - 2% before conversion to DI but increased to 96% after 55 °C conversion. We then compared the sensitivity to GR of the DI potential of GI with that of DI itself by measuring the DI potential of the irradiated GI samples depicted in Figs. 2 and 4C, 4D (Fig. 5, logarithmic plot). The ‘mean DI repaired (55 °C) after GR of DI’ data points from Fig. 4A are included for comparison, although these were only available up to 25 kGy. Fig. 5 shows no obvious difference in the GR sensitivity of the inulin chains able to form DI, whether they were originally present in GI or DI formats. Thus, as expected, it is the polymer chain lengths that represent the GR target rather than the polymorphic form in which they reside.
Fig. 5.
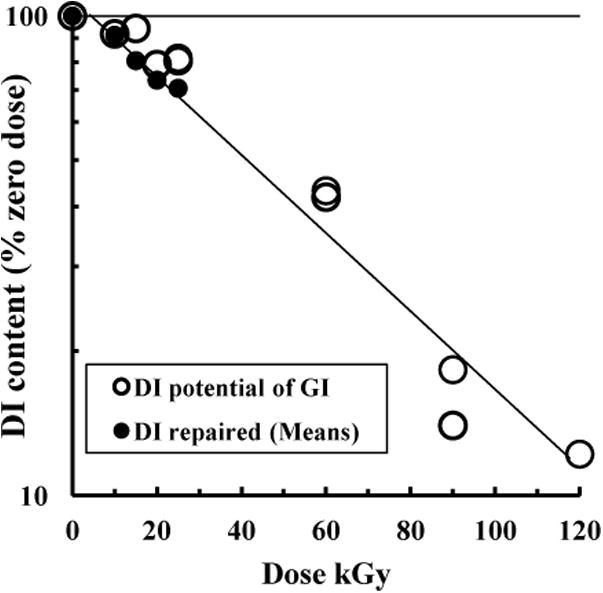
Sensitivity to GR of the potential of GI to be heat-converted to DI. Portions of the irradiated GI samples of Fig. 4C, 4D were converted to DI (2 h, 50 mg/ml, 55 °C) and the OD700nm of diluted samples (0.5 mg/ml PBS heated 10 min at 50 °C) expressed as % DI per dose (○). The mean values of repaired DI (●) are from Fig. 4 A.
3.6. GR effect on reducing activity
We probed the chemical basis of GR effects on MPI by measuring changes in ability to reduce tetrazolium blue chloride [26], expecting that reducing groups may be generated by inulin chain cleavage yielding new terminal fructose residues, or by damage to furanose rings yielding groups functioning as aldehydes or ketones. This method benefits from a rapid single reaction combining redox and colour chemistries. The untreated RM itself had innate reducing activity and yielded a standard curve that was reproducibly linear with inulin concentration between 0.1 and 2.0 mg/ml, measuring absorbance (Abs660nm). Lacking appropriate standards, all assays compared parallel standard RM curves and the results expressed as a percentage of the equivalent RM absorbance. Samples tested were the dry RM powder either untreated, or sterilized by GR (GR-RM, 25 kGy) or with ethylene oxide (ETO-RM), and DI then prepared from the RM, GR-RM or ETO-RM by the standard production protocol [2].
These samples were compared with 12 routine lots (‘standard DI’) prepared in the same way from untreated RM (Fig. 6), whose mean properties were normalized at 100%. The small error bars of the standard DI values show the reproducibility of the routine production method. All DI batches had typical DI content (mean ± SD of 92 ± 5 %) and solubility properties. Standard DI had a mean ± SD of 53 ± 3% of the reducing activity of the RM, from which DI was recovered in a yield of 38 ± 2% by dry weight. The reducing activity of the RM was unaffected by ETO as expected, while GR increased it more than 2-fold. DI made from ETO-RM had the same reducing activity as standard DI with normal overall dry weight recovery but DI made in the same way from GR-RM had ∼4-fold the reducing activity of standard DI with only ∼25% of standard DI dry weight recovery. The DPn of GR-DI was 48.2 as measured by end-group analysis using 1H nuclear magnetic resonance spectroscopy [33], compared with 40 ± 1 (38.9 - 41.1) for standard DI. GR had no significant effect on the NMR 1H resonance spectrum.
