Abstract
Radiation sterilization has now become a commonly used method for sterilization of several active ingredients in drugs or drug delivery systems containing these substances. In this context, many applications have been performed on the human products that are required to be sterile, as well as on pharmaceutical products prepared to be developed. The new drug delivery systems designed to deliver the medication to the target tissue or organ, such as microspheres, nanospheres, microemulsion, and liposomal systems, have been sterilized by gamma (γ) and beta (β) rays, and more recently, by e-beam sterilization. In this review, the sterilization of new drug delivery systems was discussed other than conventional drug delivery systems by γ irradiation.
Keywords: e-beam sterilization, neutron radiation (e-beam) sterilization, new drug delivery systems, radiation sterilization, sterilization by gamma radiation
Introduction
The method of sterilization by gamma (γ) radiation is preferred due to the special nature of the active and auxiliary substances prepared or designed to apply in humans and its advantages over other methods. Sterilization by γ radiation is used for the sterilization of active substances as well as drug delivery systems. The new studies on radiation sterilization of new drug delivery systems have especially focused on the protection of the system designed to carry the active substance as well as the protection of the structure of the active ingredient after irradiation, determining the suitable irradiation (sterilization) dose and analyzing the changes as a result of the irradiation through appropriate methods. According to the source of radioactivity used in the radiation sterilization, e-beam, γ rays, or beta (β) rays may be used.
The new drug delivery systems that most studies have focused on include microspheres, nanospheres, various carrier polymers, gels, liposomes, niosomes, gelosomes, nanoparticles, and microparticles. Radiation sterilization is also used in the development and sterilization of vaccine formulations.
Microspheres (Microparticles)
One of the new drug delivery systems which is the focus of the research is microspheres. Examples of this study are summarized below.
Polyester microspheres with different biodegradability and which have a general structure of poly D,L-lactide-co-glycolide (PLG) including captopril as active ingredient were examined. Prepared by a spray drying technique, captopril microspheres, active substance, and polymers were irradiated with γ irradiation at the doses of 6.9, 15.0, 27.7, and 34.8 kGy, using a 60Co source. While captopril showed no oxidation after irradiation process, differential scanning calorimetry (DSC) and in vitro release studies demonstrated that interactions between drug and polymer during γ irradiation are of critical importance. Microspheres prepared from polymers with various polymer weights (such as 16.500, 51.500, and 66.000 kDa) showed different properties depending on the applied dose. The researchers suggested that sterilization of parenteral delivery systems from biodegradable polyesters was not a straightforward process but required careful optimization of PLG molecular weights. In the study, it was observed that the stability of the drug against high energy radiation was also influenced by polymer properties and the distribution of drug in the device. The authors concluded that solid solution type microspheres were exceptionally sensitive towards γ irradiation [1].
The in vitro properties of poly lactide-co-glycolic acid (PLGA) (at the ratio of 50:50; molecular weight 34,000 or 88,000 kDa) microspheres incorporating naproxen sodium (NS) and diclofenac sodium were investigated. These microspheres were irradiated at the doses of 5, 15, and 25 kGy using a 60Co source. Drug loading of irradiated and nonirradiated microspheres with different molecular weight were found to be similar. The most significant difference was found in the particle sizes of the irradiated as compared to the nonirradiated formulations. The in vitro release studies demonstrated that the amount of active substance released from PLGA microspheres increased with the increasing radiation dose. The DSC studies detected that the glass transition temperatures (Tg) were slowly influenced by the increasing radiation dose [2].
In another study carried out on the PLGA microspheres loaded with clonazepam as active ingredient, the microspheres were prepared by means of a spray-drying method at 15% (v/v) ratio. Gamma irradiation was carried out either under vacuum or in air, at a dose of 25 kGy, by using a 60Co source. The drug-loaded microspheres were evaluated over a 6-month period on the basis of drug content and dissolution profile. Radiolysis mechanisms were investigated by using electronic paramagnetic resonance (EPR) studies. The microspheres irradiated under vacuum remained stable longer than expected. After irradiation in air, active ingredient release rate increased by approximately 10% and did not change further in the storage period. The EPR analysis showed some radicals arising from both the polymeric matrix and the active ingredient. It was found that, in the loaded microspheres, the intensity in time of radical signal is sufficient for use as irradiation marker [3].
An injectable system of levonorgestrel (LNG) was prepared using biodegradable polymer of natural origin. The microparticular system prepared with microsphere formulation was optimized for particle size and drug loading. For the microparticulate system tests such as scanning electron microscopy, encapsulation efficiency, moisture content, infrared (IR), DSC, X-ray diffraction (X-RD), residual solvent content, sterility, toxicity, and pyrogenity were performed. The microparticles were sterilized by γ irradiation of 25 kGy, and then it was injected intramuscularly in rabbits, and the blood levels of LNG were determined by radioimmunoassay method. An optimized drug to polymer ratio of 0.3–1.0 (w/w ratio) gave improved drug loading of about 52%. In vivo studies in rabbits showed that the drug was released in a controlled manner for 1 month. It was found that, in the preparation of microspheres as progestin, only long-term contraceptive was a better alternative with improved user compliance for the synthetic and expensive polymeric carriers [4].
In another study examining the influence of γ irradiation on the characteristics of chitosan microparticles, the active ingredient of diclofenac sodium was incorporated into chitosan microparticles by spray-drying method. The placebo and drug-loaded microspheres were irradiated at doses of 5, 15, and 25 kGy using a 60Co source. The microparticles were characterized by Fourier transform infrared (FTIR) spectroscopy, EPR, X-RD, DSC, scanning electron microscopy (SEM), and atomic force microscopy (AFM). Furthermore, microparticles were also investigated in terms of their sizes, drug content, swelling, and drug release behavior. Drug encapsulation efficiency of placebo and drug-loaded microparticles was found to be the same. Especially, surface roughness of placebo microparticles decreased significantly after γ irradiation when compared with nonirradiated placebo microparticles. The γ-irradiated microspheres showed a significantly higher drug release rate and lower swelling capacity than the nonirradiated microspheres [5].
