Abstract
Type 1 fimbriae are filamentous structures on Escherichia coli. These structures are important adherence factors. Because binding to the host cells is the first step of infection, type 1 fimbria is an important virulence factor of pathogenic E. coli. Expression of type 1 fimbria is regulated by a phase variation in which each individual bacterium can alternate between fimbriated (phase-ON) and nonfimbriated (phase-OFF) states. The phase variation is regulated by the flipping of the 314-bp fimS fragment, which contains the promoter driving the expression of the genes required for the synthesis of type 1 fimbria. Thus, the bacterial proteins able to interact with fimS are likely to be involved in regulating the expression of type 1 fimbria. To identify novel type 1 fimbria-regulating factors, we used an E. coli K12 proteome chip to screen for the bacterial factors able to interact with a 602-bp DNA fragment containing fimS and its adjacent regions. The Spr protein was identified by the proteome chip-based screening and further confirmed to be able to interact with fimS by electrophoretic mobility shift assay. Deletion of spr in the neonatal meningitis E. coli strain RS218 significantly increased the ratio of the bacterial colonies that contained the type 1 fimbria phase-ON cells on agar plates. In addition, Spr interfered with the interactions of fimS with the site-specific recombinases, FimB and FimE, which are responsible for mediating the flipping of fimS. These results suggest that Spr is involved in the regulation of type 1 fimbria expression through direct interaction with the invertible element fimS. These findings facilitate our understanding of the regulation of type 1 fimbria.
Type 1 fimbriae of E. coli are filamentous surface organelles, which mediate bacterial adherence to biotic and abiotic surfaces, leading to formation of biofilm and colonization on infected hosts (1–3). Because adherence of pathogens to host cells is the initial step to establish infections, type 1 fimbriae have been shown to contribute to E. coli-caused infections (2–4). For example, it has been shown that type 1 fimbriae of the neonatal meningitis E. coli RS218 contribute to bacterial adherence to human brain microvascular endothelial cells (HBMECs), which constitute the blood-brain barrier (BBB). Adherence to human brain microvascular endothelial cells is the essential step for the pathogen to cross the BBB from the bloodstream to infect the central nervous system (2). Type 1 fimbriae are composed of the major subunit FimA and a small tip structure containing FimF, FimG, and the adhesin FimH (5). The genes that encode type 1 fimbria's structure proteins and the factors required for its biogenesis are located in the fim operon which is directed by the fim promoter (5).
The expression of type 1 fimbria by E. coli is regulated by a process known as phase variation that allows individual bacterial cells reversibly switching between a fully fimbriated state (phase-ON) and a nonfimbriated state (phase-OFF) (6). Because of the variation, the bacterial population in a culture comprises a mixture of the type 1 fimbria phase-ON and phase-OFF cells. The phase variation is mediated by the inversion of the 314-bp invertible chromosomal element fimS that contains the fim promoter (6–8). The inversion of fimS alters the orientation of the fim promoter that drives the expression of the fim operon only when its orientation is in the “phase-ON” position (Fig. 1). The inversion of fimS is a process of site-specific recombination catalyzed by specific recombinases. FimB and FimE are the most well-known recombinases that mediate the fimS inversion and are commonly present in E. coli strains. The genes encoding these two recombinases are located at the upstream of fimS and directed by their own promoters (Fig. 1). The site-specific recombinases mediate the inversion of fimS by recognizing the 9-bp inverted repeats bordering this fimS, named the left inverted repeat and right inverted repeat (Fig. 1) (9).
Fig. 1.
The invertible element fimS and its surrounding regions. The 314-bp fimS invertible element contains the fim promoter that drives the transcription of the fim operon. fimA is the first gene of this operon and encodes the major subunit of type 1 fimbria. fimB and fimE encode the site-specific recombinases mediating the flipping of fimS. In the phase-ON orientation, the fim promoter directed to fimA drives the transcription of the fim operon. In the phase-OFF orientation, the fim promoter is directed opposite to fimA and the transcription is off. The 602-bp fimS-plus fragment contains fimS and its upstream and downstream regions. Pfim, fim promoter; PfimB, the promoter of fimB; PfimE, the promoter of fimE; IRL, left inverted repeat; IRR, right inverted repeat.
The expression of type 1 fimbria or the phase variation is affected by the conditions of culture (10–13). For example, subculture in static broth results in a higher ratio of phase-ON cells, whereas growth of most E. coli strains on agar causes few or no phase-ON bacteria in the population (11–13). It has been shown that some regulator proteins directly interact with fimS and its adjacent region to regulate the fimS inversion (9, 14, 15). However, it remains incompletely understood how E. coli regulate the inversion of fimS so as to control the phase variation of type 1 fimbria expression in response to the environmental cues. Identification of novel bacterial factors that can interact with fimS will benefit our understanding the mechanism by which E. coli regulate type 1 fimbria phase variation.
Proteome chips are effective high-throughput tools to determine DNA-protein interactions of both eukaryotic and bacterial cells. For example, eukaryotic proteome chips have been used to systemically investigate DNA-protein interactions, resulting in: (1) identification of novel transcription factors and their target genes (16), (2) determination of novel DNA-protein interactions and their biological functions (17), and (3) identification of numerous human transcription factors with methylated CpG-dependent DNA-binding activities (18) etc. As for bacterial proteome chips, we have previously constructed the E. coli K12 proteome array that contains the proteins encoded in 4256 of the 4288 genes in the E. coli K12 genome (19). This array has been used for investigating DNA damage (19), identifying serological biomarkers of inflammatory bowel disease (20), and revealing the inhibition mechanism of lactoferricin B (21). In the present study, we used the E. coli K12 proteome chip to screen for novel E. coli proteins that can interact with the promoter-containing fimS.
