Abstract
Background
The relationship between inadequate sleep and mood has been well-established in adults and is supported primarily by correlational data in younger populations. Given that adolescents often experience shortened sleep on school nights, we sought to better understand the effect of experimentally-induced chronic sleep restriction on adolescents’ mood and mood regulation.
Methods
Fifty healthy adolescents, ages 14 to 17, completed a three-week sleep manipulation protocol involving a baseline week, followed by a sleep restriction (SR) condition (6.5 hours in bed per night for five nights) and healthy sleep duration (HS) condition (10 hours in bed per night for five nights). The study used a randomized, counterbalanced, cross-over experimental design. Participants’ sleep was monitored at home via self-report and actigraphy. At the end of each condition, participants and their parents completed questionnaires of mood and mood regulation. To assess for expectancy effects, we also analyzed parent and teen ratings of hyperactivity/impulsivity, which prior research suggests is not sensitive to SR in adolescents. Wilcoxon Signed Rank tests compared questionnaire outcomes across the two conditions.
Results
Participants averaged 2.5 more hours of sleep per night during HS relative to SR. Compared to HS, adolescents rated themselves as significantly more tense/anxious, angry/hostile, confused, and fatigued, and as less vigorous (p = .001–.01) during SR. Parents and adolescents also reported greater oppositionality/irritability and poorer emotional regulation during SR compared to HS (p < .05). There were no cross condition differences in depression or hyperactivity/impulsivity (p > .05).
Conclusions
Findings complement prior correlational study results to show that after only a few days of shortened sleep, at a level of severity that is experienced regularly by millions of adolescents on school nights, adolescents have worsened mood and decreased ability to regulate negative emotions.
Keywords: Adolescence, sleep, anxiety, mental health, paediatrics
At least half of U.S. adolescents regularly sleep less than eight hours on school nights, generally sleeping one to three fewer hours per night during the school week than during the weekend or school vacations (National Sleep Foundation, 2006). This appears to be the product of early school start times, combined with increasingly later bedtimes in response to biological, psychological, and social development (Carskadon, Wolfson, Acebo, Tzischinsky, & Seifer, 1998; Dahl & Lewin, 2002; Jenni, Achermann, & Carskadon, 2005). While many sleep researchers have framed the resulting short sleep on school nights as psychologically harmful, others have questioned whether there is adequate evidence showing harm (Eide & Showalter, 2012; Matricciani, Olds, Blunden, Rigney, & Williams, 2012). In response, this paper examines the causal impact of multiple nights of experimentally shortened sleep on adolescents’ mood and ability to regulate emotions.
Multiple correlational and experimental studies have established that whole-night sleep deprivation or markedly curtailed sleep across multiple nights (chronic sleep restriction) adversely affects adults’ emotional functioning and self-regulation (Babson, Trainor, Feldner, & Blumenthal, 2010; Dinges et al., 1997; Franzen, Siegle, & Buysse, 2008; Talbot, McGlinchey, Kaplan, Dahl, & Harvey, 2010). Chronic sleep restriction in adults may even affect mood more than cognitive or motor functioning (Pilchur & Huffcutt, 1996). Sleep-deprived adults seem to experience both an increase in negative affect (Dinges et al., 1997; Drake et al., 2001) and a reduction in positive affect (Babson et al., 2010; Franzen et al., 2008; Talbot et al., 2010).
Analogous pediatric studies are fewer and almost exclusively correlational. In school-age children, sleep duration is negatively correlated with behavior and mood problems (Nixon et al., 2008; Paavonen, Porkka-Heiskanen, & Lahikainen, 2009). In adolescents, symptoms of anxiety and depression are positively correlated with reported sleep problems, short sleep on school nights, and a bigger gap between school night and weekend sleep duration (Gregory & O’Connor, 2002; Wolfson & Carskadon, 1998). However, such correlational findings can be confounded, and the direction of causation is not always clear (Beebe, 2011; Cousins et al., 2011; Dahl & Lewin, 2002).
Cause-effect relationships can be more easily inferred from experimental studies. To our knowledge, only one published study has used experimental methods to test the causal impact of short sleep on adolescent mood and emotion regulation. Harvey and Dahl’s group found that sleep restriction, characterized by one night of 6.5 hours in bed at home, followed by a night of two hours of sleep as an inpatient, reduced adolescents’ self-ratings of positive affect, changed their vocal patterns in a manner suggesting increased negative affect, and made them more vulnerable to negative moods in response to a challenge (Dagys et al., 2012; McGlinchey et al., 2011; Talbot et al., 2010). That study was groundbreaking, and its rigorous experimental methods allow for the clear interpretation that the sleep manipulation caused a change in mood and emotion regulation. However, the sleep restriction protocol was quite different from what adolescents experience in normal life with respect to the sleep setting (inpatient vs. home) and the pattern (2 nights vs. 5 consecutive school nights) and severity (2 hours the night before data collection vs. 6–7 hours) of sleep restriction.