The PLGA microspheres including acyclovir were added to gelatin (with a 2:2:10 acyclovir–gelatin–polymer ratio) and sterilized by γ irradiation at the dose of 25 kGy. While loading efficiency and morphology were characterized by studies including particle size, SEM, physicochemistry, IR, DSC, X-RD, and gel permeation chromatography (GPC), in vitro release assays for 73 days were performed to evaluate the sterilization effect on microsphere characteristics. After irradiation, no surface changes were observed by SEM and GPC measurements showed a decrease in molecular weight of the polymer. The sterilization method was found to be suitable as no change in microspheres was seen after γ irradiation. It was concluded that considering the favorable properties of the acyclovir-loaded microspheres sterilization by γ irradiation was a suitable system for the intravitreal treatment of herpes virus infections in an animal model [6].
Seventeen β-estradiol-loaded PLG microspheres were prepared by spray-drying method and sterilized by γ irradiation at the doses ranging from 5.1 to 26.6 kGy. The active drug substance showed excellent stability against γ irradiation in the investigated dose range, whereas microencapsulated estradiol seemed to be converted to conjugation products with PLG and, to a lesser extent, to the degradation product 9,11-dehydroestradiol. The weight-average molecular weight of the PLG polymers decreased with increasing irradiation dose. As a result, the efficacy of γ irradiation as terminal sterilization method for PLG microspheres was demonstrated and it was concluded that the sterilization conditions should be adjusted for the final dosage form [7].
In another study conducted on double-walled reservoir type microspheres with etanidazole solid crystals, the characterization, in vitro release, and the effects of irradiation on this class of microspheres were investigated. This highly water-soluble active ingredient was also examined for the suitability in the double-walled microsphere system. Release profiles of irradiated and nonirradiated microspheres were compared, and the dosage of 50 kGy was found to have noticeable effects on the polymer and its release profiles, while sterilization dosages of 25 kGy lowered the glass transition temperatures and crystalline melting point, indirectly indicating a decrease in molecular weight [8].
In the study carried out on the tetracycline-HCl-loaded microspheres prepared from PLGA by spray drying, the effects of γ irradiation on the formation of free radicals in polymer and drug and the mechanism of chain scission after sterilization were examined. Gamma irradiation was performed at 26.9 and 54.9 kGy using a 60Co source. The EPR studies conducted on placebo and drug-loaded microspheres characterized the formation of free radicals after irradiation. It was concluded that EPR spectroscopy in combination with GPC, DSC, and high-performance liquid chromatography (HPLC) could allow a detailed characterization of the impact of γ sterilization on biodegradable parenteral drug delivery systems [9].
Gamma irradiation was used for controlling the drug release from poly(D,L-lactide) microspheres. The relationship between the polymer decomposition caused by the γ irradiation to adjust the Tg and the drug release rate was examined. The initial release rate of progesterone microspheres decreased as Tg increased. It was reported that the release rate can be controlled by irradiation dose. A slower initial release followed by a rapid release occurred after the Tg was lowered to 37 °C. It was concluded that the time period before the start of rapid release could be controlled by altering the irradiation dose [10].
The study by Faisant et al. examined the effect of different γ-irradiation doses on the release kinetics from 5-fluorouracil (5-FU)-loaded PLGA microspheres, analyzing the obtained experimental data with a new mathematical model. Drug release was found to depend significantly on the applied γ-irradiation dose. Interestingly, the obtained release profiles were all biphasic (a rapid initial drug release phase followed by a slower) constant drug release phase. Naturally, only the initial rapid drug release was accelerated by γ irradiation; the subsequent zero-order phase was almost unaffected. In addition, the γ-irradiation dose and quantitatively calculated drug release rate were affected. It was concluded that diffusion was the dominating release rate controlling mechanism in all cases, with a significant contribution of the polymer degradation process [11].
In another study investigating the effects of sterilization by ionizing radiation on hydroxyethylcellulose–trehalose microspheres containing vancomycin, it was found that γ rays did not modify the active substance, producing no toxic products, and did not affect the kinetic behavior of drug release from microspheres. Moreover, no significant changes in the shape and in the size distribution of microspheres were found after irradiation. The experimental results showed that the therapeutic application of the pharmacological system investigated was not compromised by irradiation, and the authors concluded that ESR spectroscopy could be used to distinguish irradiated from nonirradiated products [12].
In the study which carried out the collagen–PLGA microspheres containing gentamicin as active ingredient, various sterilization methods were compared using β and γ irradiation. All methods resulted in a decrease of molecular weight and glass transition temperature of polymer raw material and microparticles. Free radicals, which could only be detected in gentamicin drug substance and at marginal level in gentamicin-loaded microspheres, disappeared within 4 weeks. Additional microbiological testing verified the microbiological activity of gentamicin liberated from β-sterilized composites. The pearls and pitfalls of all sterilization techniques were determined, but based on drug release profile and chemical changes of gentamicin, it was concluded that irradiation treatment was more suitable for collagen–gentamicin-loaded PLGA microspheres [13].
Controlled drug release systems
In their literature review regarding the use of radiation sterilization in controlled drug delivery and controlled drug release systems, the Razems pointed out that the research of radiation effects on drugs over the past 60 years has mainly dealt with radiation sterilization of individual active pharmaceutical ingredients or injectable solutions. The use of irradiation in controlled drug delivery systems has increased with the manufacturing and modification of a number of polymeric carriers with an added advantage of reducing the microbial load of products at the same time. The authors emphasizing the developments over the past 15 years discussed the radiation sterilization, crosslinking, and degradation of the principal forms of drug carrier systems. They also accentuated the need for regulatory aspects pertaining to radiation sterilization of drugs summarizing the relevant results in tabular form (Figs 1–5) [14].