EXPERIMENTAL PROCEDURES
Proteome Microarray Construction
E. coli proteome microarrays were prepared as previously described (19). Briefly, 4256 proteins encoded by E. coli K12 were first purified by using an expression clone library that was constructed by Dr. Mori and his colleagues (22). All purified proteins were spotted in duplicate on a FullMoon slides (FullMoon Biosystems, Sunnyvale, CA) by using a ChipWriter Pro (Bio-Rad, Hercules, CA) with 48 pins. The printed microarrays were stored at −80 °C until required.
Synthesis of the fimS-related DNA Fragments
The fimS-plus fragment was amplified by PCR using the primers, Ch1-F (5′-AGTAATGCTGCTCGTTTTGC-3′) and Ch1-R (5′-GACAGAGCCGACAGAACAAC-3′) as previously described (2). The digoxigenin (DIG)1 labeled fimS-plus, which served as the probe for the E. coli proteome chip assay, were synthesized with 3′-end DIG-labeled Ch1-F and Ch1-R. The fimS fragment was amplified with the primers, FimS-F (5′-TTGGGGCCAAACTGTCTATA-3′) and FimS-R (5′-TTGGGGCCATTTTGACTCAT-3′). The F1 fragment was amplified with Ch1-F and FimS-F. The F2 fragment was produced with FimS-R and Ch1-R. The F3 fragment was amplified with FimS-F and Ch1-R. The F4 fragment was produced with Ch1-F and FimS-R. The PCR reactions were carried out in a Mastercycler gradient.
The E. coli Proteome Chip Assays
To reduce nonspecific binding, the chips were blocked with 1% bovine serum albumin (Sigma-Aldrich Co.). The DIG-labeled fimS-plus DNA in TBS-T (0.05% Tween20) and 1% bovine serum albumin were used to probe the chips in a hybridization chamber with shaking at room temperature (RT) for 1 h. After incubation, Cy3-labeled anti-DIG antibody (Mission Biotech, Taiwan) was used to probe the chips at RT for 30 min. Finally, the chips were washed several times with TBS-T and distilled water. After the final wash, the chips were dried by centrifugation at 201 × g and then scanned by a microarray scanner (GenePix® 4000B, Axon Instruments, Union City, CA). The proteins binding to the DIG-labeled fimS-plus were detected at an excitation wavelength of 532 nm and emission wavelength of 570 nm.
Bioinformatics Analysis of the Chip Assays
To analyze the images of the chip assay results, GenePix Pro 6.0 was used to align each protein spot and export all of the images to text files. For data preprocessing, the protein signal larger than two standard deviation plus mean was defined as positive signal. The protein showing positive signals in all three experiments was defined as the final hit.
Electrophoretic Mobility Shift Assay (EMSA)
To confirm the interactions between fimS-plus and E. coli proteins, the fimS-plus DNA fragment was used as probes. The 6xHis-tagged recombinant proteins, including Spr, YjcZ, and AckA, were purified as described earlier (19). For binding reactions, the mixtures of the 6xHis-tagged recombinant proteins, fimS-plus, and 0.5% BSA (Sigma-Aldrich Co.) were incubated for 1 h at RT, and then subjected to agarose gel electrophoresis (1.2% agarose, 4 °C). Then the gel was stained with SYBR® Gold (InvitrogenTM), and visualized by UV illumination. Similarly, to investigate the interactions of Spr with the DNA fragments fimS, F1, F2, F3, and F4, these DNA fragments were used to incubate with the 6xHis-tagged Spr, respectively.
Invertible Element Orientation Assays
The invertible element orientation assay uses the asymmetrical digestion site of SnaBI within fimS to determine the orientation of the fim promoter (Fig. 5A). The SnaBI restriction fragments of the fimS-plus PCR products derived from the E. coli cells with fim promoter in the phase-ON orientation are 404 bp and 198 bp. The SnaBI restriction fragments of the fimS-plus derived from the phase-OFF cells are 160 bp and 442 bp. The fimS-plus PCR products derived from the E. coli cells cultivated in LB or on agar plates were purified and then subjected to SnaBI (New England Biolabs) digestion. The resulting fragments were separated in a 2.8% agarose gel, stained with RedSafeTM (iNtRON Biotechnology, Gyeonggi-do, Korea), and then visualized by UV illumination. The images of the bands corresponding to the 198 bp (ON-band) and 160 bp (OFF-band) were photographed using a VGIS-3 video gel image system (Top Bio co., Taiwan). To quantify the percentage of phase-ON E. coli cells in LB, we measured the intensities of each band by using Image J program (2). As the intensity of the bands directly correlates with the size of the fragment, a normalization procedure was applied for the 198 bp (ON) fragment band by using the formula: Intensity ON-normalized = (Intensity ON/198) × 160. The percentage of the phase-ON bacteria was estimated by comparing the normalized ON-band intensity value with the sum intensity of the ON- and OFF-bands. To determine whether a bacterial colony contained phase-ON cells on the agar plates, we investigated the existence of the band of the 198 bp fragment in the gel.
Fig. 5.