A convergence of factors suggests that it is particularly important to understand the relationship between sleep, mood, and emotion regulation during adolescence. The decline in sleep duration on school nights coincides with a developmental period when lifelong psychopathology can emerge (Walker, 2002) and when even healthy adolescents show increased emotional reactivity (Dahl & Gunnar, 2009). In the adolescent brain, the prefrontal-subcortical circuits that govern emotion are rapidly developing (Giedd, 2004; McRae, et al., 2012), and sleep researchers have speculated that chronic sleep restriction can alter that development (Beebe, 2011). On a more observable level, if mild to moderate amounts of inadequate sleep causes negative mood or poor emotion regulation, the resulting effects on adolescents’ behavior (e.g., risk-taking) could have lifelong consequences (Dahl, 2004). Finally, if short sleep on school nights causes harm, adolescence may be the best time to intervene, because school start times can be changed to promote more sleep (Danner & Phillips, 2008; Wahlstrom, 2002).
Even so, critics have questioned the empirical foundation for clinical and public policy interventions around adolescent sleep, noting a lack of experimental studies with findings that readily generalize to real-world settings (Eide & Showalter, 2012; Matricciani et al., 2012). These critics are well-publicized; over a year after the publication of those papers, parents searching the internet for “How much sleep does an adolescent need” could prominently find a news story from a respected national broadcast network that responds “Maybe less than you think” (Rubin, 2012). These critics also raise a valid concern: gaps in our science leave health professionals and the public alike relying heavily upon indirect evidence and opinion for guidance on adolescent sleep needs.
This study seeks to fill some of these gaps by examining the impact of 5 nights of experimental sleep restriction, compared to more optimal sleep duration, on the mood and emotion regulation of healthy adolescents. The protocol was designed to maximize real-world generalizability by mimicking the pattern and severity of sleep restriction experienced by many high school students on school nights (Beebe et al., 2008), and by asking parents and adolescents to rate the teen’s functioning in daily life. At the same time, we used a randomized, cross-over experimental design to promote the ability to draw confident conclusions about causation. Furthermore, because neither the adolescent participants nor their parents could be blinded to the sleep manipulation, we looked for potential expectancy effects by including a non-mood scale that we did not expect to be impacted by the sleep manipulation. Specifically, in addition to mood and mood regulation, we measured hyperactivity/impulsivity because (a) hyperactivity has been widely identified as a potential symptom of inadequate sleep in younger children (Beebe, 2011) and (b) poor regulation of behavioral impulses is superficially similar to poor regulation of emotions and therefore could be affected by similar response biases, yet (c) in reality, hyperactivity and impulsivity appear not to be related to sleep-disordered breathing or sleep restriction in adolescents (Beebe et al., 2008; Beebe, Ris, Kramer, Long, & Amin, 2010).
In this study, adolescents and their parents reported on the adolescents’ mood, emotion regulation, and hyperactivity-impulsivity in the context of several nights of 6.5 hours in bed (sleep restriction; SR) versus 10 hours in bed (healthy sleep; HS). We hypothesized that, during SR, teens would show more anxiety, depression, hostility, and fatigue. It was further hypothesized that teens would display poorer emotion regulation during SR than when well-rested. We expected no impact of the sleep manipulation on hyperactivity/impulsivity.
Methods
Participants
Participants were healthy adolescents, aged 14–17.9 years, who did not have a professionally-diagnosed psychiatric disorder (per parent- and self-report), a history of neurological illness or injury, a body mass index (BMI) greater than 30, an IQ score less than 70, high daily caffeine intake (greater than one coffee or energy drink or two caffeinated soft drinks), parent-suspected recurrent illegal substance use, use of medication with known effects on sleep or daytime alertness, or obligations that would require a bedtime later than 10:00pm or a wake time prior to 6:00am. Participants had to agree to refrain from driving during the week of SR. Families were recruited through flyers posted throughout a regional pediatric medical center, with particular emphasis on recruitment in a clinic that serves low-income adolescents, who are often under-recruited in research. An initial telephone screening was completed, followed by in-person verification of eligibility during the study’s baseline week.
Procedures
All study procedures were approved by the Institutional Review Board at CCHMC. Adolescent participants provided informed assent and their parents provided informed consent. The sleep manipulation protocol used in the present study was first detailed by Beebe et al. (2008) and was also used in a new sample (Beebe et al., 2013). Briefly, study participants were involved in a three-week, multi-night sleep manipulation protocol (see Figure 1) that occurred during the summer break from school to avoid any effects of the manipulation on scholastic performance. All sleep was obtained in the home environment and was monitored via sleep diaries and actigraphy.
Figure 1.