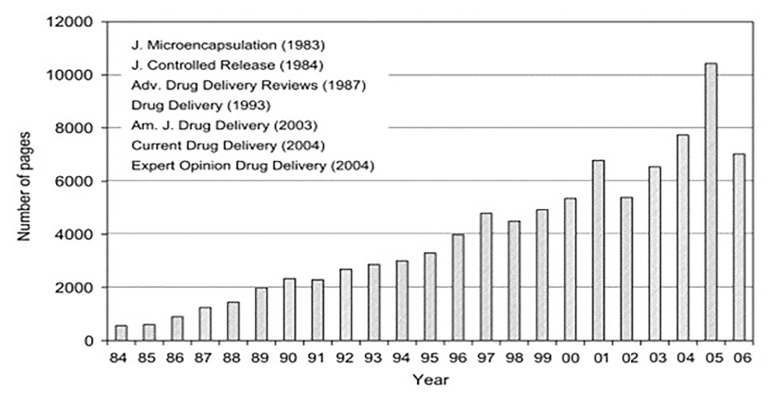
Fig. 1.
Total annual number of pages of journals dedicated to controlled drug delivery (CDD)/controlled drug release (CDR) systems [14]
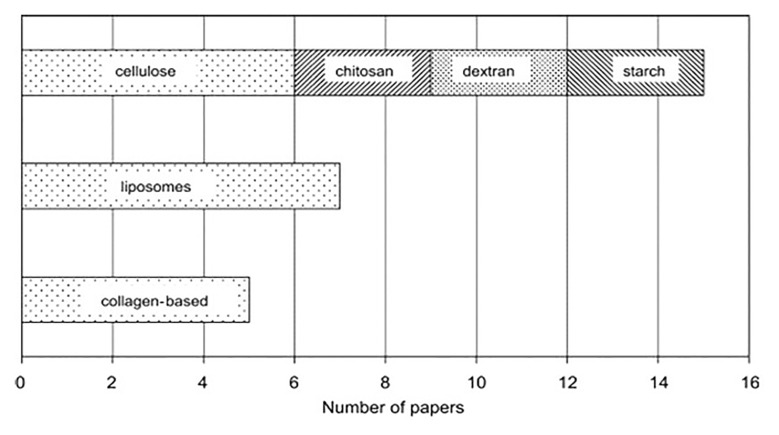
Fig. 5.
Distribution of papers on radiation effects on controlled drug delivery (CDD)/controlled drug release (CDR) systems with respect to natural polymer carriers [14]
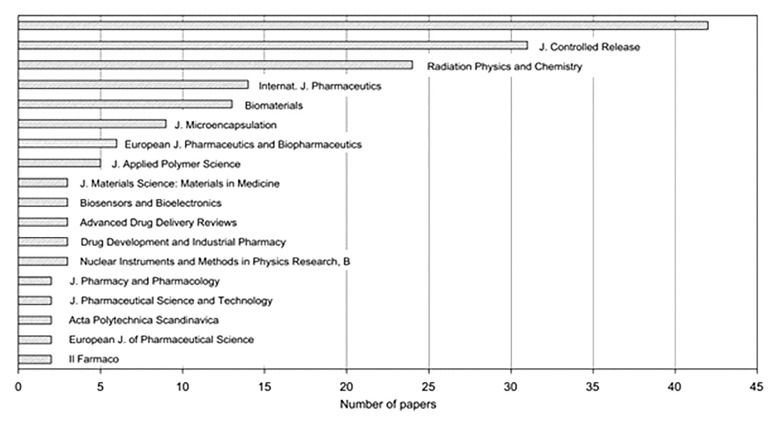
Fig. 2.
Distribution of papers on radiation effects on controlled drug delivery (CDD)/controlled drug release (CDR) systems with respect to publication outlets (the uppermost bar represents the total number of 42 different journals) [14]
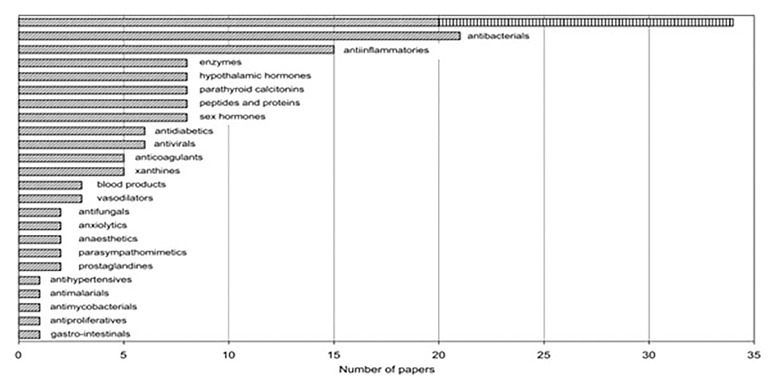
Fig. 3.
Distribution of papers on radiation effects on controlled drug delivery (CDD)/controlled drug release (CDR) systems with respect to therapeutic classes of drugs (the uppermost bar represents the total number of antineoplastic agents, 5-FU being shown separately) [14]
Fig. 4.
Distribution of papers on radiation effects on controlled drug delivery (CDD)/controlled drug release (CDR) systems with respect to synthetic polymer carriers [14]
Ocular minitablets
In one study on the properties of a bioadhesive powder mixture containing ciprofloxacin and its corresponding ocular minitablets, the influence of γ irradiation and dry heat sterilization was compared. The molecular weight characteristics, the rheological properties of the bioadhesive polymers, and the microbial activity of ciprofloxacin were studied, while the influence of the different sterilization methods on the characteristics of the ocular minitablets was investigated by measuring the crushing strength, the friability, and the in vitro release of ciprofloxacin from the minitablets. As it did not affect the physical properties of the minitablets much, γ irradiation was concluded to be a more suitable method for the sterilization ocular minitablets [15].