The effects of spr deletion on the flipping of fimS. A, The invertible element orientation assay uses the asymmetrical digestion site of SnaBI within fimS to determine the orientation of the fim promoter. The 602-bp fimS-plus fragment was PCR amplified with the primers Ch1F and Ch1R. The SnaBI restriction fragments of the phase-ON fimS-plus are 404 bp and 198 bp. The SnaBI restriction fragments of the phase-Off fimS-plus are 160 bp and 442 bp. B, The ratios of the phase-ON bacterial cells of WT-RS218 and Δspr-RS218 statically grown in LB broth, which were determined by the invertible element orientation assay as described under “Experimental Procedures.” C, The representative image results of the invertible element orientation assay of WT-RS218, Δspr-RS218, and Δspr-RS218 trans-complemented with the spr gene. Ten colonies of each strain of the bacteria were randomly selected from the agar plates and then subjected to the invertible element orientation assay. The 198-bp (phase-ON) and 168-bp (phase-OFF) bands are shown in the results. D, The ratios of the phase-ON cell-containing colonies of WT-RS218, Δspr-RS218, and Δspr-RS218 trans-complemented with the spr gene. The results were derived from 5 independent experiments. For each independent experiment, 10 colonies of each strain were selected from the agar plates and then subjected to the invertible element orientation assay. The results are shown as the mean ± S.D.
Construction and Complementation of the spr Deletion Mutant of E. coli K1 RS218
The spr deletion mutant of E. coli K1 RS218 was constructed by deleting the spr gene using the procedure described by Datsenko and Wanner (23). To complement the spr mutant of E. coli K1, the plasmid p-spr (also named pCA24N-spr) that harbors the spr gene was obtained from ASKA clone GFP minus library (22) and was used to transform the spr mutant.
The fimS-binding Competition Assays
The purified fimS-regulating proteins, FimB, FimE, Lrp, and HbiF, were coated onto the 96-well plates (NuncTM) by incubation at RT for 1 h. After unbound proteins were removed by rinsing each well with 100 μl PBS for 3 times, the protein coated wells were blocked with 3% BSA for 1 h at RT. Meanwhile, fimS was pre-incubated with Spr or PBS (negative control) for 1 h at RT. The Spr-pre-incubated fimS or PBS-pre-incubated fimS was added to the protein-coated wells and incubated for 1 h at RT. The fimS bound to the protein coated on the wells was stained by RedSafeTM for 30 min at RT in the dark. The wells were rinsed again with PBS and then subjected to signal detection by Synergy2 (BioTek) at 485 nm/528 nm (excitation/emission).
Immunofluorescence Labeling of Type 1 Fimbriae on E. coli
Surface presentation of type 1 fimbria on bacteria was assessed by immunofluorescence microscopy as described previously with a minor modification (2). Briefly, the bacteria cells from selected colonies on the agar plate were resuspended in 100 μl of Milli-Q (MQ) water and then washed three times with MQ water. Ten microliters of each bacteria samples was placed on a poly-l-lysine-coated slides and air dried. Ten microliters of a 1:100 dilution of anti-type 1 fimbria rabbit serum in PBS was placed on top of each sample, and the samples were incubated for 1 h at RT in a moist chamber. After washing three times with PBS, each slide was incubated with 10 μl of Alexa 594-conjugated anti-rabbit antibody at a dilution of 1:200 in PBS for 30 min at RT in a moist chamber. Finally, 10 μl of 4′,6-diamidino-2-phenylindol (DAPI) (0.2 μl/ml) was applied to each sample to stain the nuclei of the bacteria.
RESULTS
Screening for Bacterial Factors Able to Interact with the fimS Containing DNA Fragment by E. coli Proteome Chip Assays
An E. coli protein able to bind to fimS or/and its adjacent regions is likely to be involved in the inversion of fimS. Therefore, to identify novel bacterial factors that contribute to the regulation of type 1 fimbria phase variation, we initially used the E. coli K12 proteome chip, which contains at least 88% of entire E. coli K12 proteome (19), to screen for the proteins that may directly interact with fimS or its adjacent regions. The schematic diagram of the protein chip screening is shown in Fig. 2A. The 602-bp DNA fragment fimS-plus that contains fimS (314 bp) and its 125-bp upstream and 163-bp downstream sequences (Fig. 1) were used as the DNA probe to incubate with the protein chip. The fimS-plus fragment was synthesized by PCR amplification of the chromosomal DNA of the neonatal meningitis E. coli strain RS218 with primers that were labeled with DIG at the 3′-ends (see Experimental procedures). Thus the PCR product (fimS-plus) is DIG-labeled. After probed with fimS-plus, the protein chip was incubated with the Cy3-labeled anti-DIG antibody to identify the protein spots bound with fimS-plus. The 19 proteins recognized by fimS-plus are shown in Table I. The top five proteins and their corresponding chip images are shown in Fig. 2B. AckA is one of the proteins showing no apparent interaction with fimS-plus (Fig. 2B). This protein would be used as the negative control in the later experiments.
Fig. 2.
E. coli proteome chip assays. A, Schematic diagram of chip assays. A proteome microarray containing ∼ 4000 proteins of E. coli K12 were used to screen for the proteins that interact with the fimS-plus fragment. The proteome microarrays were probed with DIG-labeled fimS-plus. The Cy3-labeled anti-DIG antibody was used to identify the proteins binding with fimS-plus. B, The chip assay images of the labeled fimS-plus binding to the E. coli proteins. Each protein was printed in duplicate on the proteome microarray. The green spots show the interactions between fimS-plus and the proteins. The fimS-plus fragment showed apparent interactions with YjcZ, YdaV, TrpL, YebN, and Spr, whereas this DNA fragment showed no interaction with AckA.
