Abstract
Anorexia nervosa (AN) is a condition of severe low weight that is associated with low bone mass, impaired bone structure and reduced bone strength, all of which contribute to increased fracture risk., Adolescents with AN have decreased rates of bone accrual compared with normal-weight controls, raising addition concerns of suboptimal peak bone mass and future bone health in this age group. Changes in lean mass and compartmental fat depots, hormonal alterations secondary to nutritional factors contribute to impaired bone metabolism in AN. The best strategy to improve bone density is to regain weight and menstrual function. Oral estrogen-progesterone combinations are not effective in increasing bone density in adults or adolescents with AN, and transdermal testosterone replacement is not effective in increasing bone density in adult women with AN. However, physiologic estrogen replacement as transdermal estradiol with cyclic progesterone does increase bone accrual rates in adolescents with AN to approximate that in normal-weight controls, leading to a maintenance of bone density Z-scores. A recent study has shown that risedronate increases bone density at the spine and hip in adult women with AN. However, bisphosphonates should be used with great caution in women of reproductive age given their long half-life and potential for teratogenicity, and should be considered only in patients with low bone density and clinically significant fractures when non-pharmacological therapies for weight gain are ineffective. Further studies are necessary to determine the best therapeutic strategies for low bone density in AN.
Keywords: Anorexia nervosa, eating disorders, adolescents, adults, bone density, microarchitecture, strength, fracture, growth hormone, IGF-1, estrogen, testosterone, bisphosphonates, leptin, ghrelin, PYY, adipokines
Introduction
Anorexia nervosa (AN) is a condition of severe low weight associated with impaired body image and an intense fear of gaining weight that is reported in 0.2–1% of women (Lucas, et al. 1991). Although most commonly diagnosed in women, in one study, 5–15% of all individuals diagnosed with AN were male (Andersen and Holman 1997). The 2013 DSM V criteria eliminated the requirement of amenorrhea for the diagnosis of AN. With the revised diagnostic criteria, the prevalence of AN in women is expected to increase to as much as 4% (Smink, et al. 2013). Subtypes of AN include the restrictive and the binge-purge subtypes, both of which are associated with low body weight. Most available data on effects of AN on bone metabolism are based on studies that used DSM IV criteria for diagnosis of AN, and the effect of the use the new DSM V criteria remains unknown. This review will discuss the impact of AN on bone density, microarchitecture and strength estimates, as well as fracture risk. We will also discuss the determinants of impaired bone metabolism in AN, and possible therapeutic interventions to optimize bone health in this condition.
Impact of Anorexia Nervosa on Bone
Numerous studies have reported the deleterious effects of AN on bone health (Bachrach, et al. 1990; Biller, et al. 1989a; Grinspoon, et al. 2000; Jagielska, et al. 2002; Misra, et al. 2004a) associated with increased fracture risk (Espallargues, et al. 2001; Faje, et al. 2014; Lucas, et al. 1999). Whereas earlier studies examined effects of AN on bone density parameters, more recent studies have reported on the effects of this disorder on measures of bone microarchitecture and strength estimates using state-of-the-art methodologies.
Bone Mineral Density
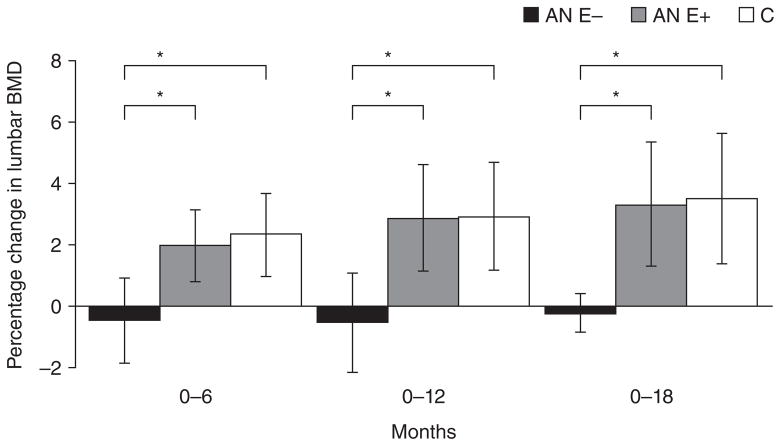
Low bone mineral density (BMD) is characteristic of AN and affects both adults (Biller et al. 1989a; Grinspoon et al. 2000) and adolescents (Bachrach et al. 1990; Jagielska et al. 2002; Misra et al. 2004a) with this condition. Both trabecular and cortical bone sites are affected in AN, although data overall suggest that in females sites of trabecular bone (such as the lumbar spine) are affected more severely than sites of predominantly cortical bone (such as the hip and whole body). This has been attributed to the profound estrogen deficiency that typically accompanies this disorder. In community dwelling adult women with AN, more than 90% have T-scores of < −1, and close to 40% have T-scores of < −2.5 at one or more sites (Grinspoon et al. 2000). Adolescent girls with AN are also at high risk for low bone density (Figure 1), and in one study, more than 50% had BMD Z-scores of < −1 at one or more sites (Misra et al. 2004a). In addition to cross sectional reports of low BMD, adolescents with AN have significant reductions in bone accrual rates over time compared with controls (Misra, et al. 2008b; Soyka, et al. 2002) (Figure 2). Reduced bone accrual is a major concern during the adolescent years because this is a time when marked increases occur in bone accrual in healthy teenagers towards attainment of peak bone mass (Bachrach 2001; Theintz, et al. 1992), an important determinant of future fracture risk. Adolescence thus represents a window in time during which bone accrual needs to be maximized in order to attain an optimal peak bone mass, and deficits incurred during this time may be permanent. In fact, women with AN who develop AN during the adolescent years tend to have lower bone density than those who develop this condition in adult life, despite a similar duration of amenorrhea (Biller, et al. 1989b).
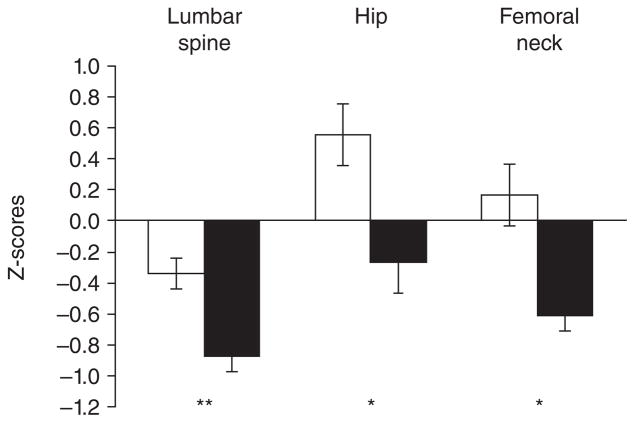
Figure 1.
Z scores for lumbar spine, hip, and femoral neck bone mineral density (BMD) in girls with anorexia nervosa (AN) (black bars) and healthy control subjects (white bars). Girls with AN had significantly lower Z-scores at each site than healthy adolescents. *P< .01; **P≤.001. From Misra et al. Pediatrics 2004;114:1574–1583 Copyright © 2004 by the American Academy of Pediatrics.
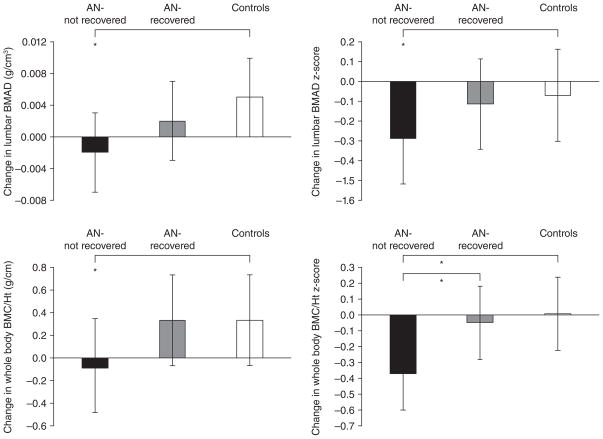
Figure 2.