Nanospheres (Nanoparticles)
Polymeric carriers and especially poly(ε-caprolactone) nanoparticles have already shown promising results in the optimization of the ophthalmic bioavailability of drugs. Any formulation instilled in the eye must be sterile and, preferentially, isotonic. Nanoparticles were formulated with Synperonic PE/F68, Synperonic PE/F127, or Cremophor RH40. A tonicity agent, a preservative, and, in some cases, a viscosifiant were then added. The pH was finally adjusted to pH 4 or buffered to pH 7. The influence of different sterilization processes on the physicochemical characteristics of carriers was studied. Autoclaving did not induce any modification on polymer molecular weight or synperonic nanospheres diameter but catalyzed some reactions with surfactants and tonicity agents. In γ sterilization, this interaction was diminished. In conclusion, it was reported that sterile filtration was a process that ensured the conservation of physicochemical integrity of nanoparticles, and it could be successfully applied on nonviscosified carriers with a sufficiently small diameter [16].
Various drug-loaded PLGA nanoparticles were prepared using an emulsification–solvent evaporation technique, and different emulsion systems were employed for a variety of model compounds. Sterilization of the drug-loaded nanoparticles was performed by γ irradiation at the dose of 25 kGy, and it showed no adverse effect on particle size, drug release behavior, as well as ex vivo arterial uptake of the nanoparticles. In conclusion, this study demonstrated that a wide variety of water soluble and insoluble bioactive agents can be incorporated into PLGA nanoparticles with a high efficiency and adjustable drug loadings. It was found that the drug release kinetics from the nanoparticles could be controlled by choosing the composition and the molecular weight of the polymeric matrix. Drug-loaded PLGA nanoparticles showed great potential in intravascular local drug delivery [17].
Another study investigating the use of nanoparticles for therapeutic purposes is the study by Kreuter and Gelperina, which used nanoparticles in the treatment of cerebral cancer. Radiation sterilization is the method of choice for the sterilization of nanoparticles. For both types of irradiation, the nanoparticles showed an excellent stability in the investigated dose range of 10 to 35 kGy. The irradiation did not influence the physicochemical parameters of the drug-loaded and empty nanoparticles and did not lead to the radiolysis of the doxorubicin [18].
In another study, sterilization of doxorubicin-loaded poly butyl cyanoacrylate (PBCA) nanoparticles using either γ irradiation or electron beam irradiation was investigated. The irradiation doses ranged from 10 to 35 kGy. It was found that the irradiation dose of 15 kGy was sufficient for sterilization of the nanoparticles and the irradiation did not influence the physicochemical parameters of the nanoparticles [19].
Hydrogels
In the study examining a series of pH-responsive hydrogels, for the protection of insulin from the acidic environments, hydrogels based on poly(vinyl alcohol) networks grafted with acrylic acid or methacrylic acid were prepared and then sterilized by γ irradiation at the doses of 5–20 kGy. The graft hydrogels showed pH-sensitive swelling behavior and were used as carriers for the controlled release of insulin. The release behavior of insulin in vivo in a rat model confirmed the effectiveness of the oral delivery of insulin to control the level of glucose [20].
The researchers prepared hydrogel sponges using hydroxyethyl methacrylate (HEMA) and ethylene glycol dimethacrylate (EGDMA) for iontophoretic drug delivery, and the optimal sterilization process was chosen by evaluating three sterilization methods: γ irradiation, ethylene oxide, and autoclave sterilizations. Sterilization by γ irradiation was found to be the most suitable method as it caused minimal alteration of hydrogel properties [21].
In another study on hydrogels, the influence of different sterilization methods on the thermo-gelation and structural properties of xyloglucan hydrogels was investigated. Xyloglucan samples were treated by either 70% ethanol, 70% isopropanol, γ irradiation at the dose of 10 kGy at room temperature, γ irradiation at the dose of 10 kGy in dry ice, or autoclaving. According to the sterility test results on these samples, xyloglucan hydrogels could only be sterilized by autoclaving or by γ irradiation either at room temperature or in dry ice. When rheology measurements of hydrogels were analyzed, it was seen that γ irradiation at room temperature significantly changed the polymer structure, preventing thermogelation. Only autoclaving and γ irradiation in dry ice preserved the rheological properties of the polymer. The solution–gel transition as a function of the temperature was found to be similar for these samples and the control sample [22].
Topical Drug Delivery Systems
In their study, Bosela and El-Bagory investigated the sensitivity of hydrocortisone to γ radiation in aqueous and organic solvents to predict the feasibility of sterilizing the topical preparations by radiation. The hydrocortisone showed a higher sensitivity to radiation in aqueous solutions. They determined that adding different types of surfactants resulted in a considerable protective effect (sodium lauryl sulfate, cetomagrogol 11000, cetyl trimethyl ammonium bromide). It was found that the stability of the drug in the formulated ointments was considerably affected by the propylene glycol content and slightly protected by cetyl alcohol [23].
Bosela et al., in a similar study, investigated the reactivity of prednisolone to γ radiation in aqueous and organic solutions using radiation doses of 0.25, 0.5, 1.0, 2.0, 3.0, 4.5, 6, and 9 KGy. The role of adding different types of surfactants such as sodium lauryl sulfate, Tween 80, and benzalkonium chloride was also examined. The study revealed that prednisolone was significantly more sensitive to γ radiation in aqueous solutions than organic solvents. The degradation occurred consistent with zero-order kinetic. The protective effect of added surfactants was found to be in the descending order: Tween 80, sodium lauryl sulfate, benzalkonium chloride [24].
Certain drug carrier systems other than new drug delivery systems have also been sterilized by γ irradiation. One of these techniques is called cryo-irradiation, which is irradiation of drugs in frozen aqueous solution. The frozen aqueous solutions of metoclopramide and metoprolol were sterilized by γ irradiation, and the resulting changes and radiolysis products were quantified by various methods. For both drugs, radiation sterilization of frozen solutions, even at high doses (25 kGy) was found to be possible [25].