Table I. The proteins identified from the E. coli K12 proteome chip assay with fimS-plus.
| Accession Number | Name | Function |
|---|---|---|
| NP_418534 | YjcZ | Mutational suppressor of yhjH motility mutation |
| NP_415878 | YdaV | Predicted DNA replication protein |
| NP_415781 | TrpL | Trp operon leader peptide |
| NP_416335 | YebN | Manganese export protein, conserved inner membrane protein |
| NP_416680 | Spr | Peptidoglycan DD-endopetidase and LD-carboxypeptidase |
| NP_415195 | YleB | 2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinone hydroxylase |
| NC_000913 | YaiX | Predicted acyl transferase (pseudogene) |
| NP_414786 | YafZ | CP4–6 prophage |
| NP_414592 | ApaG | Protein associated with Co2+ and Mg2+ efflux |
| NP_415912 | PaaG | Predicted ring 1,2-epoxyphenylacetyl-CoA isomerase (oxepin-CoA forming) |
| NP_417164 | ProW | Glycine betaine / proline ABC transporter - membrane subunit |
| NP_415785 | YciL | 23S rRNA pseudouridine 2605 synthase |
| NP_416249 | CelD | Repressor of chb operon for N,N′-diacetylchitobiose utilization |
| NP_417399 | YggB | Mechanosensitive channel protein |
| NP_417573 | YqjF | Predicted quinol oxidase subunit |
| NP_415468 | YcbY | Fused 23S rRNA m2G2445 methyltransferase and 23S rRNA m7G2069 methyltransferase |
| NP_418508 | YjcT | d-allose kinase |
| NP_415858 | YdaN | Predicted Zn(II) transporter |
| NP_417776 | RplB | 50S ribosomal subunit protein L2 |
Confirmation of the Interactions Between the Bacterial Proteins and the fimS-plus Fragment
We used EMSA to further validate the interactions between the fimS-plus fragment and the proteins screened out by the proteome chip assay. To perform EMSA, the top 5 proteins identified by chip assays (YjcZ, YdaV, TrpL, YebN, and Spr) and the negative control protein AckA were overexpressed and purified. However, in addition to that of AckA, only the amounts of the purified Spr and YjcZ proteins were high enough to be visualized on the Coomassie blue-stained SDS-PAGE (Fig. 3A). Thus, we focused on analyzing these two proteins with EMSA. As shown in Fig. 3B, addition of Spr induced band shift of the fimS-plus fragment, whereas addition of YjcZ did not, suggesting that Spr can directly interact with fimS-plus and that YjcZ may not be able to bind to this DNA fragment. In addition, Spr dose dependently induced band shift of fimS-plus (Fig. 3C). These results demonstrate the specificity of the interaction between Spr and fimS-plus.
Fig. 3.
EMSA confirms the interaction between Spr and fimS-plus. A, Coomassie Blue staining of the recombinant 6xHis-tagged Spr and YjcZ proteins, which were purified with nickel-bound resin and were used in the EMSA. B, Spr, but not YjcZ and AckA, retarded the mobility of the fimS-plus fragment in the gel electrophoresis. C, Spr dose-dependently induced band shift of fimS-plus. The protein amounts of Spr preincubated with fimS-plus: lane 1–4: 0.49 (+), 0.97 (++), 1.46 (+++), and 1.94 (++++) μg; lane 7–9: 0.97, 1.46, and 1.94 μg. The amount of AckA pre-incubated with fimS-plus was 1.5 μg (lane 6). FP, free probe.
The retarded bands of the fimS-plus-Spr complex were located close to the sample-loading wells of the agarose gels (Figs. 3B and 3C), suggesting no or low mobility of the protein-DNA complex during the electrophoresis. This is likely because of the charge of the Spr protein. The deduced isoelectric point of Spr is about 10. Because the pH value of the buffer in the gel electrophoresis was 8, Spr was likely carrying a net positive charge in the band shift experiments. Such net positive charge may significantly interfere with the mobility of the protein–nucleotide complex.
Spr Protein Mainly Interacts with fimS
The molecules of fimS-plus, which were PCR amplified from the E. coli chromosome, actually contained two types of DNA, in which fimS is oriented in either ON or OFF position (Fig. 4A), because the E. coli culture contained both type 1 fimbriated and nonfimbriated cells (data not shown). To further investigate whether the interaction between the fimS-plus fragment and Spr is affected by the orientation of fimS and whether the up- and down-stream flanking sequence of fimS is required for the interaction, we PCR synthesized DNA fragments containing combinations of fimS and it flanking sequences (Fig. 4A). The interactions of these DNA fragments with Spr were investigated with EMSA. As shown in Fig. 4B, the fimS fragment alone and the fimS in combination with any of the flanking sequences in ON and OFF orientations were able to interact with the purified Spr protein. These findings suggest that Spr mainly interacted with the fimS fragment, and that the interaction is not affected by its flanking sequences and its orientation relative to the flanking sequences.
Fig. 4.

Interactions between Spr and fimS in combination with its upstream and downstream sequences. A, Schematic representation of fimS in combination with its upstream and downstream sequences. Both fragments F1 and F4 contained fimS and its upstream region, but fimS was oriented ON in F1 and OFF in F4. Both fragments F2 and F3 contained fimS and it downstream region, but fimS was oriented ON in F2 and OFF in F3. B, The results of EMSA with the DNA fragments. FP, free probe.