Fig.6.
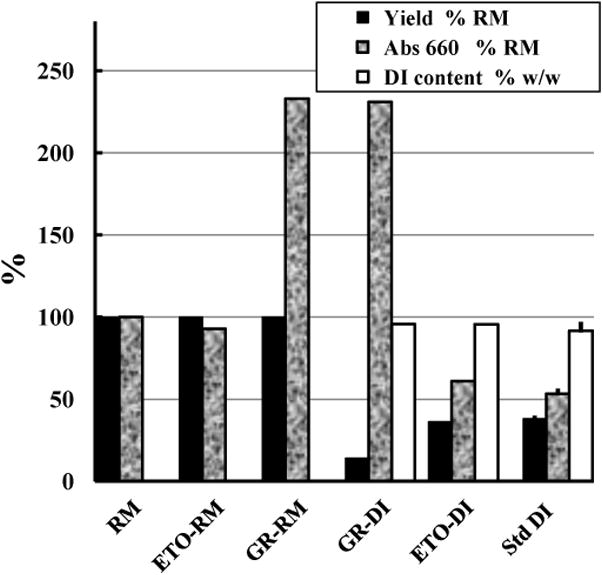
Reducing activities and physical properties of inulin preparations. The RM was compared with the RM commercially sterilized with ethylene oxide (ETO-RM) or with 25 kGy (GR-RM), and with DI made from these three sources using the standard production protocol. In the cases of yields and absorbencies the results are standardized taking the value of the RM as 100%. ‘Yield’ is compared w/w, ‘Abs660’ is the absorbance of the colour produced after reduction of tetrazolium blue (Methods), and ‘DI content’ = (OD700nm at 49 °C/OD700nm at 40 °C) × 100 of a 2 mg/ml suspension [2]. Error bars are SD of a set of 12 replicate routine standard DI preparations.
4. Discussion
Functionally, adjuvant activity and overt phenotype of DI MPI were unaffected by normal sterilizing doses of GR (15 - 25 kGy). Internal structural changes at 10 - 25 kGy were shown in all samples by isoform analysis.
The lack of effect of free radical scavengers or chelating agents makes GR damage by indirect oxidation an unlikely cause of these changes. The linear fructose polymer backbone does not pass through the furanose ring but comprises a polyethylene oxide chain (- C - C -O -) of the anomeric C2 carbon, the C1 carbon and its attached oxygen. Covalent bond breakage could occur by direct atomic ionization either in the chain backbone creating lower DP material, or in the furanose moiety affecting H-bonding, chain folding or charge distribution. Local thermal hydrolysis of the glycosidic bond is possibly the most likely damage event, a conclusion echoed for GR of β-glucan [34] and of lignocellulose [35].
The GR changes were dose-dependent with first-order (‘single-hit’) characteristics, resembling the random ‘lethal hit’ of classical target theory [31] where one component is made completely non-functional by a single damage event. Target theory holds that a ∼37% (e-1) ‘survival’ of any component means not only that by definition 37% received zero lethal hits, but by Poisson distribution statistics the overall distribution of lethal hits is on average one per unit. The 37% survival doses for GI, DI and EI were about 80, 60 and 40 kGy respectively (Figs. 2 and 5). These rough estimates indicate that the number of lethal hits delivered by 25 kGy to MPI is not large, apparently on average well under 1 per unit for the smaller targets of GI and DI.