Change in lumbar bone mineral apparent density (BMAD) and whole body bone mineral content/height (WB BMC/Ht) measures in anorexia nervosa (AN)-not recovered (black bars), AN-recovered (gray bars), and healthy adolescents (white bars) over 1-year. AN-not recovered continued to lose bone mass over the 1-year follow-up period, and change in bone density measures was significantly lower in this group compared with controls (Tukey-Kramer test for multiple comparisons). AN-recovered did not differ from controls for change in bone density parameters and differed significantly from AN-not recovered for change in whole body bone density Z-scores. *, P <0.05. From Misra et al. J Clin Endocrinol Metab 2008;93: 1231–1237. Copyright © 2008 by The Endocrine Society.
In addition to women, males with AN are at high risk for low bone density, and we have reported BMD Z-scores of < −1 at the femoral neck and lumbar spine in 65% and 50% of boys with AN 12–19 years old, compared with only 18 and 24 % of normal-weight boys in the same age range (Misra, et al. 2008a). Therefore, in contrast to women, males with AN have greater involvement of sites of predominantly cortical bone.
Most earlier studies used dual energy x-ray absorptiometry (DXA) to measure BMD, and DXA reports ‘areal’ BMD (aBMD) (bone mineral content/cross-sectional area of bone), rather than ‘volumetric’ BMD (vBMD) (bone mineral content/bone volume). Areal BMD is susceptible to artifactual changes based on body size, such that shorter individuals have lower reported aBMD than taller individuals, even when volumetric BMD (vBMD) is similar. Surrogates for vBMD, such as lumbar bone mineral apparent density (BMAD) are also significantly lower in AN than in a normal-weight control population (Misra et al. 2004a; Misra et al. 2008b).
Quantitative computed tomography (QCT) is a methodology that allows measurement of vBMD in vivo, and in 2014 remains a research tool. QCT can measure vBMD at the spine and hip, while peripheral QCT (pQCT) and high resolution pQCT (HRpQCT) have been used to measure vBMD at the distal radius in both adult women and adolescent girls with this disorder. Adult women with AN have decreases in cortical vBMD (Milos, et al. 2005), and we have reported lower total and trabecular vBMD at the distal radius in adolescent girls with AN compared with controls (Faje, et al. 2013b)(Figure 3).
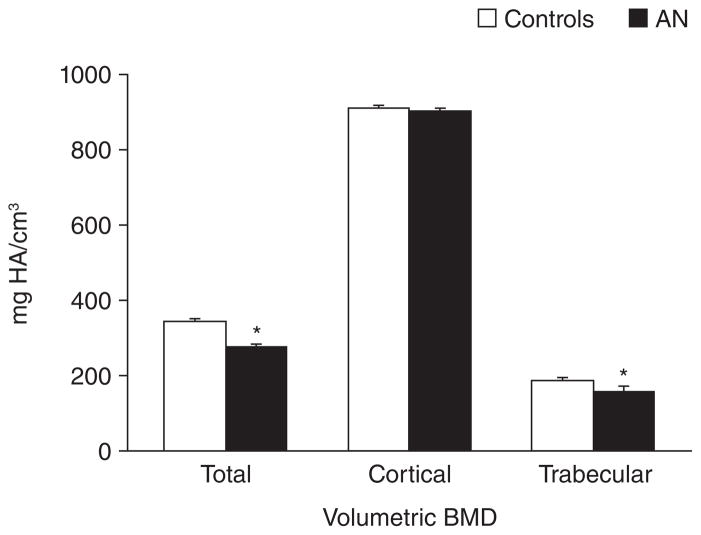
Figure 3.
Volumetric bone mineral density (BMD) assessed by HRpQCT in adolescent girls with anorexia nervosa (black bars) and normal-weight controls (white bars). Girls with anorexia nervosa had significantly lower total and trabecular volumetric BMD than controls. * p<0.05. Adapted from Faje et al. J Clin Endocrinol Metab 2013; 98(5):1923–9. Copyright © 2013 by The Endocrine Society.
Bone Geometry and Microarchitecture
Both pQCT and HRpQCT can measure size parameters of bone, and adult and adolescent women with AN have reductions in cortical thickness and cortical area, with coincident increases in trabecular area (Faje et al. 2013b; Lawson, et al. 2010; Milos et al. 2005). This is likely consequent to estrogen deficiency in AN. Estrogen prevents endosteal bone resorption (Riggs, et al. 2002), and low estrogen levels would thus be expected to lead to an increase in endosteal bone resorption and reductions in cortical area, exactly what is observed in AN. Because cortical thickness is an important determinant of bone strength (Faje et al. 2013b), reductions in cortical thickness likely contribute to increased fracture risk.
HRpQCT can assess bone microarchitecture, and we have reported that adolescent girls with AN have increased cortical porosity and decreased trabecular thickness compared with normal-weight controls (Faje et al. 2013b). Similarly, adult women with AN have reductions in trabecular number and thickness with increased trabecular separation (Lawson et al. 2010; Milos et al. 2005). Abnormalites in microarchitecture may precede changes observed using DXA scanning. Using flat panel ultra-high resolution volumetric CT, we reported that bone microarchitecture may be affected in adolescent girls with AN even before significant reductions occur in DXA reports of aBMD (Bredella, et al. 2008).
Data are currently lacking regarding bone geometry and microarchitecture changes using QCT in males with AN. However, hip structural analysis (HSA) (from DXA) is a validated technique to measure bone geometry at the hip and assess fracture risk (Schousboe, et al. 2013). Using HSA we have reported lower cross-sectional area, cross-sectional moment of inertia and section modulus in boys with AN at the narrow neck, trochanteric region and femoral shaft, compared to normal-weight controls after controlling for age and height (Misra, et al. 2013). Lower cortical thickness at the narrow neck and trochanteric region, and greater buckling ratio at the trochanteric region were also shown (Misra et al. 2013). These changes suggest reduced bone strength at the hip and femoral neck in males with AN. Future studies using QCT techniques are necessary to confirm these findings.
Bone Strength Estimates and Fracture Risk
Micro finite element analysis (μFEA) allows estimation of bone strength using data from HRpQCT and advanced engineering modeling techniques. Strength estimates (stiffness and failure load) of the distal radius assessed using μFEA are markedly lower in adult and adolescent women with AN compared with normal-weight controls (Faje et al. 2013b; Milos et al. 2005). Consistent with these studies, an increase in fracture risk has been reported in both adults and adolescents with AN (Espallargues et al. 2001; Faje et al. 2014; Lucas et al. 1999). In adolescents, we have reported historical occurrence of one or more fractures in 31% of girls compared with 19% of normal-weight healthy controls (Faje et al. 2014). Interestingly, we found that fracture risk was increased in girls with AN compared with controls even at relatively normal BMD Z-scores of < −1 (and not just < −2) (Faje et al. 2014), raising concerns that changes in bone geometry and structure occur at relatively ‘normal’ BMD Z-scores in adolescents with AN to increase fracture risk. Consistent with these findings, another study reported that spine areal BMD and measures of disease duration and severity did not predict occurrence of incident vertebral fractures in adolescents and young adults with AN using the Genant semiquantitative technique. Of note, in this study, 12.5% of the women developed incident vertebral fractures over 18 months of follow-up (Divasta, et al. 2014b).
Surrogate Markers of Bone Turnover
In order to better understand the pathophysiology underlying changes in bone density and structure in AN, it is important to examine surrogate markers of bone turnover. Normal adults and adolescents differ in patterns of biochemical markers of bone turnover and patterns are different in AN as well. Adult women with AN have a decrease in markers of bone formation (Grinspoon, et al. 1996; Hotta, et al. 1998) and an increase in markers of bone resorption (Grinspoon et al. 1996; Hotta et al. 1998; Zipfel, et al. 2001), consistent with an uncoupling of bone turnover leading to impaired bone metabolism. In contrast, adolescent girls and boys with AN have a “low turnover state” with decreases in both bone formation and bone resorption markers (Misra, et al. 2011; Soyka et al. 2002), reflective of a coupled decrease in bone turnover. These findings are in contrast to the increased bone turnover state of normal adolescence (Mora, et al. 1999).