The fimS Orientation of Wild-type, spr Deletion Mutant, and spr Deletion Mutant Transformed with spr
Because Spr can directly interact with fimS, we further investigated whether Spr is involved in the flipping of fimS. The spr deletion mutant of the neonatal meningitis E. coli strain RS218 (Δspr-RS218) was constructed. The populations of the phase-ON cells in the wild-type RS218 (WT-RS218) and Δspr-RS218 cultures were determined by using the invertible element orientation assay (2, 24).
The invertible element orientation assay used the asymmetrical digestion site of SnaBI within the invertible element to determine the percentages of the bacterial cells with the fimS oriented phase-ON and phase-OFF (Fig. 5A). The fimS-plus fragment amplified from the E. coli cultures were purified and digested with SnaBI. When fimS is in the phase-ON position, the restriction enzyme digestion produces fragments of 404 and 198 bp. Otherwise in the phase-OFF position, the sizes of the digested fragments became 442 and 160 bp (Fig. 5A).
First we investigated whether deletion of spr affects the percentages of phase-ON cells grown in the LB broth. The WT-RS218 and Δspr-RS218 colonies, whose fimS orientation was confirmed to be in the phase-OFF, were selected and cultured in LB broth at 37 °C statically. Then the percentages of the phase-ON populations of the wild-type and mutant cell cultures were determined after different periods of culture (Fig. 5B). The percentages of the phase-ON and phase-OFF bacterial cells can be determined by measuring and comparing the intensities of the digested DNA bands in the gel. The percentages of the phase-ON populations in the WT-RS218 and Δspr-RS218 cultures were similar, suggesting that Spr is not involved in the fimS flipping when the bacteria were grown in broth.
Next, we assessed the role of Spr in regulating the fimS orientation when the bacteria were grown on the LB agar plate. The bacterial colonies were investigated with the invertible element orientation assay. It has been known that most of the E. coli cells are tend to be at the nonfimbriated state when grown on agar plate (i.e. the fimS in the cells grown on the solid surface is tend to be in the phase-OFF orientation) (11–13). Similarly, our results showed that less than 10% of the wild-type E. coli colonies (5% ± 5%) contained phase-ON bacteria. However, 57% ± 8% of the Δspr-RS218 colonies contained the phase-ON bacteria. This means that deletion of spr significantly increases the frequency of the colonies containing phase-ON bacteria (Figs. 5C and 5D). Trans-complementation of Δspr-RS218 with the plasmid harboring the spr gene decreased the frequency of the phase-ON containing colonies (6% ± 5%) to the level of WT-RS218 (Figs. 5C and 5D). These results suggest that the Spr protein is involved in mediating the flipping of fimS.
The fimS Orientation Reflects the State of Type 1 Fimbria Expression in Δspr-RS218
To investigate whether the orientation of fimS still reflects type 1 fimbria expression on bacterial surface after deletion of spr, the immunofluorescence microscopy with anti-type 1 fimbria serum was performed to detect the type 1 fimbria on the Δspr-RS218 cells from the colonies grown on the LB agar plates. As shown in Fig. 6A and 6B, the Δspr-RS218 cells from the colonies whose bacterial cells' fimS were in phase-OFF (such as colony #2) showed no type 1 fimbria expression on the surface (no red color). On the other hand, in the majority of the Δspr-RS218 cells from the colonies, most of whose bacterial cells' fimS were in phase-ON (such as colony #1) showed type 1 fimbria expression on the surface (red color). These results demonstrate that the orientation of fimS in the spr deletion mutant of E. coli can still reflect the state of type 1 fimbria expression.
Fig. 6.
Immunofluorescence microscopy analysis of type 1 fimbria expression on Δspr-RS218 grown on the agar plate. A, The Δspr-RS218 colonies were analyzed with the invertible element orientation assay to determine whether these colonies contained the phase-ON cells or not. The results derived from the type 1 fimbria locked-ON and locked-OFF RS218 mutants whose fimS have been modified to be fixed in the phase-ON and phase-OFF positions, respectively (2), were shown as the size markers of the 198-bp (phase-ON) and 168-bp (phase-OFF) bands. B, Then, their surface expression of type 1 fimbria was investigated by immunofluorescence microscopy with the anti-type 1 fimbria serum (red). The nuclei of the bacteria were labeled with 4′,6-diamidino-2-phenylindole (DAPI) (blue).
Spr Blocks the Binding of fimS with the Bacterial Factors That Have Been Known to be able to Interact with This DNA Fragment
Because Spr was involved in regulation of the fimS flipping, we further investigated whether Spr is involved in the interactions between fimS and the E. coli proteins that mediate the flipping of fimS. The E. coli proteins, FimB, FimE, HbiF, and Lrp were assessed in this study. FimB, FimE, and HbiF are the site-specified recombinases that mediate the inversion of fimS. FimB and FimE have been shown to be able to directly interact with fimS. Because HbiF is homologous to FimB and FimB with 55 and 50% of identity (7), it is very likely that HbiF also direct interact with fimS to mediate the fimS flipping. In addition, Lrp has been shown to be capable of binding to fimS to increase the flipping rates of fimS (14).
To investigate whether Spr affects the interactions of the bacterial factors with fimS, the purified proteins FimB, FimE, HbiF, and Lrp were purified and coated on the 96 well plates, respectively (see Experimental procedures). Then, the fimS fragment was added in the wells to interact with the proteins on the plates with or without the presence of Spr. The presence of the Spr protein significantly decreased the levels of fimS binding to FimE and FimB (Fig. 7). These results suggest that Spr can interfere with the interactions between fimS and the recombinases FimB and FimE. It is likely that Spr regulated fimS expression through the control of dynamic interactions between fimS and these recombinases.