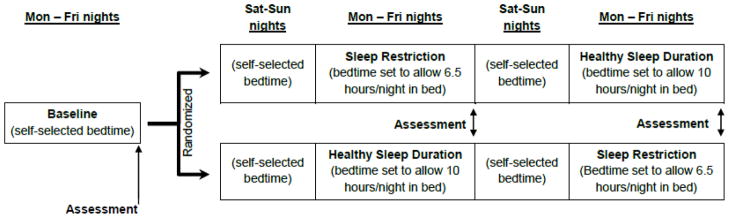
Design chart of sleep manipulation study design
At the time of initial enrollment, teens and parents were asked the time the teen would need to awaken to arrive at the research location by 8:30am; their response determined the teen’s wake time throughout the study. During the first (baseline) week, teens were asked to awaken at that predetermined time, but were permitted to self-select their bedtimes. Participants who were found to be unable to consistently rise at the prescribed time were dropped prior to randomization.
During the subsequent two weeks, eligible teens were asked to systematically change their bedtimes to accommodate SR and HS conditions. A within-subjects, randomized cross-over design was used, such that all teens who completed the study were seen in both the SR and HS conditions, with the order of conditions counterbalanced across participants. Adolescents adjusted their bedtime on weeknights to allow for 6.5 hours in bed during the SR condition and 10 hours in bed during the HS condition. A nightly sleep opportunity of 10 hours has been shown to promote a well-rested state in adolescents (Beebe et al., 2008; Carskadon et al., 1980; Fallone, Acebo, Seifer, & Carskadon, 2005). Teens were asked to be fully ready for bed, with lights out and all electronics with screens turned off, and parents were asked to support their teens in achieving this goal. The 6.5 hour requirement for SR is in line with Beebe et al. (2008) and Fallone et al. (2005), who reported that this level of sleep restriction is feasible to sustain across five nights and induces measurable sleepiness in adolescents. Further, 6.5 hours in bed a night is similar to what roughly 20% of adolescents report occurring on school nights (Eide & Showalter, 2012; National Sleep Foundation, 2006). Saturdays and Sundays were a two-night wash-out period, where teens were required to rise at their prescribed times, but could self-select their bedtimes.
Participants were instructed not to nap and to consume no more than one coffee or energy drink, or no more than two caffeinated sodas per day. Sleep was monitored throughout the study, while primary data collection occurred at the Saturday morning sessions following each experimental condition.
Measures
Demographics and Screening
During the baseline week visit, parents provided demographic information via questionnaires, and participant eligibility was confirmed via testing with the Kaufman Brief Intelligence Test, Second edition (Kaufman & Kaufman, 2004) and a brief physical examination that included screening questions and anthropometry.
Sleep monitoring
Throughout the study, participants wore an actigraph (Micro Motionlogger SleepWatch®, Ambulatory Monitoring, Inc.; AMI) and reported their bedtime, sleep onset latency, and wake time on a daily sleep diary. The actigraph was worn on the non-dominant wrist, functioning outwardly as a simple wristwatch while collecting data on movement patterns. Default settings were used to collect data in “zero crossing” mode. Sleep-wake estimates derived from AMI hardware and algorithms have >90% agreement with EEG-defined sleep parameters in healthy adolescents (Sadeh, Sharkey, & Carskadon, 1994). Participants were asked to wear them at all times except during participation in contact sports, bathing/showering, and swimming. Trained coders uploaded the actigraph data weekly and, with the support of the sleep diaries and follow-up queries to participants and parents, identified artifact-free periods for processing via AMI software. Sleep onset, offset, and duration were the primary sleep monitoring outcomes. The four participants whose actigraphs did not show an average of at least one hour longer nightly sleep during HS than SR were considered non-adherent to the protocol and dropped from analyses.
Mood
At each Saturday assessment, adolescents completed the Profile of Mood States (POMS; McNair, Lorr, & Droppelman, 1971), a well-validated, 65-item self-report measure comprised of six subscales: Tension/Anxiety, Depression/Dejection, Anger/Hostility, Energy/Activity, Fatigue/Vigor, and Confusion/Bewilderment. Adolescents reported on their mood “over the past 3 days, including today” using a 5-point scale (“Not at all” to “Extremely”).
Secondary mood measures were derived from short forms based upon the Vanderbilt Assessment Scale (Wolraich et al., 2003), a validated parent-report measure that assesses symptoms of common childhood psychiatric disorders, each rated on a 4-point scale (“Never” to “Very Often”). As described by Beebe et al. (2008), a parallel self-report version was created by making minor wording changes (e.g., “your behaviors” versus “your child’s behaviors”). For validation purposes, during the baseline assessment, parents and adolescents completed the original Vanderbilt, which asks about behaviors “over the past six months,” and a short form version that inquired about “the past five days, including today.” Only the short form versions were administered following the two conditions. For this paper, two subscales were used: the 8-item Oppositionality/Irritability (anger, arguing with adults, blaming others for one’s own mistakes, and spitefulness), and 7-item Anxiety/Depression subscale (fear, worry, guilt, sadness, and feelings of worthlessness).