Effects of Weight Gain and Menstrual Recovery on Bone Parameters
Recovery from AN is key to improving bone health. In adults with AN, our studies have shown increases in bone density following weight gain and/or menstrual recovery. Weight gain leads to increases in bone density at the total hip, a predominantly cortical site, whereas menstrual recovery leads to increases in bone density at the spine, a predominantly trabecular site (Miller, et al. 2006) (Figure 4). Weight gain with menstrual recovery results in a mean annual increase in BMD of 3.1% at the spine and 1.8% at the hip (Miller et al. 2006). In adolescents with AN, we have demonstrated a modest improvement in bone accrual rates following weight gain and menstrual recovery, and not to the extent seen in normal-weight healthy controls (Misra et al. 2008b). This, however, is an improvement to the reductions in bone density observed over time in those who do not gain weight and/or recover menses (Misra et al. 2008b) (Figure 2). Thus, while bone density Z-scores continue to decrease in non-recovered adolescents, the decrease in Z-scores is attenuated in those who gain weight and resume menses. We have also shown that biochemical markers of bone turnover increase with weight gain in adolescents with AN, and an improvement in bone turnover markers over the initial six months predicts increases in bone mineral content in the subsequent six months (Soyka et al. 2002). These data suggest that with persistent weight recovery, one is likely to see a significant improvement in bone parameters. Data are currently lacking regarding the impact of weight gain and/or resumption of menses on bone geometry, microarchitecture, strength estimates and fracture risk.
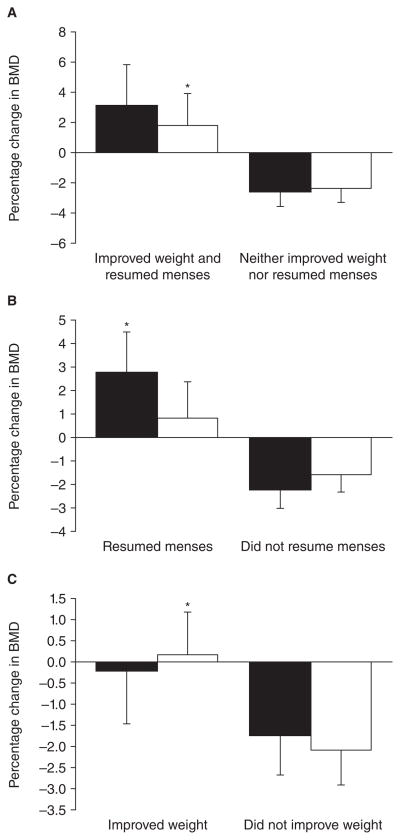
Figure 4.
Impact of weight and/or menstrual recovery on bone density parameters in adult women with anorexia nervosa not receiving oral contraceptives.
A. Women who both improved weight and resumed menses increased BMD at the PA spine and hip, compared with those who neither improved weight nor resumed menses.
B. Women who resumed menses increased PA spine BMD (but not hip BMD), compared with those who did not improve menstrual function.
C. Women who improved weight increased hip BMD (but not PA spine BMD), compared with those who did not improve weight.
Black bars, PA spine BMD; white bars, hip BMD. *, P< 0.05.
From Miller et al. J Clin Endocrinol Metab 91: 2931–7; 2006. Copyright © by The Endocrine Society 2006.
Factors Contributing to Impaired Bone Metabolism in Anorexia Nervosa
This section discusses the body composition, nutritional and hormonal changes that contribute to poor bone health in AN. Knowing the determinants of low bone density and impaired bone structure in AN is essential to develop the appropriate therapeutic strategies to optimize bone density and structure in adults and adolescents with AN.
Changes in Body Composition
AN is characterized by marked reductions in fat mass, and less marked but significant reductions in lean mass (Faje et al. 2013b; Miller et al. 2006; Misra et al. 2004a; Soyka et al. 2002). Lower lean mass is an important determinant of lower bone density and impaired bone structure in adults and adolescents with AN (Faje et al. 2013b; Miller et al. 2006; Misra et al. 2004a; Soyka et al. 2002). We have also shown that increases in lean mass following weight gain are strongly predictive of coincident increases in bone density in adolescents with AN (Soyka et al. 2002). Similar to females, lean mass is an important determinant of bone density in males with AN, and of HSA parameters (Misra et al. 2013; Misra et al. 2008a).
Recent studies have examined compartmental fat stores in AN, and our group has reported higher marrow adiposity using magnetic resonance spectroscopy in women with AN compared with normal-weight controls (Figure 5) and women who have recovered from AN (Bredella, et al. 2009; Fazeli, et al. 2012). Increased marrow adiposity in AN is associated with lower aBMD (Bredella et al. 2009), consistent with reports of a reciprocal relationship between marrow fat and bone in studies of healthy children and adults (Lewiecki, et al. 2008). Preadipocyte factor-1 (Pref-1) is a member of the epidermal growth factor like family of proteins that inhibits differentiation of the mesenchymal progenitor stem cell along the osteoblast pathway, and women with AN have higher Pref-1 levels than controls and recovered women (Fazeli et al. 2012; Fazeli, et al. 2010a). Higher Pref-1 levels in AN are associated inversely with aBMD and positively with marrow fat (Fazeli et al. 2010a). Increased marrow adiposity is believed to reduce biomechanical strength of bone (compared to hemopoietic marrow) (Schellinger, et al. 2001), and may contribute to increased fracture risk in AN.
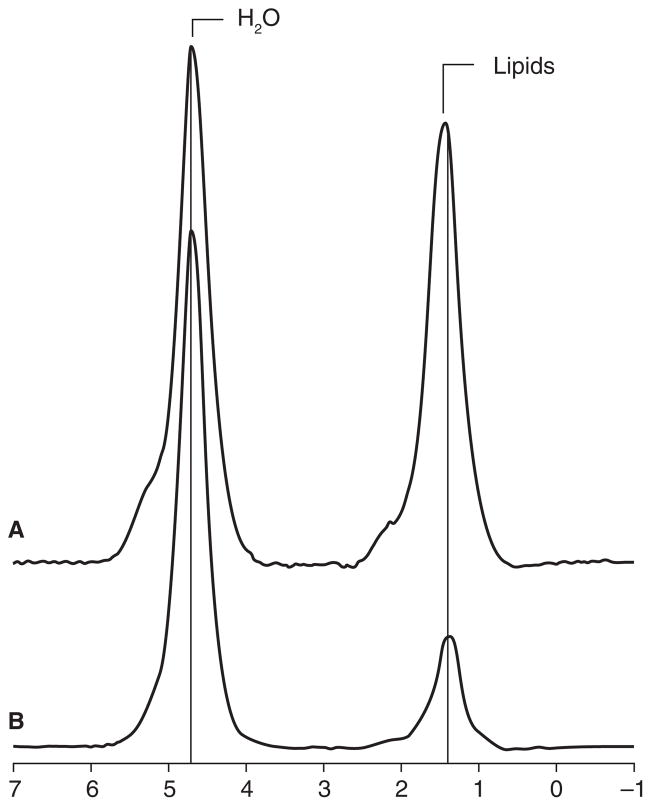
Figure 5.
1H-magnetic resonance spectra of L4 marrow obtained at 3.0T from a subject with anorexia nervosa (A) and a age-matched healthy control (B). The prominent lipid resonance in (A) indicates a relatively higher marrow fat content. From Bredella et al. J Clin Endocrinol Metab 2009; 94(6): 2129–2136. Copyright © 2009 The Endocrine Society.