MPI behaves like a multiple structure in which some component(s) maintain the basic formation even though another component is disabled by GR. The minimal lethal hit is inferred to comprise a single event in just one chain, but is not detected until the particle approaches its Tc. There its entire structure abruptly becomes unstable and begins to disassemble, while GR change exposes the structure to greater instability at temperatures just below its Tc. Irradiated preparations are partly repaired by a process resembling reshuffling H-bonding to re-assort damaged and un-damaged chains. Original variant-type material is rescued in an as-new state, almost exactly halving the amount lost at each dose point. A 50% recovery of lost material implies some duplex arrangement in the pre-damage structure, i.e. some kind of pair-wise ‘special relationship’ between two adjacent chains. While the crystal unit cell of inulin involves complex H-bonding between two anti-parallel helical chains [21], it is not yet clear whether this arrangement is sufficient to account for the two-fold repair observed here.
Colorimetric analysis shows innate reducing activity of the dry RM that is increased further by GR, where oxidative radiation effects are negligible without participation of water. The RM is free of short-chain fractions, has a DPn ∼30 and negligible free fructose. Unfractionated chicory inulin contains small amounts of glucose-free fructose polymers [18], presumably offering reducing end-groups. The RM may well have retained some of these, while DI preparation appears to decrease their proportion. To estimate the scale of reducing-end content, we find the absorbance from 1 mg/ml RM (∼ 0.25) equals that of 2.5 μg/ml fructose, representing hexose-hexose molar ratios of ∼400: 1. Fructose or reducing di- and tri- saccharides of [26] behaved similarly. A DPn of ∼30 then implies that the RM chains carrying a reducing group are a small minority. The multiplier effect of GR on this proportion (∼2-fold) is not significant. The DPn of GR-DI (48 ± 1) compared with 40 ± 1 for standard DI suggests that some longer glucose-free chains may be retained to increase the fructose: glucose ratio on which DPn assay depends, but the aggregate thermal properties were not affected.
5. Conclusions
Bond vulnerability, the losses of DI remaining after annealing rescue and reducing-group assay make minor chain cleavage seem most likely as the GR damage event. GR-DI appears not to differ qualitatively from standard DI. Overall, GR is a viable strategy for terminal sterilization of Advax™ microparticulate inulin adjuvants. These studies provide a basis for the parameters to be used for commercial application.
Highlights.
Advax™ adjuvant (delta inulin-based) has uniform particles ∼1-2 μm diameter
Micro particles this size cannot be terminally sterile-filtered
60Co sterilization (25 kGy) does not overtly affect adjuvant action or structure
Isoform and reducing-end analyses show single-hit cleavage of some inulin chains
Gamma irradiation appears a viable strategy for Advax™ terminal sterilisation
Acknowledgments
We are grateful to Connie Banos, Justin Davies and the staff at the Australian Nuclear Science and Technology Organization, Lucas Heights, NSW, for advice and radiation services and for reviewing the manuscript. We acknowledge Dr Bruce Lyons for performing the HBsAg immunization study as an employee of Vaxine. We thank Dr Doug Taupin for invaluable support throughout the course of this work and Timothy Jose for excellent technical assistance. P.C. is an Emeritus Visiting Fellow of the Australian National University Medical School and of the Department of Immunology, John Curtin School of Medical Research, ANU, Canberra, Australia. This work was supported in part by Federal funds from the National Institutes of Health (NIH; Collaborative Research Contract No. U01AI061142) and the US Department of Health and Human Services (HHSN272200800039C). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was also supported by the Australian Research Council through a Linkage grant (LP0882596) and a LIEF grant (LE0668489).