Emotion Regulation
To assess emotion regulation, three items were added to the short forms. These items, which assessed how easily upset the adolescent was and whether emotional reactions were unprovoked or disproportionately large, were validated during the baseline week against the Emotion Control subscale of the parent- and self-report Behavior Rating Inventory of Executive Functioning (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000; Guy, Isquith, & Gioia, 2004). Because of its sensitivity to real-world executive functioning difficulties, the BRIEF has been widely adopted within the pediatric neuropsychological community (c.f., Baron, 2004). The Emotional Control subscale on both the parent- and self-report BRIEF is psychometrically strong (Gioia et al., 2000; Guy et al., 2004), but asks about a longer (6-month) timeframe than our 5-night sleep manipulation period.
Hyperactivity/Impulsivity
Parents and adolescents completed short-form versions of the Hyperactivity/Impulsivity subscale from the Vanderbilt, which inquires about nine hyperactive and impulsive symptoms of attention-deficit/hyperactivity disorder (Beebe et al., 2008; Wolraich et al., 2003). Beebe et al. (2008) found that, although this short-form scale had good psychometric support, it was not sensitive to sleep restriction in adolescents.
Statistical analyses
Preliminary analyses
Prior to analyses, the distributions of all variables were inspected. The sleep variables were roughly normal in distribution, but several of the mood and mood regulation variables were highly skewed (absolute value of skew > 2 and more than twice the standard error of skew) and could not be readily transformed to approximate the normal distribution. Sample descriptives are presented in the form of mean ± standard deviation for continuous, normally-distributed variables, as median (25th, 75th percentile) for non-normal continuous variables, and as percentages for categorical data. The adequacy of our outcome measures was assessed via coefficient alpha (internal consistency) at all assessment points, as well as Spearman correlations between short-form scales and their respective validation measures at the baseline assessment. Sleep onset, offset, and duration were roughly normal in distribution, and compared across conditions using paired-sample t tests.
Primary and exploratory analyses
Wilcoxon Signed Rank Tests compared the SR and HS conditions on the six adolescent-report POMS subscales, and the parent and adolescent-report Oppositionality/Irritability, Anxiety/Depression, and Emotion Regulation subscales, as well as the Hyperactivity/Impulsivity subscale. In all cases, paired-sample t tests also yielded similar findings (not presented for parsimony). An alpha threshold of .05 was adopted.
Interpretation of the primary analyses could be complicated by period effects (i.e., if simply repeating the measures caused an effect) or carryover effects (i.e., the effect of the sleep manipulation differed depending on which sleep condition came first). To explore possible period effects, we repeated our primary analyses, substituting experimental week for sleep condition as the independent variable. To explore possible carryover effects and moderation based upon demographic factors, we first computed difference scores for each dependent variable across the two sleep manipulation conditions (e.g., POMS anxiety during HS minus POMS anxiety during SR). We then used a series of independent-sample Wilcoxon tests to assess whether these difference scores were systematically associated with the order of sleep conditions (HS vs. SR first), sex, or race (white vs. non-white). To assess whether these difference scores were systematically associated with the continuous variables of age and sleep duration in each condition, we conducted Spearman correlations.
Results
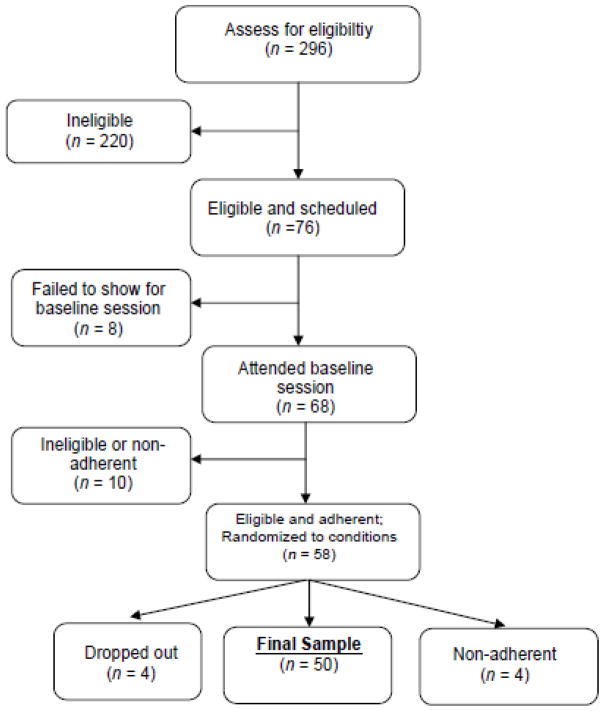
Figure 2 provides a consort diagram. Of the 68 adolescents who attended the baseline session, 10 did not meet eligibility criteria or were non-adherent to the wake time instructions for the baseline week, so they were not randomized to the experimental conditions. Of those randomized, four dropped out before completion, and four were non-adherent to the sleep regimen. The final sample of 50 was 15.5 ± 0.9 years old, had a median family income of $50–60,000 USD, was 50% female, and 52% self-identified as Caucasian, 40% as African-American, and 6% as Multiracial.
Figure 2.