Cold induced brown adipose tissue (BAT) activity can now be assessed using PET-CT and PET-MRI scans, and young women with AN are less likely to have cold induced BAT activity than controls (Bredella, et al. 2012). Because BAT increases energy expenditure and shivering thermogenesis, reductions in BAT activity likely represent an adaptive mechanism to conserve energy in AN, a state of very low energy stores (as indicated by marked reductions in body fat mass). Of importance, BAT activity has also been demonstrated to be bone anabolic (Lee, et al. 2013; Ponrartana, et al. 2012), thus reductions in BAT activity in women with AN may also contribute to lower BMD. In fact, positive associations of lower BAT activity and lower BMD are reported in women with AN (Bredella et al. 2012).
Exercise Activity
There are limited data regarding the impact of exercise activity on bone metabolism in AN. Although mechanical loading is known to be beneficial to bone health, because exercise typically leads to increases in energy expenditure, management of AN includes limiting exercise activity to conserve energy stores.
Calcium and Vitamin D Status
Although it is clear that optimizing calcium and vitamin D status are essential to optimize bone mineralization, most adults and adolescents with AN have a higher calcium and vitamin D intake than a control population due to increased use of supplements (Hadigan, et al. 2000; Misra, et al. 2006b). In adolescents with AN, we have shown that vitamin D intake is lower than the RDA in only 23% of girls with AN compared with 50% controls, and calcium intake is lower than the RDA in 41% girls with AN compared to 70% of controls (Misra et al. 2006b). Consistent with increased supplement intake, 25-hydroxy vitamin D levels are higher in AN than in controls (Misra et al. 2011). One study has reported that a daily calcium intake of <600 mg is associated with lower bone density (Castro, et al. 2000). However, most studies have failed to demonstrate a relationship between calcium or vitamin D intake and bone parameters in AN.
Changes in Hormonal Axes
AN is associated with marked changes in almost every endocrine axis, and most of these are adaptive changes that help conserve energy for vital functions, stimulate food intake or maintain euglycemia. Many of these hormonal changes, however, have potential deleterious effects on bone. This section will discuss the impact of known hormonal changes in AN on bone.
Hypothalamic-Pituitary- Gonadal (HPG) Axis
Although amenorrhea is no longer required for the diagnosis of AN per DSM V criteria, hypothalamic amenorrhea is a common finding in women with AN. Suppression of the HPG axis is advantageous in a state of very low energy availability, as reproduction would divert available energy from that required for maintenance of vital body functions. In adolescents with AN, menarche may be delayed, and menarchal delay is an important determinant of low bone density (Misra et al. 2004a).
The gonadal steroids, both estrogen and testosterone, are critical for bone accrual during adolescence, and for maintaining bone density in adults. Estrogen inhibits osteoclastic bone resorption (Riggs 2000), and also inhibits sclerostin and Pref-1 (Divasta et al. 2014b; Faje, et al. 2013a), effects that should lead to an increase in bone density. Sclerostin is a product of osteocytes which inhibits wnt signaling and thus osteoblastic activity (Modder, et al. 2011). Pref-1, as previously discussed, decreases differentiation of the mesenchymal progenitor stem cell along the osteoblast pathway (Wang and Sul 2009). Testosterone acts primarily to prevent osteoclastic bone resorption following its aromatization to estrogen, and also has proposed direct bone anabolic effects (Riggs et al. 2002). During adolescence, rising estradiol levels in girls and aromatization of testosterone to estradiol in boys inhibit endosteal bone resorption leading to increased cortical thickness, while rising testosterone levels in boys [along with rising levels of growth hormone (GH) and insulin like growth factor-1 (IGF-1)] contribute to periosteal bone apposition. In women and adolescent girls with AN, lower estradiol levels and duration of amenorrhea (Bachrach et al. 1990; Baker, et al. 2000; Biller et al. 1989a; Castro et al. 2000; Misra et al. 2004a) are key determinants of low bone density. In boys with AN, low testosterone levels predict low spine BMD whereas BMI and lean mass predict total hip and femoral neck BMD (Misra et al. 2008a).
Growth Hormone- Insulin like Growth Factor-1 Axis
Puberty is characterized by increases in GH and IGF-1, both of which are bone anabolic and facilitate periosteal bone apposition. In contrast, AN is associated with marked reductions in IGF-1 levels in both adolescents and adults, and low IGF-1 levels correlate with lower levels of bone formation markers and lower BMD (Grinspoon, et al. 2002; Misra, et al. 2003a; Soyka et al. 2002). Furthermore, IGF-1 levels correlate positively with measures of bone microarchitecture (Faje et al. 2013b; Lawson et al. 2010). Despite low IGF-1 levels, GH concentrations are increased in AN, indicative of a nutritionally acquired hepatic GH resistance (Argente, et al. 1997; Misra et al. 2003a; Scacchi, et al. 1997; Stoving, et al. 1999). Whereas GH concentrations are strongly associated with concentrations of biochemical markers of bone turnover in normal-weight adolescents, these associations are lost in girls with AN, suggesting GH resistance in bone (in addition to the liver) (Misra et al. 2003a). Furthermore, administration of supraphysiologic doses of recombinant human (rh) GH to adult women with AN fails to increase IGF-1 levels or levels of bone turnover markers (Fazeli, et al. 2010b), further corroborating the concept of GH resistance.
Hypothalamic-Pituitary-Adrenal Axis
Both adults and adolescents with AN have higher serum and urinary cortisol levels compared with normal-weight controls (Lawson, et al. 2009; Misra, et al. 2004b). This state of relative hypercortisolemia may be an adaptive mechanism in AN, as cortisol is a gluconeogenic hormone. However, hypercortisolemia has multiple deleterious effects on bone, and girls and women with AN and higher cortisol levels have lower measures of bone formation markers and lower BMD (Lawson et al. 2009; Misra et al. 2004b).
Adipokines
Leptin is an adipokine that is anorexigenic and has effects on bone. Whereas central leptin is deleterious to the axial skeleton (Ducy, et al. 2000; Hamrick, et al. 2004), peripheral leptin has bone anabolic effects (with possible osteoclast inhibitory effects as well), particularly on the appendicular skeleton (Hamrick, et al. 2005; Hamrick et al. 2004). Levels of leptin are low in AN (Mehler, et al. 1999; Misra, et al. 2005b), likely an adaptive mechanism to increase appetite, and lower leptin levels are associated with lower fat mass and bone density measures (Lawson et al. 2010). Adiponectin is another adipokine that is deleterious to bone based on studies in post-menopausal women and adult men (Biver, et al. 2011; Jurimae, et al. 2008). Levels of adiponectin have been variably reported to be high, normal or low in adults and adolescents with AN (Amitani, et al. 2013; Housova, et al. 2005; Misra, et al. 2007; Tagami, et al. 2004). At least one study reported that all adiponectin isoforms should be evaluated in AN (in addition to total adiponectin) and that percent high molecular weight adiponectin correlates positively with body mass index (Amitani et al. 2013). We have reported that higher adiponectin levels corrected for fat mass in girls with AN predict lower spine BMD and BMAD (Misra et al. 2007).
Enteric Hormones
Insulin and amylin are bone anabolic, and levels of both hormones are reduced in AN and are associated with lower levels of bone formation markers and lower BMD (Misra et al. 2007; Wojcik, et al. 2010). Ghrelin is an orexigenic hormone and GH secretagogue, secreted by the gastric fundus (Kojima, et al. 1999). Ghrelin increases osteoblastic activity in cell cultures, suggestive of bone anabolic effects (Kim, et al. 2005). Levels of ghrelin are increased in AN compared with normal-weight controls (Lawson, et al. 2011b; Misra, et al. 2004a; Misra, et al. 2005a), likely an adaptive change to increase appetite and thus caloric intake. Whereas ghrelin levels positively predict bone density in healthy individuals, we found no associations of ghrelin levels with bone measures in girls with AN, likely representing a state of ghrelin resistance in AN (Misra et al. 2005a). Finally, peptide YY (PYY) is an enteric anorexigenic hormone secreted by the endocrine L-cells of the distal gut (Batterham, et al. 2002) that inhibits osteoblast activity and is thus deleterious to bone (Wong, et al. 2012). PYY levels are paradoxically higher in girls with AN than controls (Misra, et al. 2006a) and therefore may not be adaptive to the low energy state. In addition, in contrast to many endocrine abnormalities which reverse with weight gain, PYY levels may be persistently abnormal even with weight recovery. Higher PYY levels are an independent predictor of lower levels of bone turnover markers in adolescents and lower bone density measures in adults with AN (Misra et al. 2006a; Utz, et al. 2008).