Fig. 7.
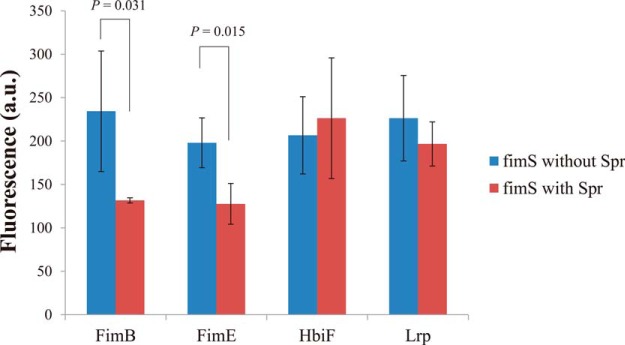
Analysis of Spr effect on the interactions between fimS and known fimS regulating proteins. The fluorescence signals reflected the levels of the fimS fragment bound to the known fimS regulating proteins (FimB, FimE, HbiF, and Lrp) coated on the 96-well plates with or without the presence of the Spr protein.
DISCUSSION
This study is the first utilization of the proteome chip to identify the protein involved in the regulation of cell structure components. Type 1 fimbria expression is phase variable, which is controlled by the flipping of the fim promoter-containing fimS at the transcriptional level (6). The phase variation is sensitive to the environmental cues the bacteria encounter, such as amino acids, temperature, and the medium forms etc (10–13). Although bacterial factors, such as FimB, FimE, HbiF, and Lrp etc., have been identified (FimB, FimE, and Lrp) or suggested (HbiF) to be able to regulate the flipping of fimS through direct interaction with fimS and its close adjacent regions (7, 14, 25, 26), understanding of type 1 fimbria phase variation in response to different environmental cues remains incomplete. Thus, identification of novel bacterial factors involved in regulating the fimS flipping would facilitate the understanding of this complex regulation circuit. Because of the environment-dependent expression of type 1 fimbria, some bacterial factors involved in regulating type 1 fimbria expression may also environment-dependently carry out their regulating function; i.e. they may affect type 1 fimbria expression only under specific growth conditions. It may not be a proper strategy to comprehensively screen for such bacterial factors by using the traditional mutant library screening. Taking into account of all possible combinations of a large bacterial mutant numbers and the environmental variables, it would be very labor-consuming and inefficient. Therefore, an approach that can narrow down the number of potential type 1 fimbria-regulating factors in the beginning may be a proper strategy. With a smaller number of candidate bacterial factors, the following investigation focusing on identifying the functional conditions of these potential factors would be relatively economical of time and resource. Given the fact that the bacterial factors able to interact with the fimS region are likely to be involved in regulating the flipping of the fim promoter so as to regulate type 1 fimbria expression, screening for such bacterial factors would be a good narrow-down strategy. A potential tool for screening for the interaction between the DNA fragment and E. coli protein has been developed in one of our recent studies (19). The proteome array that contains ∼90% of the proteins encoded by the E. coli K12 genome has been constructed. It has been used to investigate DNA damage (19), identify serological biomarkers of inflammatory bowel disease (20), and reveal the inhibition mechanism of lactoferricin B (21). The proteome chip is a good tool to comprehensively screen for bacterial proteins potentially able to interact with fimS or its adjacent regions.
In this study, the E. coli proteome array was used to screen for E. coli proteins able to interact with the fimS-plus DNA fragment that contains fimS and its adjacent regions. Among the screened-out proteins, the Spr protein's interaction with the DNA fragment was further confirmed by EMSA analysis. Deletion of spr in the neonatal meningitis E. coli strain RS218 significantly increased the frequency of the bacterial colonies that contained the type 1 fimbria phase-ON cells on agar plates. However, deletion of spr did not affect the type 1 fimbria expression when the bacteria were grown in the broth medium. Spr were shown to interact with fimS alone, and with fimS in any combinations with its upstream and downstream sequences. Also, Spr interfered with the interactions of fimS with the site-specific recombinases, FimB and FimE, which are responsible for mediating the flipping of fimS. These results suggest that Spr involves in the regulation of type 1 fimbria expression through direct interaction with the invertible element fimS.
The Spr protein has been known to be involved in biogenesis of peptidoglycan of the bacterial cell wall (27). Spr is predicted to belong to the NlpC/p60 peptidase superfamily (28) and exhibits a significant DD-endopeptidase and a weak LD-carboxypeptidase activities which are able to cleave the peptide cross-links of the peptidoglycan. The bacterial peptidoglycan layer protects bacterial cells from intracellular turgor pressure and to maintain cell shapes (29–31). The cleavage of the peptide cross-link is one of the essential steps to synthesize peptidoglycan during the process of bacterial growth, in which cell shape would change accordingly (27). Because Spr is involved in regulating the expression of both type 1 fimbriae and peptidoglycan, this protein may play a role in coordinating the expression of these bacterial components. Type 1 fimbria structures are anchored in the E. coli cell wall that contains the peptidoglycan layer and the outer membrane. It has been shown that the expression of type 1 fimbria coordinates with the expression of cell wall components. For example, the constitutive expression of type 1 fimbria decreases the expression of flagella, bacterial structures also anchored in the cell wall (32). Additionally, inactivation of the outer membrane protein OmpX up-regulates the expression of type 1 fimbria (33). Such coordination in expressions, including the one potentially mediated by Spr, may be required for maintaining the integrity of the cell wall, and for reconciling the functions of the cell-wall associated components.