In another study investigating cryo-irradiation, it was emphasized that this method was especially important for the sterilization of protein systems which could be infected by dangerous viruses (hepatitis B, etc.), blood plasma, and protein preparations manufactured from donor’s blood [26].
While γ radiation is widely used in more and more areas, researchers have been publishing detailed information regarding its use in various fields such as medical materials, polymers forming them, human tissue grafts, food, and the impact they have created so far [27–29].
In the studies on sterilization by γ radiation of drug delivery systems, different methods such as e-beam radiation are also investigated [30–33].
In this context, a study conducted a comparative evaluation of advantages and disadvantages of e-beam sterilization and γ sterilization methods [34–36]. Although γ irradiation has been used for many years in sterilization process, e-beam sterilization is a relatively new process for the sterilization of products, materials, and some pharmaceuticals. Since e-beam was commercialized over 40 years ago, a great deal of research has been performed on its effects on pharmaceuticals. The products of the process can be detected and evaluated for safety by using some instruments in analytical chemistry. As a result, it was concluded that sterilization by radiation was a better alternative for several complex pharmaceutical products that are not suitable for heat or steam sterilization [34].
In one of the sterilization studies using new radiation types, poly l-lactide (PLLA) nanoparticles loaded with dirhenium decacarbonyl (Re2(CO10)) were neutron-irradiated (450 kGy), and in vivo degradation characteristics were examined via targeted multitherapy (TMT) technique. It was concluded that these nanoparticles were a novel interesting candidate for local intratumoral radiotherapy [37].
Liposomes–Niosomes and Lipogelosomes–Niogelosomes
Özer’s review [38] discussing γ radiation, described applications of γ irradiation in the field of healthcare, mainly focusing on its use in pharmaceuticals, cosmetics, and medical equipment industry.
Pharmaceutical dosage forms that can be sterilized by γ radiation are as follows:
Ophthalmic ointments, whose sterile active ingredients and excipients are mixed aseptically and packaged in sterile containers;
Sterile injectable powders that are unstable in aqueous solutions, dissolved prior to use or dispersed to produce a suspension;
Aerosols, whose sterile active ingredients and additional excipients are filled under pressure;
Injectable liposomal, nanospheric, and microspheric controlled drug delivery systems;
Polymeric drug delivery systems that can be applied by implantation.
By stating “In general, as the presence of moisture in the environment reduces the radiation stability of the drug, solid dosage forms or nonaqueous formulations are more stable than the aqueous ones,” Özer concluded that new drug delivery systems could be sterilized by γ irradiation [38].
In some of the studies, the structure forming the new drug delivery systems, active ingredient, or its components were exposed to γ irradiation and the resulting changes were monitored and evaluated to develop new formulations or create a drug delivery system. The study investigating the effects of γ radiation is an example of liposomal phospholipids [39]. In this study, solid phospholipids were irradiated at doses lower than 25 kGy prior to liposome production, which did not cause any significant changes in the structure of the phospholipids.
Another study conducted on niosomes containing nystatin demonstrated that the 25-kGy γ-irradiation dose was sufficient for the sterilization and niosomal encapsulation provided means for parenteral administration of nystatin and reduced its toxicity, making it a more efficient agent [40].
The study investigating the effects of γ irradiation on diclofenac sodium-loaded liposomes–niosomes and lipogelosomes–niogelosomes for the treatment of rheumatoid arthritis, concluded that sterilization using an irradiation dose of 25 kGy did not affect the efficacy of the treatment [41].
Vaccines prepared by the new drug delivery systems