Other Hormones
Oxytocin is now known to have anorexigenic and bone anabolic effects (Tamma, et al. 2009), and oxytocin levels are lower in women with AN than controls, and are associated with lower BMD (Lawson, et al. 2011a).
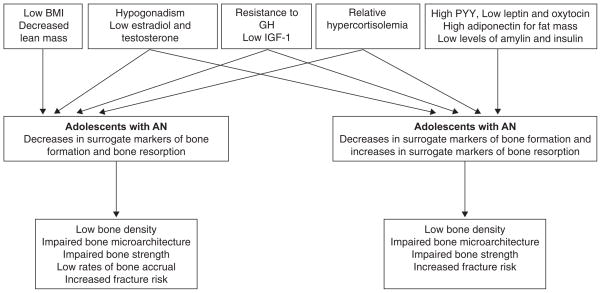
Figure 6 summarizes the various hormonal contributions to low bone density in AN.
Figure 6.
Pathophysiology contributing to impaired bone metabolism in adolescents and adults with anorexia nervosa (Adapted and reprinted with permission from Curr Opin Endocrinol Diabetes Obes, 2011; 2011;18(6):376–82. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins.
Treatment Strategies to Optimize Bone Density in Adults and Bone Accrual in Adolescents
All individuals with AN should be assessed for bone density by DXA at diagnosis and at periodic intervals thereafter depending on disease course. However, DXA results should be adjusted for body size and interpreted carefully based on guidelines from the International Society of Clinical Densitometry (Lewiecki et al. 2008; Schousboe et al. 2013). The future role of clinical role of assessment of bone microarchitecture and strength remains to be determined, but is likely important based on the poor correlation of areal BMD measures with fractures. Management strategies include behavioral modifications, hormone replacement, and pharmacological therapy.
Weight Gain and Restoration of Menstrual Function
The most important and most effective strategy to improve bone density in AN is normalization of weight and restoration of menstrual function. We have reported increases in spine and hip BMD by 3.1 and 1.8% following weight gain and menstrual recovery in adult women with AN (Miller et al. 2006). Adolescents with AN also have an improvement in bone density measures following weight gain and menses restoration, although complete catch-up does not occur (Misra et al. 2008b). This may be because of intermittent relapses or because of persistence of certain hormonal alterations (such as hypercortisolemia (Misra et al. 2004b)) that are deleterious to bone.
Calcium and Vitamin D Supplementation
Because calcium and vitamin D intake is typically higher in girls and women with AN than in controls (Hadigan et al. 2000; Misra et al. 2006b), calcium and vitamin D supplementation alone is not effective in increasing bone density in this condition (Klibanski, et al. 1995; Soyka et al. 2002). However, given the known beneficial effects of calcium and vitamin D (which increases gut absorption of calcium) on bone mineralization, it is important to optimize intake of these micronutrients in AN if suboptimal.
Hormonal Strategies
An important consideration in managing low bone density in AN is the replacement of hormones that are low in AN, such as the gonadal steroids and IGF-1. Other hormones that are low in AN and contribute to low BMD include insulin, leptin, amylin and oxytocin. However, replacement of these hormones is not currently indicated because of associated deleterious effects, such as hypoglycemia with amylin, and because of the anorexigenic effects of leptin. Increases in cortisol, adiponectin and PYY are more challenging to address. Medications that reduce cortisol levels are associated with various adverse effects including the risk of hypocortisolemia, and there are currently no PYY or adiponectin antagonists available for use in humans.
Gonadal Steroid Replacement
Several studies have examined the effects of estrogen replacement on bone density in adults and adolescents with AN. Most studies have demonstrated that oral estrogen replacement as the estrogen-progesterone combination pill is not effective in increasing bone density in AN in adults or adolescents (Golden, et al. 2002; Klibanski et al. 1995; Strokosch, et al. 2006). This has variably been attributed to the IGF-1 suppressive effects of oral estrogen from its hepatic first pass effects, and to the dose and type of estrogen used. In contrast, we have demonstrated in an 18-month randomized controlled trial (RCT) that physiologic estrogen replacement as transdermal 17-β-estradiol, which is not IGF-1 suppressive, with cyclic progesterone, increases bone accrual rates at the spine and hip in adolescents with AN to approximate rates in normal-weight controls, even after controlling for age and weight changes over time (Misra et al. 2011) (Figure 7). This results in the maintenance of BMD Z-scores in girls with AN, whereas there is a decrease in BMD Z-scores over time in girls with AN randomized to placebo. However, physiologic estrogen replacement does not lead to an increase in BMD Z-scores; thus ‘catch-up’ does not occur, likely because other hormonal alterations persist.
Figure 7.
Impact of physiologic estrogen replacement on bone density in adolescent girls with anorexia nervosa (AN). Girls with AN randomized to physiologic estrogen administration (AN-E+) had significant increases in bone density at the lumbar spine over 6, 12 and 18 months compared with those randomized to placebo (AN-E−), to approximate bone accrual rates observed in controls (C) (adjusted for baseline age and weight). From Misra et al. J Bone Mineral Metab. 26; 2430–2438; 2011. Copyright © by The American Society for Bone and Mineral Research, 2011.
Women with AN are also deficient in testosterone, and in adolescent girls with AN, we have shown that increases in testosterone levels following weight gain are strongly predictive of increases in bone density (Soyka et al. 2002). However, testosterone replacement using the low dose patch to attain testosterone levels in the upper half of the normal range was not effective in increasing bone density over a one-year period in adults with AN, despite increases in lean mass and an initial increase in bone formation markers (Miller, et al. 2011).
Finally, some (Gordon, et al. 1999), though not all (Soyka et al. 2002), studies have reported low levels of the adrenal hormones, DHEA/S, in women with AN, and one study reported maintenance of BMD Z-scores (Divasta, et al. 2012) and an improvement in femoral cross sectional area, section modulus, and cortical thickness (by hip structural analysis) (DiVasta, et al. 2014a) using a combination of oral estrogen-progesterone (anti-resorptive) and DHEA (weakly bone anabolic) for 18-months in young women with AN.
Low testosterone levels are an important determinant of low bone density in young men and boys with AN (Misra et al. 2008a), suggesting a role for testosterone replacement in males with this disorder. However, data are lacking regarding the effects of testosterone replacement on bone in males with AN.
Supraphysiological Doses of Recombinant Human Growth Hormone or IGF-1 Replacement
Women and girls with AN are in a state of GH resistance with high concentrations of GH, but low levels of IGF-1 (Argente et al. 1997; Misra et al. 2003a; Scacchi et al. 1997; Stoving et al. 1999). We performed a RCT to determine whether administration of supraphysiologic doses of rhGH vs. placebo to adult women with AN would be effective in overcoming the state of GH resistance and lead to an increase in IGF-1 levels and bone formation markers (Fazeli et al. 2010b). We found that supraphysiologic doses of rhGH were not effective in increasing IGF-1 levels or levels of bone turnover markers in adult women with AN. Conversely, these high GH doses led to a further reduction in fat mass, likely consequent to the direct lipolytic effects of GH. A better strategy to address the GH resistant state in AN is to administer rhIGF-1 to normalize IGF-1 levels. Utilizing this strategy, we have demonstrated that rhIGF-1 replacement in the short-term leads to an increase in bone formation markers in adults and adolescents with AN (Grinspoon et al. 1996; Misra, et al. 2009), and when given for 9-months with oral estrogen-progesterone is effective in increasing bone density significantly in adult women with AN compared with double placebo (Grinspoon et al. 2002) (Figure 8). We are currently conducting an RCT to determine whether addition of rhIGF-1 to transdermal estradiol replacement (vs. transdermal estradiol alone) is effective in increasing BMD Z-scores in adolescent girls with AN, and whether this will enable them to ‘catch-up’ such that their BMD Z-scores approximate that in normal-weight healthy controls.