Consort chart outlining participant recruitment
As shown in Table 1, all outcome measures showed good to strong internal consistency at all three measurement points. The short-form subscales created for this study were also significantly, but moderately correlated with their respective validation measures, which is appropriate given the different time-frames for ratings (past 6 months versus past 5 days; c.f. Beebe et al., 2008). Descriptive data on baseline sleep, POMS, and short-form mood measures are reported in Table S1 (see online appendix). We caution against overinterpreting these data because of the circadian shifts that often occurred during the baseline week. The primary goal of the baseline week was to allow sufficient time for teens to adjust their summer sleep schedules (which are often phase-delayed) to align with a schedule that better resembles sleep during the school year. In the process, many teens appeared to experience a temporary “jet lag” during the baseline week that is idiosyncratic to the study and not necessarily representative of teens’ “normal” sleep on school nights or non-school nights.
Table 1.
Internal Consistency Reliability of Outcome Measures and Correlation of Short-Form Subscales against Validation Measures at the Baseline Assessment
| Correlation with validation questionnaire | Internal Consistency (α) | |||
|---|---|---|---|---|
|
| ||||
| Baseline | Week 2 | Week 3 | ||
| Self-report | ||||
| POMS Tension/Anxiety | n/a | .83 | .79 | .87 |
| POMS Depression/Dejection | n/a | .93 | .93 | .95 |
| POMS Anger/Hostility | n/a | .89 | .91 | .88 |
| POMS Vigor/Activity | n/a | .86 | .78 | .85 |
| POMS Fatigue/Inertia | n/a | .84 | .83 | .81 |
| POMS Confusion/Bewilderment | n/a | .66 | .64 | .50 |
| Short form Anxiety/Depression | .68 | .86 | .81 | .83 |
| Short form Oppositionality/Irritability | .73 | .87 | .87 | .84 |
| Short form Emotion Regulation | .52 | .87 | .67 | .86 |
| Short form Hyperactive/Impulsive | .77 | .80 | .83 | .87 |
| Parent Report | ||||
| Short form Anxiety/Depression | .65 | .86 | .72 | .83 |
| Short form Oppositionality/Irritability | .68 | .92 | .83 | .90 |
| Short form Emotion Regulation | .44 | .90 | .67 | .92 |
| Short form Hyperactive/Impulsive | .61 | .71 | .78 | .82 |
Note: POMS = Profile of Mood States
Adolescents obtained 2.5 ± 0.7 more hours of sleep per night during the HS condition as compared to the SR condition (p < .001; range = 1.0 – 4.1 hours). Actigraphy data showed that adolescents slept 8.8 ± 0.9 hours per night when in the HS condition, compared to 6.3 ± 0.5 hours of sleep obtained during SR. Consistent with the prescribed sleep schedule, the difference in sleep duration was due to significant differences in sleep onset time (HS = 10:20 ± 0:51 pm, SR = 12:50 ± 0:48 am, p < .001), whereas wake time was unchanged across the two experimental conditions (HS = 7:09 ± 0:25 am, SR = 7:07 ± 0:36 am, p > .05).
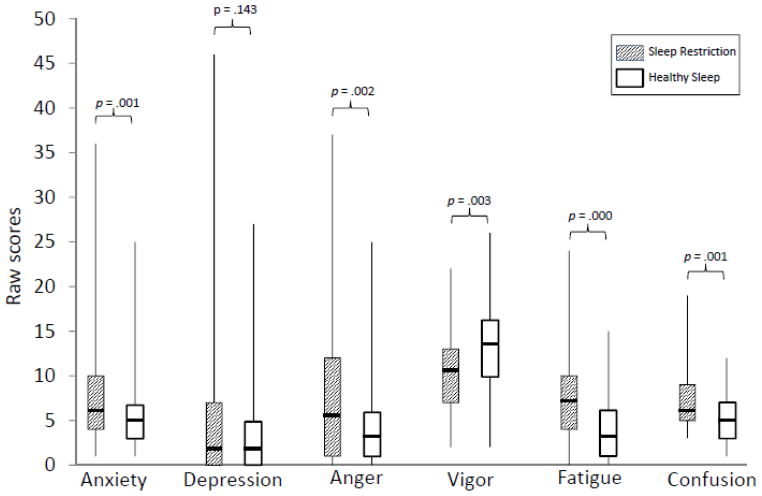
Results of the cross-condition comparisons on the POMS subscale scores are illustrated in Figure 3. Compared to HS, during SR adolescents rated themselves as having increased levels of Tension/Anxiety (Z = −3.25, p = .001), Anger/Hostility (Z = −3.10, p = .002), Fatigue/Inertia (Z = −4.78, p < .001), and Confusion/Bewilderment (Z = −3.37, p = .001), and significantly less vigor (Z = −3.02, p = .003). There were no significant differences across conditions on the Depression/Dejection subscale, p > .05.
Figure 3.