Today, new drug delivery systems have been widely used for various purposes such as to increase stability of the vaccines, to protect the active ingredients, to provide targeted delivery, to enhance their properties, and to ensure controlled drug release, while sterilization of these systems by γ irradiation has been gaining popularity in the recent years.
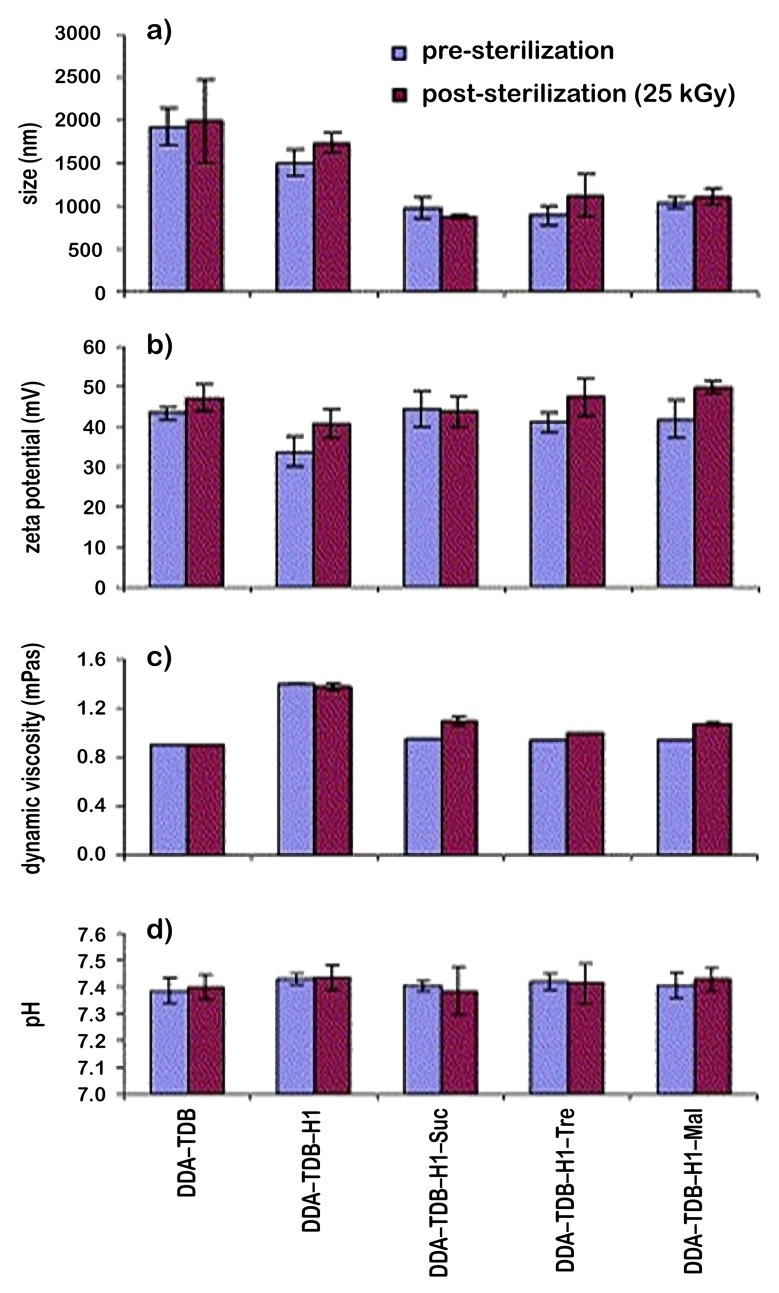
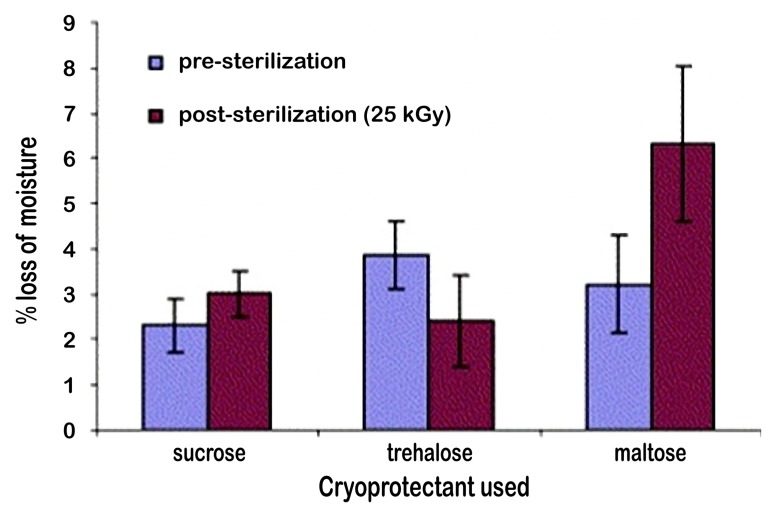
In this context, several studies have been conducted on microspheres [42] and liposomes [43]. In their study, Mohammed et al. investigated techniques for the lyophilization, cryoprotection, and sterilization of liposomal vaccines used as delivery systems for peptide, protein, and DNA vaccines. For the sterilization process, γ irradiation was used and then the liposomes were monitored for the changes caused by γ sterilization (Fig. 6 and 7). The effects of sterilization process were evaluated by analyzing the physicochemical parameters such as particle size of liposomes, zeta potential, dynamic viscosity, and pH, while these parameters were examined before or after sterilization [43]. It was concluded that the values obtained before or after sterilization showed sterilization by γ irradiation did not alter the properties of the liposomes (Fig. 6). In the same study, it was found that the sugars added to the formulation did not change any changes in the physicochemical characteristics of the liposomes. According to the thermogravimetric studies, the loss of moisture increased for sucrose and maltose after sterilization, while it decreased for trehalose (Fig. 7).
Fig. 6.
Physicochemical characterization of DDA–TDB MLV liposomes incorporating mycobacterial fusion protein Ag85B-ESAT-6 (H1; 10 μg) in the absence and presence of sucrose (8:1 mol/mol), maltose (4:1 mol/mol), or trehalose (10:1). (a) Measurement of mean volume diameter of liposomal suspension prior to and post-sterilization. (b) Surface charge measurements of liposomal suspension before and after exposure to γ sterilization. (c) Dynamic viscosity measurements were recorded to study the influence of γ sterilization. The hydrated liposomal solutions were filled into the glass capillary tube, and the measurements recorded at an angle tilt of 50° and –50°. The measurements were carried out at 20 °C. (d) The influence of γ sterilization on pH measurements. The hydrated liposomal samples were measured for the pH after calibration with the known standard pH solutions. All values reported are the average values from at least three independent samples ± standard deviation [43]
Fig. 7.
Thermogravimetric studies were carried out to measure the moisture content of the freeze dried liposomes as described in Fig. 2. The samples were investigated between the temperature ranges of 50 to 140 °C at a scan rate of 10 °C/min. All the measurements were carried out with the baseline subtraction of the empty sample pan weight. The values reported are the % loss in weight of the product [43]
The number of research carried out regarding the new drug delivery systems using different types of radiation sterilization indicates that radiation sterilization methods, especially sterilization by γ irradiation, has gained importance over the recent years. In parallel with the development of new drug delivery systems, the sterilization of these systems has become equally important. Therefore, we have concluded that the research into radiation sterilization of active ingredients, structures carried in a system, or polymer components, etc. will be a preliminary step for implementation in drug delivery systems. The studies (from 1990 to 2013) included in this paper, which investigated the effects of various radiation types on drug delivery systems, suggest that there is still plenty of room for improvement in this subject and it will clearly gain more importance with every future study on the drug delivery systems.
Funding Statement
Funding sources: None.
Footnotes
Author's Contributions: The manuscript has been written by GA, under the supervision of AYÖ. The authors are taking the scientific responsibility together.
Conflict of interest: None.
Contributor Information
Gürhan Abuhanoğlu, Department of Radiopharmacy, Faculty of Pharmacy, Hacettepe University, Sıhhiye, Ankara, Turkey.