Spr may regulate the flipping of the fimS fragment through interfering with the interactions between this fragment and its interacting recombinases in vivo. Our result indicated that Spr was able to interfere with the in vitro interaction between fimS and the site-specific recombinases FimB and FimE. It is known that both FimB and FimE bind to the sequences adjacent to and in the invert repeats at both ends of fimS. The recombinase binding sites contain the essential core dinucleotide 5′-CA (9). Thus, the Spr binding site on fimS may be close to or overlapping with the recombinase binding sites, so that Spr can interfere with the recombinase-fimS interactions. Given that these recombinases mediate the flipping of the fimS fragment through direct interaction with this fragment (9), it is likely that a novel regulatory role of Spr exists in the control of dynamic interactions between fimS and these recombinases. FimB is known to be able to mediate bi-directional flipping of fimS (ON-to-OFF and OFF-to-ON), whereas FimE mediates only the On-to-Off flipping of fimS (7, 25, 26). Therefore, it is reasonable to speculate that Spr contributes to facilitating the ON-to-OFF flipping by interfering more of the in vivo FimB-fimS interaction than the FimE-fimS interaction, as deletion of Spr facilitated the tendency of OFF-to-ON flipping in vivo (Fig. 5C). However, further in-depth investigation on the regulatory role of Spr-caused interference on the FimB- and FimE-mediated flipping of fimS may be required to validate the speculation, as our evidence support that Spr could interfere with the in vitro interaction of fimS with FimB and FimE.
The involvement of Spr in regulating the flipping of fimS is environment-dependent, because the negative effect of spr deletion on type 1 fimbria expression was seen only in the bacteria grown on agar, not in those grown in liquid medium. The majority of E. coli strains express few or no type 1 fimbria when grown on agar (11–13). Spr may contribute to the suppression of the fimbria expression on agar. Because Spr directly interacted with fimS, the conditional involvement of Spr in the fimbria expression may be regulated by the accessibility of Spr to fimS; i.e. Spr may be allowed to access to fimS only on certain conditions, such as growth on agar. Alternatively, to regulate the flipping of fimS in vivo, Spr may need to cooperate with an unidentified factor (or factors) that are available only when the bacteria are grown on agar. Thus, the environment-dependent availability of the factor determines when Spr is involved in regulation of type 1 fimbria expression. A further study is required to reveal the responsible mechanism.
In summary, by using the E. coli K12 proteomic array, we screened for the bacterial proteins able to interact with the DNA fragment containing fimS whose orientation regulates the expression of type 1 fimbria. The E. coli protein Spr was involved in the negative regulation of type 1 fimbriae expression. However, its negative regulatory function was growth condition-dependent, which happened when bacteria were grown on agar. These findings facilitate our understanding of regulation of type 1 fimbria and also demonstrate a new strategy to identify bacterial factors that environment-dependently contribute to a specific phenotype by using this E. coli protein chip.
Acknowledgments
We thank Kuan-Yi Lu and F. X. Reymond Sutandy for engaging the discussion and providing helpful suggestions.
Footnotes
Author contributions: C.T. and C.C. designed research; Y.C., Y.H., T.H., W.H., and M.H. performed research; Y.C., C.T., Y.H., and C.C. analyzed data; Y.C., C.T., Y.H., T.H., I.C., and C.C. wrote the paper.
* This research was supported in part from National Science Council, Taiwan (NSC101-2320-B-008-004-MY3 and NSC102-2627-M-008-001 to C-S Chen; NSC99-2320-B-006-005-MY3 to C-H Teng); The Aim for the Top University Project, National Central University and Landseed Hospital Join Research Program (NCU-LSH-102-A-019) and National Central University and Cathay General Hospital Join Research Program (102NCU-CGH-07).
1 The abbreviations used are:
- DIG
- digoxygenin
- EMSA
- electrophoreticmobility shift assay.