Self-reports on Profile of Mood States (POMS) by condition
*Median scores indicated by solid line; the Vigor subscale is reverse scored, with higher scores reflecting more vigor/energy
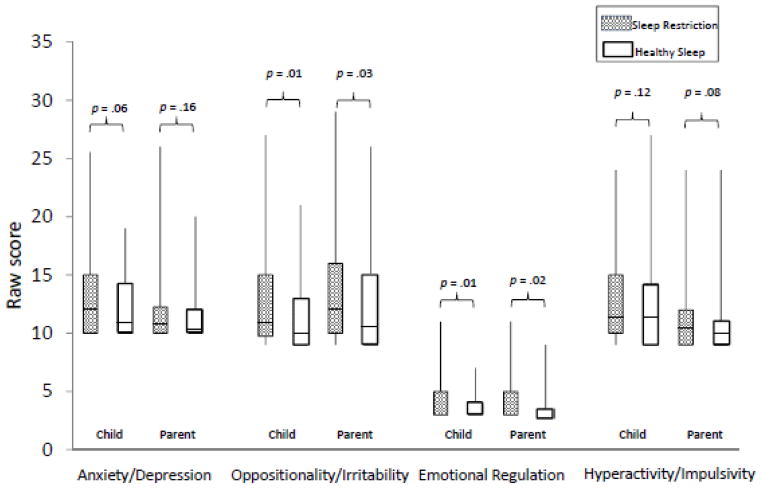
As shown in Figure 4, adolescents also reported higher levels of Oppositionality/Irritability (Z = −2.47, p = .01) and Emotion Regulation problems (Z = −2.71, p = .007) during SR compared to HS. Parent reports of their adolescent’s mood and emotion regulation were similar. Scores on the Oppositionality/Irritability (Z = −2.22, p = .03) and Emotion Regulation (Z = −2.40, p = .02) subscales were all significantly higher following the SR compared to HS, indicating greater oppositional behaviors, irritability, and emotional regulation problems. There were no differences between SR and HS on parent or self-reported Anxiety/Depression or Hyperactive/Impulsive symptoms, p > .05.
Figure 4.
Parent and self-reports of behavior and emotional control
*Median scores indicated by solid line; Emotional Regulation subscale medians = 25th percentile.
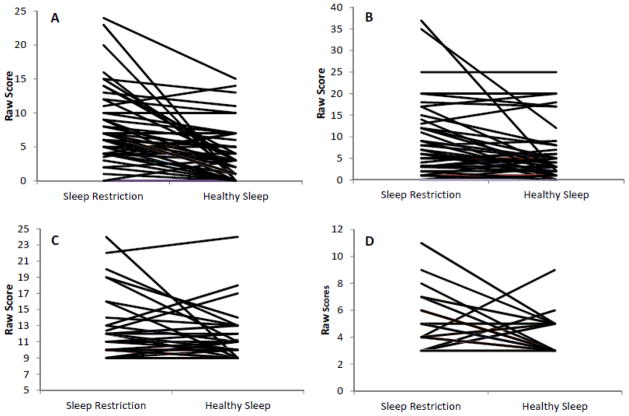
It is worth noting that, even though the vast majority of the mood and emotion regulation outcome variables were significantly impacted by the sleep manipulation, not all teens responded the same. Individual changes in select outcome variables are illustrated in Figure 5. This raises questions of whether any of these individual differences could be systematically explained by other factors. However, of the nearly 100 exploratory analyses conducted to assess for carryover effects, period effects, and the effects of sex, race, age, and variation in sleep duration during each condition only three had a p < .05. Self-report ratings on the POMS Anger/Hostility subscale were somewhat lower at the end of the study regardless of sleep condition, teens who slept less during HS showed a greater effect of the sleep manipulation on self-reported Fatigue/Inertia, and parent-reported Anxiety/Depression. Although these three effects may be worth examining in follow-up work, we caution against overinterpretation; given the sheer number of exploratory analyses, some or all three effects may be due entirely to chance. Importantly, there was no evidence that age, gender, race, or order of sleep conditions impacted the effect of the sleep manipulation.
Figure 5.
Intraindividual change by condition on selected dependent variables.
A-Self-report of Fatigue/Inertia on the Profile of Mood States (POMS); B - Self-report of Anger/Hostility on the POMS; C - Parent report of Hyperactivity/Impulsivity; D - Parent report of Emotional Control
Discussion
This study confirmed that several nights of sleep restriction adversely affects adolescents’ mood and ability to regulate their emotions. These effects did not appear to be due to simple expectancy effects or rater biases, as hyperactivity/impulsivity and depression scores did not show the same effect, and both parents and teens provided strikingly similar reports.
Our sleep restriction protocol –6.5 hours in bed per night– was much milder than the complete absence of sleep used in many adult research studies, or even the marked curtailment in sleep time that characterizes most chronic sleep restriction experiments. Yet, after only a few days of shortened sleep, adolescents experienced decreased energy and increased fatigue and confusion. They reported feeling less alert, less efficient, and more helpless, forgetful, and exhausted. In addition to lacking energy, during sleep restriction the adolescents also reported increased feelings of tension, anger, and anxiety. They had more feelings of being “on edge,” nervous, and restless when sleep-restricted compared to when they had ample opportunity to sleep (10 hours in bed).