A. Yekta Özer, Department of Radiopharmacy, Faculty of Pharmacy, Hacettepe University, Sıhhiye, Ankara, Turkey.
References
- 1.Volland C, Wolff M, Kissel T. The influence of terminal gamma-sterilization on captopril containing poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 1994;31(3):293–305. doi: 10.1016/0168-3659(94)90012-4. [DOI] [Google Scholar]
- 2.Caliş S, Bozdag S, Kaş HS, Tunçay M, Hincal AA. Influence of irradiation sterilization on poly(lactide-co-glycolide) microspheres containing anti-inflammatory drugs. Farmaco. 2002 Jan;57(1):55–62. doi: 10.1016/s0014-827x(01)01171-5. [DOI] [PubMed] [Google Scholar]
- 3.Montanari L, Cilurzo F, Valvo L, Faucitano A, Buttafava A, Groppo A, Genta I, Conti B. Gamma irradiation effects on stability of poly(lactide-co-glycolide) microspheres containing clonazepam. J Control Release. 2001 Aug 10;75(3):317–330. doi: 10.1016/s0168-3659(01)00401-1. [DOI] [PubMed] [Google Scholar]
- 4.Puthli S, Vavia P. Gamma irradiated micro system for long-term parenteral contraception: An alternative to synthetic polymers. Eur J Pharm Sci. 2008 Nov 15;35(4):307–317. doi: 10.1016/j.ejps.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Desai KG, Park HJ. Study of gamma-irradiation effects on chitosan microparticles. Drug Deliv. 2006 Jan-Feb;13(1):39–50. doi: 10.1080/10717540500309123. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Sancho C, Herrero-Vanrell R, Negro S. Study of gamma-irradiation effects on aciclovir poly(D,L-lactic-co-glycolic) acid microspheres for intravitreal administration. J Control Release. 2004 Sep 14;99(1):41–52. doi: 10.1016/j.jconrel.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Mohr D, Wolff M, Kissel T. Gamma irradiation for terminal sterilization of 17beta-estradiol loaded poly-(D,L-lactide-co-glycolide) microparticles. J Control Release. 1999 Aug 27;61(1-2):203–217. doi: 10.1016/s0168-3659(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 8.Lee TH, Wang J, Wang CH. Double-walled microspheres for the sustained release of a highly water soluble drug: characterization and irradiation studies. J Control Release. 2002 Oct 30;83(3):437–452. doi: 10.1016/s0168-3659(02)00235-3. [DOI] [PubMed] [Google Scholar]
- 9.Bittner B, Mäder K, Kroll C, Borchert HH, Kissel T. Tetracycline-HCl-loaded poly(D,L-lactide-co-glycolide) microspheres prepared by a spray drying technique: influence of gamma-irradiation on radical formation and polymer degradation. J Control Release. 1999 May 1;59(1):23–32. doi: 10.1016/s0168-3659(98)00170-9. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka S, Aso Y, Kojima S. Drug release from poly(dl-lactide) microspheres controlled by γ-irradiation. J Control Release. 1995;37(3):263–267. [Google Scholar]
- 11.Faisant N, Siepmann J, Oury P, Laffineur V, Bruna E, Haffner J, Benoit J. The effect of gamma-irradiation on drug release from bioerodible microparticles: a quantitative treatment. Int J Pharm. 2002 Aug 21;242(1-2):281–284. doi: 10.1016/s0378-5173(02)00188-6. [DOI] [PubMed] [Google Scholar]
- 12.Bartolotta A, D'Oca MC, Campisi M, De Caro V, Giandalia G, Giannola LI, Brai M, Calderaro E. Effects of gamma-irradiation on trehalose-hydroxyethylcellulose microspheres loaded with vancomycin. Eur J Pharm Biopharm. 2005 Jan;59(1):139–146. doi: 10.1016/j.ejpb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Friess W, Schlapp M. Sterilization of gentamicin containing collagen/PLGA microparticle composites. Eur J Pharm Biopharm. 2006 Jun;63(2):176–187. doi: 10.1016/j.ejpb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Ražem D, Ražem BK. The effects of irradiation on controlled drug delivery/controlled drug release systems. Radiat Phys Chem. 2008;77(3):288–344. [Google Scholar]
- 15.Weyenberg W, Vermeire A, D'Haese E, Vanhaelewyn G, Kestelyn P, Callens F, Nelis HJ, Remon JP, Ludwig A. Effect of different sterilisation methods on the properties of bioadhesive powders and ocular minitablets, and clinical evaluation. Eur J Pharm Sci. 2004 Sep;23(1):77–87. doi: 10.1016/j.ejps.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Masson V, Maurin F, Fessi H, Devissaguet JP. Influence of sterilization processes on poly(epsilon-caprolactone) nanospheres. Biomaterials. 1997 Feb;18(4):327–335. doi: 10.1016/s0142-9612(96)00144-5. [DOI] [PubMed] [Google Scholar]
- 17.Song CX, Labhasetwar V, Murphy H, Qu X, Humphrey WR, Shebuski RJ, Levy RJ. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J Control Release. 1997;43(2–3):197–212. doi: 10.1016/S0168-3659(96)01484-8. [DOI] [PubMed] [Google Scholar]
- 18.Kreuter J, Gelperina S. Use of nanoparticles for cerebral cancer. Tumori. 2008 Mar-Apr;94(2):271–277. doi: 10.1177/030089160809400220. [DOI] [PubMed] [Google Scholar]