Figure 8.
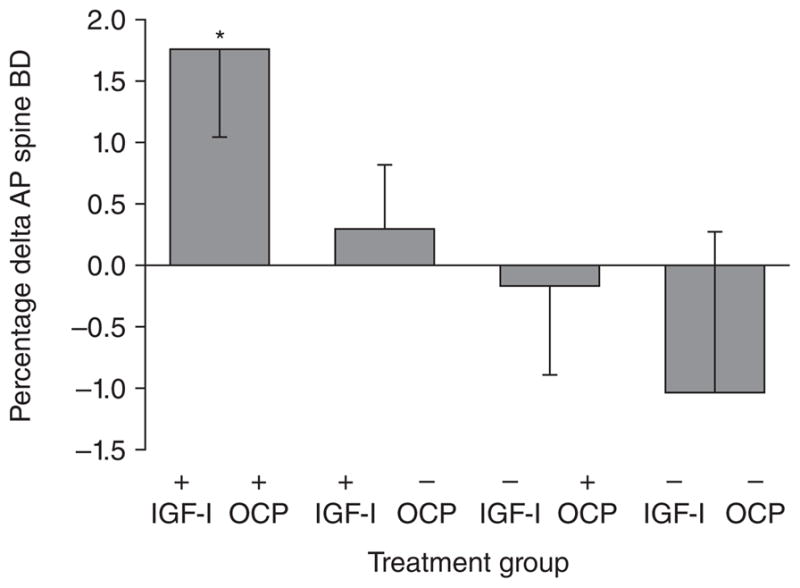
Effect of rhIGF-I +/− OCP on BMD. Administration of recombinant human insulin like growth factor-I (IGF-I) (30 mcg/kg b.i.d. subcutaneously) increased bone density in adult women with anorexia nervosa. The effect was most marked when IGF-I was given with a birth control pill (Reprinted with permission from the Journal of Clinical Endocrinology and Metabolism, 2002;87:2888. Copyright © 1999 The Endocrine Society. All rights reserved)
Recombinant Human Leptin
Because individuals with AN are leptin deficient and leptin has bone anabolic, a potential strategy to improve bone density in AN is to administer rh-leptin. This strategy is intriguing because metreleptin administration has been demonstrated to also restore menses in 60–70% of normal weight women with hypothalamic amenorrhea because of its effects on kisspeptin neurons and therefore GnRH pulsatility (Sienkiewicz, et al. 2011; Welt, et al. 2004). In fact, a study has examined effects of metreleptin administration on bone in a 9-month RCT in adult women with hypothalamic amenorrhea, and reported a significant increase in bone mineral content (but not BMD) with metreleptin (Sienkiewicz et al. 2011). However, leptin administration leads to reductions in appetite and significant reductions in body weight and fat mass (Sienkiewicz et al. 2011; Welt et al. 2004), which would be of major concern in AN.
Other Therapeutic Options for Treating Low Bone Density
Other options to treat low bone density in AN include pharmacological therapies such as bisphosphonates, teriparatide and denosumab.
Biphosphonates
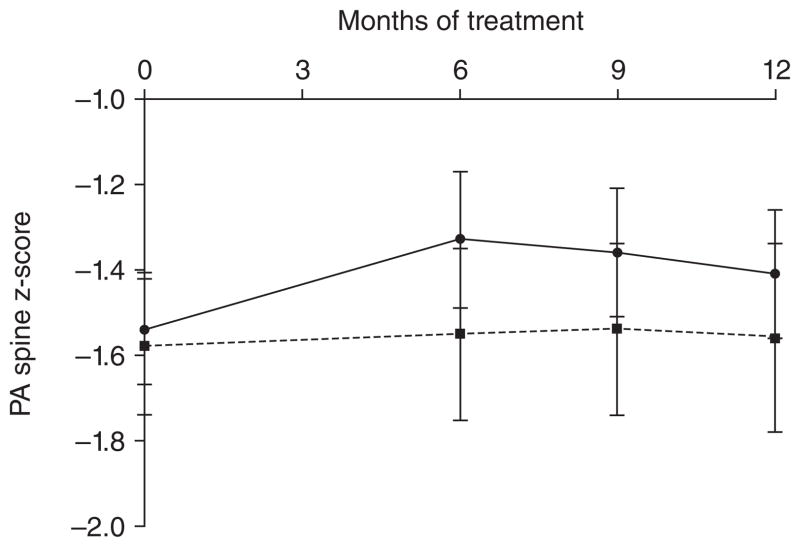
These drugs inhibits osteoclastic bone resorption, and we have reported a significant increase in BMD in adult women with AN with risedronate compared with placebo in a one-year RCT (Miller et al. 2011) (Figure 9). In this study, spine and hip BMD increased by 3% and 2% in women with AN following use of risedronate. However, in adolescents with AN, a one-year RCT of alendronate vs. placebo reported no improvement in spine BMD with alendronate (Golden, et al. 2005). These differing results in adults vs. adolescents with AN may reflect differences in bone turnover in the two age groups. Whereas bone resorption is increased in adult women with AN (Golden et al. 2005) (which would suggest that anti-resorptive therapies such as bisphosphonates may be effective in improving bone density), bone resorption is decreased in adolescents with AN with an overall reduction in bone turnover (Misra et al. 2011; Misra, et al. 2003b). Thus, further reductions in bone turnover with bisphosphonates may not be as effective in improving bone density in an adolescent population. Also, bisphosphonates have an extraordinarily long half-life, which has raised concerns regarding their use in adolescents and young women of reproductive age. At this time, bisphosphonate use should be limited to women with osteoporosis who are having fractures, and should not be used for low bone density alone.
Figure 9.
PA spine BMD increased in women receiving risedronate (solid line) over a 12-month period compared with those receiving placebo (dotted line) (P< 0.0001). Z-scores are shown. (Reprinted with permission from the J Clin Endocrinol Metab. 2011 July; 96(7): 2081–2088. Copyright © 2011 The Endocrine Society. All rights reserved).
Teriparatide
Teriparatide is known to be effective in increasing bone density in post-menopausal women, and we have recently reported an increase in spine BMD following 6-months of teriparatide vs. placebo in an RCT in older adult women with AN (Fazeli, et al. 2014). This was the first study to show the effectiveness of teriparaptide in this population and after only 6 months had an effect much greater than all prior therapies used to increase bone mass in this population. Teriparatide, however, has a black box warning related to reports of osteosarcoma in animal studies, and should not be used in those with an increased baseline risk of osteosarcoma, such as children with open epiphyses, individuals with unexplained elevations of ALP, Paget’s disease, a prior history of external beam radiation therapy or implant radiotherapy of the skeleton (Product Information: FORTEO(R). Eli Lilly and Company, Indianapolis, IN, 2004).
Denosumab
Denosumab is effective in treating post-menopausal osteoporosis (Diab and Watts 2013a, b). However, at this time, no data are available regarding use of denosumab in AN.
General Guidelines for Treating Low Bone Density in Anorexia Nervosa
All individuals with AN should have a structured treatment team that includes (at the very least) a therapist, nutritionist and a physician who is an eating disorder specialist. Every effort should be made to optimize caloric intake and thus induce weight gain and restoration of menses. Family based therapies and cognitive behavioral therapy should be implemented as necessary.
In addition, all individuals with AN should be supplemented with calcium and vitamin D such that they meet the RDA for these micronutrients (1300 mg of elemental calcium and 600 IU of vitamin D). While it is important to optimize calcium and vitamin D intake, patients should be cautioned that supplementation alone will not be effective in increasing bone density in this condition.