Abbreviations
- AI-2
alpha-2 inulin
- BP
British Pharmacopeia
- DI
delta inulin
- DP
degree of polymerization
- DPn
number average degree of polymerization
- EDTA
ethylene diamine tetra acetate
- EI
epsilon inulin
- GI
gamma inulin
- GR
gamma radiation
- kGy
kilo Gray
- PBS
phosphate buffered saline
- RI
refractive index
- RT
room temperature (20-21 °C)
- Tc
critical temperature of an isoform/polymorphic variant
- SD
standard deviation
- USP
United States Pharmacopeia
Footnotes
Conflict of interest statement: P.C. and N.P. are employees or consultants of Vaxine Pty Ltd.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barclay T, Ginic-Markovic M, Cooper P, Petrovsky N. Inulin - a versatile polysaccharide with multiple pharmaceutical and food chemical uses. J Excipients and Food Chem. 2010;1:1–24. [Google Scholar]
- 2.Cooper PD, Barclay TG, Ginic-Markovic M, Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013;23:1131–1141. doi: 10.1093/glycob/cwt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper PD, McComb C, Steele EJ. The adjuvanticity of Algammulin, a new vaccine adjuvant. Vaccine. 1991;9:408–415. doi: 10.1016/0264-410x(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 4.Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising (β-D-[2→1] poly(fructo-furanosyl) α-D-glucose polymers. Glycobiology. 2011;21:595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper PD, Steele EJ. The adjuvanticity of gamma inulin. Immunology and Cell Biology. 1988;66:345–352. doi: 10.1038/icb.1988.45. [DOI] [PubMed] [Google Scholar]
- 6.Gordon DL, Sajkov D, Woodman RJ, Honda-Okubo Y, Cox MM, Heinzel S, Petrovsky N. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 2012;30:5407–5416. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Layton RC, Petrovsky N, Gigliotti AP, Pollock Z, Knight J, Donart N, Pyles J, Harrod KS, Gao P, Koster F. Delta inulin polysaccharide adjuvant enhances the ability of split- virion H5N1vaccine to protect against lethal challenge in ferrets. Vaccine. 2011;29:6242–6251. doi: 10.1016/j.vaccine.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda-Okubo Y, Saade F, Petrovsky N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad- based enhancement of adaptive immune responses. Vaccine. 2012;30:5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristillo AD, Ferrari MG, Hudacik L, Lewis B, Galmin L, Bowen B, Thompson D, Petrovsky N, Markham P, Pal R. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1- vaccine formulations. J Gen Virol. 2011;92:128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saade F, Honda-Okubo Y, Trec S, Petrovsky N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckersley AM, Petrovsky N, Kinne J, Wernery R, Wernery U. Improving the dromedary antibody response: The hunt for the ideal camel adjuvant. J Camel Practice Res. 2011;18:35–46. [Google Scholar]
- 12.Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, Komiya T, Toriniwa H, Petrovsky N. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010;91:1407–1417. doi: 10.1099/vir.0.019190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larena M, Prow NA, Hall RA, Petrovsky N, Lobigs M. JE-ADVAX vaccine protection against japanese encephalitis mediated by memory B cells in the absence of CD8+ T cells and pre-exposure neutralizing antibody. J Virol. 2013;87:4395–4402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrovsky N, Larena M, Siddharthan V, Prow NA, Hall RA, Lobigs M, Morrey J. An inactivated cell-culture japanese encephalitis vaccine (JE- ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile Encephalitis via cross-protective memory B cells and neutralizing antibody. J Virol. 2013;87:10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva DG, Cooper PD, Petrovsky N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol Cell Biol. 2004;82:611–616. doi: 10.1111/j.1440-1711.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- 16.Petrovsky N. Vaccine adjuvant safety: the elephant in the room. Expert Rev Vaccines. 2013;12:715–717. doi: 10.1586/14760584.2013.811198. [DOI] [PubMed] [Google Scholar]