There were no significant differences in feelings of depression across sleep conditions, but angry feelings (e.g., resentful, annoyed, spiteful) were higher with sleep restriction. This was corroborated on secondary mood measures, on which both parents and teens reported that the teens showed greater irritability and oppositionality when sleep-restricted, but with no corresponding effect on a measure that included depressive symptoms. Depressive symptoms in adolescents can manifest as irritable mood rather than sadness (American Psychiatric Association, 2000), which may be why we see effects on irritability and not depression overall. It may also be that some individuals are particularly vulnerable to the negative effects of sleep restriction, as some studies show poor sleep is associated with depressive symptoms (Gregory & O’Connor, 2002; Wolfson & Carskadon, 1998). Finally, both parents and teens also reported that, when sleep-restricted, the teens were less able to appropriately regulate their emotional reactions, leading to emotional outbursts and exaggerated responses to small triggers.
These results help to fill key gaps in the research literature. Previous correlational research has suggested that (a) negative mood is associated with shortened sleep during adolescence (Gregory & O’Connor, 2002; Wolfson & Carskadon, 1998), (b) this association may be stronger for anxiety than depression (Babson et al., 2010), and (c) short sleep is also associated with wide mood swings and poor emotion regulation (Dagys et al., 2012; Franzen et al., 2008). It was impossible to confidently conclude that short sleep was the causal factor in those studies because of the risk of confounding variables. We can now be more confident about causation; findings were similar for the current study, which used a rigorous within-subjects experimental cross-over design, and which included a relevant non-mood outcome measure to rule out general reporter biases and expectancy effects.
At the same time, the current study also overcame concerns about the real-world relevance of findings from experimental studies of adults (Babson et al., 2010; Franzen et al., 2008; Talbot et al., 2010) and the one published experimental study of adolescents (Dagys et al., 2012; McGlinchey et al., 2011; Talbot et al., 2010) that have reported a negative effect of sleep deprivation on mood and mood regulation. The vast majority of those studies, including the only relevant adolescent study, used extreme sleep restriction (e.g., sleep time <2 hours). In contrast, the current study resulted in a level of nightly sleep restriction similar to that obtained regularly on school nights by roughly 20% of healthy high school students in the U.S. (National Sleep Foundation, 2006; Eide & Showalter, 2012) and extended that across five nights, as in the typical school week. Moreover, by allowing sleep to be obtained at home, the current study avoided the potential for adverse effects of sleeping in a laboratory environment, which may impact mood even without restricting sleep (Paterson, et al., 2011).
This study found that sleep restriction of a realistic degree of severity and duration can cause adolescents to have a worse mood and weaken their ability to regulate their emotions. This has implications for developmental psychopathology research, parenting, and public policy. The developmental period of adolescence is associated with increased emotional reactivity (Dahl & Gunnar, 2009) due in part to the key maturational changes in emotional and behavioral regulation that take place during this time. Present findings suggest that shortened sleep is an important, modifiable contributor to poor emotion regulation. Emotional reactivity and associated mood problems can lead to problematic or risky behaviors, such as cigarette smoking and risky driving behavior (Dahl, 2008; Magid et al., 2009), that can have long-term negative consequences (Dahl, 2001, 2008). Furthermore, clinical mood disorders and addictive behaviors often emerge during adolescence (Dahl, 2004). It seems unlikely that sleep restriction alone is causally responsible – the rate of normative sleep restriction far exceeds adolescent incidence rates for psychopathology, and none of our healthy teens reported mood symptoms of clinical severity following sleep restriction – but inadequate sleep may exacerbate a pre-existing vulnerability to emotional and behavioral problems (Dahl, 2004)
This study also highlights the need for parents and teens to be aware of the negative effects of inadequate sleep. Mood and behavioral issues may become more apparent at home following short sleep and have a considerable impact on a teen’s ability to be successful across multiple environments (Dahl, 2008; Vorona et al., 2011). Structured sleep interventions that promote healthy sleep hygiene may be effective in improving sleep in adolescents (Tan, Healey, Gray, & Galland, 2012), with secondary benefits for mood and emotion regulation.
Beyond sleep hygiene, present findings further support the need for public policy interventions that promote adolescent sleep. School start time is a primary limiting factor of sleep duration in adolescents, with high schools in the U.S. typically starting over an hour earlier than standard “office hours” for adults (National Sleep Foundation, 2006). Particularly early school start times are associated with shorter sleep, increased daytime sleepiness, poorer concentration, and more attention problems in school-age children (Epstein, Chillag, & Lavie, 1998; Wahlstrom, 2002). Simply starting school later has been consistently demonstrated to promote longer adolescent sleep (Danner & Phillips, 2008; Wahlstrom, 2002), and there are hints that it also improves student mood (Wahlstrom, 2002), which would make sense in light of our experimental findings.