Pharmacological therapy may be considered in women with AN with low bone density and a clinically significant fracture history (per ISCD guidelines) (Lewiecki et al. 2008) if weight gain strategies are not effective despite best efforts. In addition, hormone replacement therapy using transdermal 17β-estradiol (100 mcg daily) with cyclic progesterone (micronized progesterone 100–200 mg daily for 12 days of every month) (Misra et al. 2011) may be considered in adolescents with AN whose bone density Z-scores are low and are decreasing over time despite all efforts at weight gain, given that the adolescent years are a very narrow window in time during which to optimize bone accrual, and deficits incurred at this time may be permanent.
Conclusion
Low bone density is an important consequence of AN in adults and adolescents and affects both sexes. Important causes of low bone density include body composition changes and hormonal alterations. The most important strategy to improve bone density in AN is weight gain and menstrual recovery. However, this can be difficult to attain in some women, leading to persistent reductions in bone density over time. Physiologic estrogen replacement (with cyclic progesterone) has been demonstrated to be effective in increasing bone accrual rates in adolescents with AN and to maintain bone density Z-scores, although ‘catch-up’ does not occur. Bisphosphonates are effective in increasing bone density in adult women with AN, but must be used with caution in women of reproductive age given their very long half-life and potential for teratogenicity. These medications should be reserved for women with low bone density and a clinically significant fracture history when weight gain strategies are ineffective despite best efforts. Studies are ongoing to determine the impact of other therapeutic strategies to improve bone density in adults and adolescents with AN.
Acknowledgments
This work was supported in part by NIH grants 1 R01 HD060827, 5 UL1 RR025758, K24HD071843 and 2 RO1 DK062249
Footnotes
The authors have no financial conflicts of interest to disclose
References
- Amitani H, Asakawa A, Ogiso K, Nakahara T, Ushikai M, Haruta I, Koyama K, Amitani M, Cheng KC, Inui A. The role of adiponectin multimers in anorexia nervosa. Nutrition. 2013;29:203–206. doi: 10.1016/j.nut.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Andersen AE, Holman JE. Males with eating disorders: challenges for treatment and research. Psychopharmacol Bull. 1997;33:391–397. [PubMed] [Google Scholar]
- Argente J, Caballo N, Barrios V, Munoz MT, Pozo J, Chowen JA, Morande G, Hernandez M. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab. 1997;82:2084–2092. doi: 10.1210/jcem.82.7.4090. [DOI] [PubMed] [Google Scholar]
- Bachrach L. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001;12:22–28. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86:440–447. [PubMed] [Google Scholar]
- Baker D, Roberts R, Towell T. Factors predictive of bone mineral density in eating-disordered women: a longitudinal study. Int J Eat Disord. 2000;27:29–35. doi: 10.1002/(sici)1098-108x(200001)27:1<29::aid-eat3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Biller B, Saxe V, Herzog D, Rosenthal D, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989a;68:548–554. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989b;68:548–554. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- Biver E, Salliot C, Combescure C, Gossec L, Hardouin P, Legroux-Gerot I, Cortet B. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2703–2713. doi: 10.1210/jc.2011-0047. [DOI] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Freedman LM, Calder G, Lee H, Rosen CJ, Klibanski A. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97:E584–590. doi: 10.1210/jc.2011-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Misra M, Miller KK, Madisch I, Sarwar A, Cheung A, Klibanski A, Gupta R. Distal radius in adolescent girls with anorexia nervosa: trabecular structure analysis with high-resolution flat-panel volume CT. Radiology. 2008;249:938–946. doi: 10.1148/radiol.2492080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Lazaro L, Pons F, Halperin I, Toro J. Predictors of bone mineral density reduction in adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2000;39:1365–1370. doi: 10.1097/00004583-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Diab DL, Watts NB. Denosumab in osteoporosis. Expert Opin Drug Saf. 2013a doi: 10.1517/14740338.2014.860133. [DOI] [PubMed] [Google Scholar]
- Diab DL, Watts NB. Postmenopausal osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2013b doi: 10.1097/01.med.0000436194.10599.94. [DOI] [PubMed] [Google Scholar]
- DiVasta AD, Feldman HA, Beck TJ, LeBoff MS, Gordon CM. Does hormone replacement normalize bone geometry in adolescents with anorexia nervosa? J Bone Miner Res. 2014a;29:151–157. doi: 10.1002/jbmr.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism. 2012;61:1010–1020. doi: 10.1016/j.metabol.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divasta AD, Feldman HA, Gordon CM. Vertebral fracture assessment in adolescents and young women with anorexia nervosa: a case series. J Clin Densitom. 2014b;17:207–211. doi: 10.1016/j.jocd.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, del Rio L, Setoain J, Granados A. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int. 2001;12:811–822. doi: 10.1007/s001980170031. [DOI] [PubMed] [Google Scholar]
- Faje AT, Fazeli PK, Katzman D, Miller KK, Breggia A, Rosen CJ, Mendes N, Misra M, Klibanski A. Inhibition of Pref-1 (preadipocyte factor 1) by oestradiol in adolescent girls with anorexia nervosa is associated with improvement in lumbar bone mineral density. Clin Endocrinol (Oxf) 2013a;79:326–332. doi: 10.1111/cen.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, Mendes N, Snelgrove D, Meenaghan E, Misra M, et al. Fracture Risk and Areal Bone Mineral Density in Adolescent Females with Anorexia Nervosa. Int J Eating Disord. 2014 doi: 10.1002/eat.22248. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, Meenaghan E, Goldstein MA, Bouxsein ML, Misra M, et al. Adolescent Girls With Anorexia Nervosa Have Impaired Cortical and Trabecular Microarchitecture and Lower Estimated Bone Strength at the Distal Radius. J Clin Endocrinol Metab. 2013b doi: 10.1210/jc.2012-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, Rosen CJ, Klibanski A. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012;27:1864–1871. doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010a;95:407–413. doi: 10.1210/jc.2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PK, Lawson EA, Prabhakaran R, Miller KK, Donoho DA, Clemmons DR, Herzog DB, Misra M, Klibanski A. Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2010b;95:4889–4897. doi: 10.1210/jc.2010-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PK, Wang IS, Miller KK, Herzog DB, Misra M, Lee H, Finkelstein JS, Bouxsein ML, Klibanski A. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2013-4105. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, Hertz S, Shenker IR. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:3179–3185. doi: 10.1210/jc.2004-1659. [DOI] [PubMed] [Google Scholar]
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135–143. doi: 10.1016/s1083-3188(02)00145-6. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Grace E, Emans SJ, Goodman E, Crawford MH, Leboff MS. Changes in bone turnover markers and menstrual function after short-term oral DHEA in young women with anorexia nervosa. J Bone Miner Res. 1999;14:136–145. doi: 10.1359/jbmr.1999.14.1.136. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3864–3870. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- Hadigan CM, Anderson EJ, Miller KK, Hubbard JL, Herzog DB, Klibanski A, Grinspoon SK. Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. Int J Eat Disord. 2000;28:284–292. doi: 10.1002/1098-108x(200011)28:3<284::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Hotta M, Shibasaki T, Sato K, Demura H. The importance of body weight history in the occurrence and recovery of osteoporosis in patients with anorexia nervosa: evaluation by dual X-ray absorptiometry and bone metabolic markers. Eur J Endocrinol. 1998;139:276–283. doi: 10.1530/eje.0.1390276. [DOI] [PubMed] [Google Scholar]
- Housova J, Anderlova K, Krizova J, Haluzikova D, Kremen J, Kumstyrova T, Papezova H, Haluzik M. Serum adiponectin and resistin concentrations in patients with restrictive and binge/purge form of anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2005;90:1366–1370. doi: 10.1210/jc.2004-1364. [DOI] [PubMed] [Google Scholar]
- Jagielska G, Wolanczyk T, Komender J, Tomaszewicz-Libudzic C, Przedlacki J, Ostrowski K. Bone mineral density in adolescent girls with anorexia nervosa--a cross-sectional study. Eur Child Adolesc Psychiatry. 2002;11:57–62. doi: 10.1007/s007870200011. [DOI] [PubMed] [Google Scholar]
- Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26:618–623. doi: 10.1007/s00774-008-0861-5. [DOI] [PubMed] [Google Scholar]