- 17.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 18.De Leenheer L, Hoebregs H. Progress in the elucidation of the composition of chicory inulin. Starch. 1994;46:193–196. [Google Scholar]
- 19.Garcia R, Howard B, LaRue R, Parton G, Walker J. Strategies for gamma sterilization of pharmaceuticals. Pharm Med Packaging News May. 2004;29 http://www.pmpnews.com/article/strategies-gamma-sterilization-pharmaceuticals. [Google Scholar]
- 20.Hébette CL, Delcour JA, Koch MHJ, Booten K, Reynaers HL. Crystallization and melting of inulin crystals. A small angle X-ray scattering approach (SAXS) Polimery. 2011;56:645–651. [Google Scholar]
- 21.André I, Mazeau K, Tvaroska I, Putaux JL, Winter WT, Taravel FR, Chanzy H. Molecular and crystal structures of inulin from electron diffraction data. Macromol. 1996;29:4626–4635. [Google Scholar]
- 22.André I, Putaux JL, Chanzy H, Taravel FR, Timmermans JW, de Wit D. Single crystals of inulin. Int J Biol Macromol. 1996;18:195–204. doi: 10.1016/0141-8130(95)01075-0. [DOI] [PubMed] [Google Scholar]
- 23.Brooks AL. A history of the United States Department of Energy (DOE) low dose radiation research program: 1998-2008. Draft book published online for peer review by cited authors. 2012 doi: 10.1667/RR14027.1. http://lowdose.energy.gov/pdf/albRoughDraft/doeHistoryComplete09262012.pdf. [DOI] [PubMed]
- 24.ASTM: American Society for Testing and Materials International. Standard practice for use of a ceric-cerous sulfate dosimetry system. ISO/ASTM 51205:2009. Annual Book of ASTM Standards. 2009;12:02. www.astm.org/digitallibrary. [Google Scholar]
- 25.ISO: International Organization for Standardization. Uncertainty of measurement - Part 3: Guide to the expression of uncertainty in measurement. ISO/IEC Guide. 2008:98–3. [Google Scholar]
- 26.Jue CK, Lipke PN. Determination of reducing sugars in the nanomole range with tetrazolium blue. J Biochem Biophys Methods. 1985;11:109–115. doi: 10.1016/0165-022x(85)90046-6. [DOI] [PubMed] [Google Scholar]
- 27.AAMI TIR33. Sterilization of health care products. Association for the Advancement of Medical Instrumentation Technical Information Report; Arlington VA: 2005. [Google Scholar]
- 28.Cooper PD, Carter M. Anticomplementary action of polymorphic ‘solubility forms’ of particulate inulin. Mol Immunol. 1986;23:895–901. doi: 10.1016/0161-5890(86)90075-1. [DOI] [PubMed] [Google Scholar]
- 29.Cooper PD, Steele EJ. Algammulin, a new vaccine adjuvant comprising gamma inulin particles containing alum: preparation and in vitro properties. Vaccine. 1991;9:351–357. doi: 10.1016/0264-410x(91)90063-c. [DOI] [PubMed] [Google Scholar]
- 30.Haynes RH, Eckardt F, Kunz BA. The DNA damage-repair hypothesis in radiation biology: Comparison with classical hit theory. Br J Cancer. 1984;49:81–90. [PMC free article] [PubMed] [Google Scholar]
- 31.Lea DE. Actions of radiations on living cells. second. Cambridge University Press; Cambridge, UK: 1962. [Google Scholar]
- 32.Graf E, Mahoney JR, Bryant AG, Eaton JW. Iron-catalysed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984;259:3620–3624. [PubMed] [Google Scholar]
- 33.Barclay T, Ginic-Markovic M, Johnston MR, Cooper PD, Petrovsky N. Analysis of the hydrolysis of inulin using real time 1H NMR spectroscopy. Carbohydr Res. 2012;352:117–125. doi: 10.1016/j.carres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byun EH, Kim JH, Sung NY, Choi Jil, Lim ST, Kim KH, Yook HS, Byun MW, Lee JW. Effects of gamma irradiation on the physical and structural properties of β- glucan. Radiation Phys Chem. 2008;77:781–786. [Google Scholar]
- 35.Orozco RS, Hernández PB, Ramírez NF, Morales GR, Luna JS, Montoya AGC. Gamma irradiation induced degradation of orange peels. Energies. 2012;5:3051–3063. [Google Scholar]