REFERENCES
- 1. Cookson A. L., Cooley W. A., Woodward M. J. (2002) The role of type 1 and curli fimbriae of Shiga toxin-producing Escherichia coli in adherence to abiotic surfaces. Int. J. Med. Microbiol. 292, 195–205 [DOI] [PubMed] [Google Scholar]
- 2. Teng C. H., Cai M., Shin S., Xie Y., Kim K. J., Khan N. A., Di Cello F., Kim K. S. (2005) Escherichia coli K1 RS218 interacts with human brain microvascular endothelial cells via type 1 fimbria bacteria in the fimbriated state. Infect. Immun. 73, 2923–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim J. K., Gunther N. W. T., Zhao H., Johnson D. E., Keay S. K., Mobley H. L. (1998) In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect. Immun. 66, 3303–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez J. J., Mulvey M. A., Schilling J. D., Pinkner J. S., Hultgren S. J. (2000) Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19, 2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schilling J. D., Mulvey M. A., Hultgren S. J. (2001) Structure and function of Escherichia coli type 1 pili: new insight into the pathogenesis of urinary tract infections. J. Infect. Dis. 183, S36–40 [DOI] [PubMed] [Google Scholar]
- 6. Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. (1985) An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 82, 5724–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie Y., Yao Y., Kolisnychenko V., Teng C. H., Kim K. S. (2006) HbiF regulates type 1 fimbriation independently of FimB and FimE. Infect. Immun. 74, 4039–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burns L. S., Smith S. G., Dorman C. J. (2000) Interaction of the FimB integrase with the fimS invertible DNA element in Escherichia coli in vivo and in vitro. J. Bacteriol. 182, 2953–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gally D. L., Leathart J., Blomfield I. C. (1996) Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol. Microbiol. 21, 725–738 [DOI] [PubMed] [Google Scholar]
- 10. Gally D. L., Bogan J. A., Eisenstein B. I., Blomfield I. C. (1993) Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J. Bacteriol. 175, 6186–6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ofek I., Goldhar J., Eshdat Y., Sharon N. (1982) The importance of mannose specific adhesins (lectins) in infections caused by Escherichia coli. Scand. J. Infect. Dis. Supplementum 33, 61–67 [PubMed] [Google Scholar]
- 12. Duguid J. L., Old D. C. (1980) Adhesive properties of enterobacteriaciae, p. 185–217 In Beachey E. H. (ed.), Bacterial adherence. Receptors and recognition, series B 6 Chapman and Hall, London [Google Scholar]
- 13. Hultgren S. J., Schwan W. R., Schaeffer A. J., Duncan J. L. (1986) Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect. Immun. 54, 613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roesch P. L., Blomfield I. C. (1998) Leucine alters the interaction of the leucine-responsive regulatory protein (Lrp) with the fim switch to stimulate site-specific recombination in Escherichia coli. Mol. Microbiol. 27, 751–761 [DOI] [PubMed] [Google Scholar]
- 15. Blomfield I. C., Kulasekara D. H., Eisenstein B. I. (1997) Integration host factor stimulates both FimB- and FimE-mediated site-specific DNA inversion that controls phase variation of type 1 fimbriae expression in Escherichia coli. Mol. Microbiol. 23, 705–717 [DOI] [PubMed] [Google Scholar]
- 16. Ho S. W., Jona G., Chen C. T., Johnston M., Snyder M. (2006) Linking DNA-binding proteins to their recognition sequences by using protein microarrays. Proc. Natl. Acad. Sci. U.S.A. 103, 9940–9945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu S., Xie Z., Onishi A., Yu X., Jiang L., Lin J., Rho H. S., Woodard C., Wang H., Jeong J. S., Long S., He X., Wade H., Blackshaw S., Qian J., Zhu H. (2009) Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell 139, 610–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu S., Wan J., Su Y., Song Q., Zeng Y., Nguyen H. N., Shin J., Cox E., Rho H. S., Woodard C., Xia S., Liu S., Lyu H., Ming G. L., Wade H., Song H., Qian J., Zhu H. (2013) DNA methylation presents distinct binding sites for human transcription factors. eLife 2, e00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen C. S., Korobkova E., Chen H., Zhu J., Jian X., Tao S. C., He C., Zhu H. (2008) A proteome chip approach reveals new DNA damage recognition activities in Escherichia coli. Nat. Methods 5, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen C. S., Sullivan S., Anderson T., Tan A. C., Alex P. J., Brant S. R., Cuffari C., Bayless T. M., Talor M. V., Burek C. L., Wang H., Li R., Datta L. W., Wu Y., Winslow R. L., Zhu H., Li X. (2009) Identification of novel serological biomarkers for inflammatory bowel disease using Escherichia coli proteome chip. Mol. Cell. Proteomics 8, 1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ho Y. H., Sung T. C., Chen C. S. (2012) Lactoferricin B inhibits the phosphorylation of the two-component system response regulators BasR and CreB. Mol. Cell. Proteomics : MCP 11, M111 014720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitagawa M., Ara T., Arifuzzaman M., Ioka-Nakamichi T., Inamoto E., Toyonaga H., Mori H. (2005) Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12, 291–299 [DOI] [PubMed] [Google Scholar]
- 23. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aberg A., Shingler V., Balsalobre C. (2006) (p)ppGpp regulates type 1 fimbriation of Escherichia coli by modulating the expression of the site-specific recombinase FimB. Mol. Microbiol. 60, 1520–1533 [DOI] [PubMed] [Google Scholar]
- 25. Klemm P. (1986) Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 5, 1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McClain M. S., Blomfield I. C., Eisenstein B. I. (1991) Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J. Bacteriol. 173, 5308–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh S. K., SaiSree L., Amrutha R. N., Reddy M. (2012) Three redundant murein endopeptidases catalyse an essential cleavage step in peptidoglycan synthesis of Escherichia coli K12. Mol. Microbiol. 86, 1036–1051 [DOI] [PubMed] [Google Scholar]
- 28. Anantharaman V., Aravind L. (2003) Evolutionary history, structural features and biochemical diversity of the NlpC/P60 superfamily of enzymes. Genome Biol. 4, R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weidel W., Pelzer H. (1964) Bagshaped Macromolecules–a New Outlook on Bacterial Cell Walls. Adv. Enzymol. RAMB 26, 193–232 [DOI] [PubMed] [Google Scholar]
- 30. Holtje J. V. (1998) Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. R. 62, 181–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vollmer W., Blanot D., de Pedro M. A. (2008) Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149–167 [DOI] [PubMed] [Google Scholar]
- 32. Lane M. C., Simms A. N., Mobley H. L. (2007) complex interplay between type 1 fimbrial expression and flagellum-mediated motility of uropathogenic Escherichia coli. J. Bacteriol. 189, 5523–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Otto K., Hermansson M. (2004) Inactivation of ompX causes increased interactions of type 1 fimbriated Escherichia coli with abiotic surfaces. J. Bacteriol. 186, 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]