- Kim SW, Her SJ, Park SJ, Kim D, Park KS, Lee HK, Han BH, Kim MS, Shin CS, Kim SY. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone. 2005;37:359–369. doi: 10.1016/j.bone.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Klibanski A, Biller B, Schoenfeld D, Herzog D, Saxe V. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898–904. doi: 10.1210/jcem.80.3.7883849. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, Wexler T, Herzog DB, Klibanski A. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–4716. doi: 10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, Sluss PM, Miller KK, Klibanski A. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry. 2011a;72:1546–1551. doi: 10.4088/JCP.10m06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Eddy KT, Donoho D, Misra M, Miller KK, Meenaghan E, Lydecker J, Herzog D, Klibanski A. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol. 2011b;164:253–261. doi: 10.1530/EJE-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, Rosenblum L, Donoho D, Gupta R, Klibanski A. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone. 2010;46:458–463. doi: 10.1016/j.bone.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Brychta RJ, Collins MT, Linderman J, Smith S, Herscovitch P, Millo C, Chen KY, Celi FS. Cold-activated brown adipose tissue is an independent predictor of higher bone mineral density in women. Osteoporos Int. 2013;24:1513–1518. doi: 10.1007/s00198-012-2110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, Kalkwarf HJ, Langman CB, Plotkin H, Rauch F, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43:1115–1121. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- Lucas AR, Beard CM, O’Fallon WM, Kurland LT. 50-year trends in the incidence of anorexia nervosa in Rochester, Minn.: a population-based study. Am J Psychiatry. 1991;148:917–922. doi: 10.1176/ajp.148.7.917. [DOI] [PubMed] [Google Scholar]
- Lucas AR, Melton LJ, 3rd, Crowson CS, O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999;74:972–977. doi: 10.4065/74.10.972. [DOI] [PubMed] [Google Scholar]
- Mehler PS, Eckel RH, Donahoo WT. Leptin levels in restricting and purging anorectics. Int J Eat Disord. 1999;26:189–194. doi: 10.1002/(sici)1098-108x(199909)26:2<189::aid-eat8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, Gleysteen S, Mickley D, Herzog D, Klibanski A. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91:2931–2937. doi: 10.1210/jc.2005-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, Herzog D, Klibanski A. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2081–2088. doi: 10.1210/jc.2011-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milos G, Spindler A, Ruegsegger P, Seifert B, Muhlebach S, Uebelhart D, Hauselmann HJ. Cortical and trabecular bone density and structure in anorexia nervosa. Osteoporos Int. 2005;16:783–790. doi: 10.1007/s00198-004-1759-2. [DOI] [PubMed] [Google Scholar]
- Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, Herzog DB, Klibanski A. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004a;114:1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, Goldstein MA, Ebrahimi S, Clauss L, Weigel T, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430–2438. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Katzman DK, Clarke H, Snelgrove D, Brigham K, Miller KK, Klibanski A. Hip structural analysis in adolescent boys with anorexia nervosa and controls. J Clin Endocrinol Metab. 2013;98:2952–2958. doi: 10.1210/jc.2013-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Katzman DK, Cord J, Manning SJ, Mendes N, Herzog DB, Miller KK, Klibanski A. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab. 2008a;93:3029–3036. doi: 10.1210/jc.2008-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, McGrane J, Miller KK, Goldstein MA, Ebrahimi S, Weigel T, Klibanski A. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone. 2009;45:493–498. doi: 10.1016/j.bone.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Miller K, Bjornson J, Hackman A, Aggarwal A, Chung J, Ott M, Herzog D, Johnson M, Klibanski A. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003a;88:5615–5623. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004b;89:4972–4980. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, Katzman DK, Klibanski A. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92:2046–2052. doi: 10.1210/jc.2006-2855. [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Herzog DB, Ramaswamy K, Aggarwal A, Almazan C, Neubauer G, Breu J, Klibanski A. Growth Hormone and Ghrelin Responses to an Oral Glucose Load in Adolescent Girls with Anorexia Nervosa and Controls. J Clin Endocrinol Metab. 2004a;89:1605–1612. doi: 10.1210/jc.2003-031861. [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005a;289:E347–356. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005b;289:E373–381. doi: 10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006a;91:1027–1033. doi: 10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]
- Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008b;93:1231–1237. doi: 10.1210/jc.2007-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Soyka LA, Miller KK, Herzog DB, Grinspoon S, De Chen D, Neubauer G, Klibanski A. Serum osteoprotegerin in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2003b;88:3816–3822. doi: 10.1210/jc.2003-030088. [DOI] [PubMed] [Google Scholar]
- Misra M, Tsai P, Anderson EJ, Hubbard JL, Gallagher K, Soyka LA, Miller KK, Herzog DB, Klibanski A. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. Am J Clin Nutr. 2006b;84:698–706. doi: 10.1093/ajcn/84.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res. 2011;26:27–34. doi: 10.1002/jbmr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Pitukcheewanont P, Kaufman FR, Nelson JC, Gilsanz V. Biochemical markers of bone turnover and the volume and the density of bone in children at different stages of sexual development. J Bone Miner Res. 1999;14:1664–1671. doi: 10.1359/jbmr.1999.14.10.1664. [DOI] [PubMed] [Google Scholar]
- Ponrartana S, Aggabao PC, Hu HH, Aldrovandi GM, Wren TA, Gilsanz V. Brown adipose tissue and its relationship to bone structure in pediatric patients. J Clin Endocrinol Metab. 2012;97:2693–2698. doi: 10.1210/jc.2012-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs B. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106:1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Scacchi M, Pincelli A, Caumo A, Tomasi P, Delitala G, Baldi G, Cavagnini F. Spontaneous nocturnal growth hormone secretion in anorexia nervosa. J Clin Endocrinol Metab. 1997;82:3225–3229. doi: 10.1210/jcem.82.10.4275. [DOI] [PubMed] [Google Scholar]
- Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–1627. [PMC free article] [PubMed] [Google Scholar]
- Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom. 2013;16:455–466. doi: 10.1016/j.jocd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, Arampatzi KM, Gao C, Koniaris A, Mantzoros CS. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60:1211–1221. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Smink FR, van Hoeken D, Hoek HW. Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry. 2013;26:543–548. doi: 10.1097/YCO.0b013e328365a24f. [DOI] [PubMed] [Google Scholar]
- Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, Klibanski A. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- Stoving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, Kristiansen J, Hagen C. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab. 1999;84:2056–2063. doi: 10.1210/jcem.84.6.5734. [DOI] [PubMed] [Google Scholar]
- Strokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double-blind, placebo-controlled study. J Adolesc Health. 2006;39:819–827. doi: 10.1016/j.jadohealth.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Tagami T, Satoh N, Usui T, Yamada K, Shimatsu A, Kuzuya H. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2004;89:1833–1837. doi: 10.1210/jc.2003-031260. [DOI] [PubMed] [Google Scholar]
- Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci U S A. 2009;106:7149–7154. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko P, Bonjour J. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, Klibanski A, Miller KK. Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone. 2008;43:135–139. doi: 10.1016/j.bone.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant Human Leptin in Women with Hypothalamic Amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- Wojcik MH, Meenaghan E, Lawson EA, Misra M, Klibanski A, Miller KK. Reduced amylin levels are associated with low bone mineral density in women with anorexia nervosa. Bone. 2010;46:796–800. doi: 10.1016/j.bone.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong IP, Driessler F, Khor EC, Shi YC, Hormer B, Nguyen AD, Enriquez RF, Eisman JA, Sainsbury A, Herzog H, et al. Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PLoS One. 2012;7:e40038. doi: 10.1371/journal.pone.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel S, Seibel MJ, Lowe B, Beumont PJ, Kasperk C, Herzog W. Osteoporosis in eating disorders: a follow-up study of patients with anorexia and bulimia nervosa. J Clin Endocrinol Metab. 2001;86:5227–5233. doi: 10.1210/jcem.86.11.8050. [DOI] [PubMed] [Google Scholar]