- 19.Maksimenko O, Pavlov E, Toushov E, Molin A, Stukalov Y, Prudskova T, Feldman V, Kreuter J, Gelperina S. Radiation sterilisation of doxorubicin bound to poly(butyl cyanoacrylate) nanoparticles. Int J Pharm. 2008 May 22;356(1-2):325–332. doi: 10.1016/j.ijpharm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Park SE, Nho YC, Lim YM, Kim H. Preparation of pH-sensitive poly(vinyl alcohol-g-methacrylic acid) and poly(vinyl alcohol-g-acrylic acid) hydrogels by gamma ray irradiation and their insulin release behavior. J Appl Polym Science. 2004;91(1):636–643. [Google Scholar]
- 21.Eljarrat-Binstock E, Bentolila A, Kumar N, Harel H, Domb AJ. Preparation, characterization, and sterilization of hydrogel sponges for iontophoretic drug-delivery use. Polym Adv Technol. 2007;18(9):720–730. [Google Scholar]
- 22.Amanda K, Brun-Graeppi AS, Richard C, Bessodes M, Scherman D, Naritag T, Ducouret G, Merten OW. The effect of sterilization methods on the thermo-gelation properties of xyloglucan hydrogels. Polym Degradation Stability. 2010;95(2):254–259. [Google Scholar]
- 23.Bosela AA, El-Bagory JM. Sensitivity of hydrocortisone to gamma radiation in topical preparations. J Drug Deliv Sci Technol. 2008;18(3):209–213. [Google Scholar]
- 24.Bosela AA, Salamah KK, Alsarra IA, El-Bagory IM. Reactivity of prednisolone to gamma radiation in aqueous and organic solutions. J Drug Deliv Sci Technol. 2010;20(3):225–229. [Google Scholar]
- 25.Maquille A, Habib Jiwan JL, Tilquin B. Cryo-irradiation as a terminal method for the sterilization of drug aqueous solutions. Eur J Pharm Biopharm. 2008 May;69(1):358–363. doi: 10.1016/j.ejpb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Talrose VL. Cryoradiation sterilization contemporary state and outlook. Radiat Phys Chem. 1995;46(4–6):633–637. [Google Scholar]
- 27.Silva Aquino KA. Sterilization by gamma irradiation. In: Adrovi F, editor. Gamma Radiation. 2012. pp. 171–206. [Google Scholar]
- 28.Olguner G, Özer AY. Radiation sterilization: II – radiation sterilization of drugs. FABAD J Pharm Sci. 2000;25:53–73. [Google Scholar]
- 29.Liman V. Investigation of the effect of radiation on sterility maintenance in some cephalosporins. Ankara, Turkey: Hacettepe University; 2005. Master Thesis. [Google Scholar]
- 30.Cleland MR, Beck JA. In: Electron Beam Sterilization in Encyclopedia of Pharmaceutical Technology. Swarbrick J, Boylan JC, editors. New York: Marcel Dekker; 1992. pp. 105–136. [Google Scholar]
- 31.International Atomic Energy Agency. Trends in Radiation Sterilization of Health Care Products. Vienna: IAEA Publications; 2008. [Google Scholar]
- 32.Gu L, Zablocki K, Lavelle L, Bodnar S, Halperin F, Harper I, Moghe PV, Uhrich KE. Impact of ionizing radiation on physicochemical and biological properties of an amphiphilic macromolecule. Polym Degradation Stability. 2012;97(9):1686–1689. doi: 10.1016/j.polymdegradstab.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dispenza C, Sabatino MA, Grimaldi N, Spadaro G, Bulone D, Bondì ML, Adamo G, Rigogliuso S. Large-scale radiation manufacturing of hierarchically assembled nanogels. Chem Eng Trans. 2012;27:229–234. [Google Scholar]
- 34.Silindir M, Özer AY. Sterilization methods and the comparison of e-beam sterilization with gamma radiation sterilization. FABAD J Pharm Sci. 2009;34:43–53. [Google Scholar]
- 35.Jacobs GP. Radiation sterilization of parenterals, Pharmaceutical Technology. 2007. May 1, [Google Scholar]
- 36.Yaman A. Alternative methods of terminal sterilization for biologically active macromolecules. Curr Opin Drug Discov Dev. 2001;4(6):1–5. [PubMed] [Google Scholar]
- 37.Hamoudeh M, Fessi H, Mehier H, Al Faraj A, Canet-Soulas E. Dirhenium decacarbonyl-loaded PLLA nanoparticles: influence of neutron irradiation and preliminary in vivo administration by the TMT technique. Int J Pharm. 2008 Feb 4;348(1-2):125–136. doi: 10.1016/j.ijpharm.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Özer AY. Gamma radiation and gamma radiation sterilization. Congress Book; 3rd Sterilization and Disinfection (DAS); 2003 October 2–4; pp. 228–229. [Google Scholar]
- 39.Erdoğan S, Özer AY, Ekizoğlu M, Özalp M, Çolak Ş, Korkmaz M. Gamma irradiation of liposomal phospholipids. FABAD J Pharm Sci. 2006;31:182–190. [Google Scholar]
- 40.El-Ridy MS, Abdelbary A, Essam T, El-Salam RM, Kassem AA. Niosomes as a potential drug delivery system for increasing the efficacy and safety of nystatin. Drug Dev Ind Pharm. 2011 Dec;37(12):1491–1508. doi: 10.3109/03639045.2011.587431. [DOI] [PubMed] [Google Scholar]
- 41.Turker S, Ozer AY, Kiliç E, Ozalp M, Colak S, Korkmaz M. Gamma-irradiated liposome/noisome and lipogelosome/niogelosome formulations for the treatment of rheumatoid arthritis. Interv Med Appl Sci. 2013 Jun;5(2):60–69. doi: 10.1556/IMAS.5.2013.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langer R, Cleland JL, Hanes J. New advances in microsphere-based single-dose vaccines. Adv Drug Deliv Rev. 1997 Oct 13;28(1):97–119. doi: 10.1016/s0169-409x(97)00053-7. [DOI] [PubMed] [Google Scholar]
- 43.Mohammed AR, Bramwell VW, Coombes AG, Perrie Y. Lyophilisation and sterilisation of liposomal vaccines to produce stable and sterile products. Methods. 2006 Sep;40(1):30–38. doi: 10.1016/j.ymeth.2006.05.025. [DOI] [PubMed] [Google Scholar]