Limitations
Findings are tempered by several limitations. Although an actigraphy-monitored, home-based sleep manipulation allowed us to maximize real-world applicability, this lessened experimental control over sleep environment factors and did not allow for assessment of some aspects of sleep (e.g., sleep architecture). This study also focused on mood and emotion regulation across several days, which did not allow for assessment of day-to-day changes. The use of rating scales also introduced a degree of subjectivity. Parents and teens arguably have unique insight into the teens’ mood and emotion regulation in real-world circumstances, but it also would be helpful for future work to pair chronic sleep restriction with objective measures of emotional reactivity, as well as blinded observations in different settings (e.g., school).
We also recommend a more systematic study of changes in both positive and negative affect following adolescent sleep restriction, as prior work suggests that these may show different responses to sleep restriction (e.g., Franzen et al., 2008; Talbot et al., 2010). From a theoretical perspective, the Tripartite Model of Anxiety and Depression (Clark & Watson, 1991) suggests that negative affect is a construct shared by both clinical anxiety and depression, whereas depression also involves decreased positive affect and anxiety has increased physiological arousal (Clark & Watson, 1991). In the context of increased negative affect, jitteriness and agitation in response to sleep restriction (Everson, 1998) could contribute to the subjective experience of anxiety, and a sleepiness-induced reduction in positive affect (Talbot et al., 2010) would contribute to the subjective experience of depression.
While the two experimental conditions allow for a comparison of a clear state of sleep restriction against a well-rested state, the baseline week was designed to stabilize circadian rhythm, not to reflect a true normative sleep pattern. During that baseline week, many teens shifted their sleep phase from a late-rise summer phase toward a more typical school year schedule, inducing a sort of temporary “jet lag.” The baseline week successfully resolved this phase shift, helping to prevent order/carryover effects during the experimental weeks. However, placing this phase shift during the baseline week prevented us from prospectively collecting data on mood or emotion regulation in participants’ “normal” sleep-wake schedule. Having a longer “run-in” period, or focusing on experimental sleep extension during the school year, would provide a true “baseline” condition for comparison in future research.
With respect to the sleep manipulation, limiting each sleep condition to five nights replicates the U.S. school week, but other effects on mood might be seen after longer or more intense sleep restriction (Dinges et al., 1997; Drake et al., 2001). It would be helpful for future work to parametrically vary the “dose” of sleep restriction, including even milder (and more common) levels of nightly restriction. Similarly, it would be interesting to extend the period of sleep restriction beyond 5 nights, as some teens restrict their sleep both on school nights and on weekends. Adult data suggest that long-term sleep restriction is not accompanied by successful “adaptation” in mood or emotional regulation (Pilchur & Huffcutt, 1996), but this remains to be tested in youth. Finally, a direct test of whether sleep restriction differentially affects vulnerable individuals would require a sample that moves away from the typically-developing healthy adolescents studied here, to one with greater developmental challenges or psychiatric vulnerability.
Conclusion
Although it is clear that there is more work to be done, this study adds to a growing evidence base that, despite well-publicized critiques (Eide & Showalter, 2012; Matricciani et al., 2012), there is strong reason to believe that the chronic sleep restriction experienced by millions of adolescents on school nights does indeed have adverse effects. Bridging the findings from large correlational studies and “proof of concept” experiments that used unrealistically extreme sleep deprivation, current results confirm that several nights of realistically shortened sleep can cause worsened mood and emotion regulation in adolescents.
Supplementary Material
Key points.
Restricted sleep, particularly on weekday nights, is common in adolescence.
Although we know the negative impact that sleep deprivation has on mood in adults, research in the adolescent population is largely correlational, and the few experimental studies demonstrate worsening mood following unrealistically severe sleep loss.
This study experimentally demonstrated that even a few nights of reduced sleep, similar to the amount regularly experienced by many adolescents on school nights (~6.3 hours/night), worsens mood and mood regulation.
Clinicians, parents, and teens should be educated on how even relatively “mild” sleep restriction can, after a few days, impact adolescents’ ability to emotionally manage day-to-day demands.
Acknowledgments
We would like to thank the parent and adolescent participants who gave generously of their time, as well as the multiple students and clinical research professionals who made the study possible. We would also like to acknowledge our funding source: National Institutes of Health (R01 HL092149, UL1 RR026314).
Footnotes
Conflict of interest statement: No conflict of interests declared.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition, Text Revision: DSM-IV-TR. Washington, DC: 2000. [Google Scholar]
- Babson KA, Trainor CD, Feldner MT, Blumenthal H. A test of the effects of acute sleep deprivation on general and specific self-reported anxiety and depressive symptoms: An experimental extension. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41:297–303. doi: 10.1016/j.jbtep.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron IS. Neuropsychological evaluation of the child. New York: Oxford University Press; 2004. [Google Scholar]
- Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatric Clinics of North America. 2011;58(3):649–665. doi: 10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW, Fallone G, Godiwala N, Flanigan M, Martin D, Schaffner L, Amin R. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. Journal of Child Psychology and Psychiatry. 2008;49:915–923. doi: 10.1111/j.1469-7610.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. 2010;33(11):1447–1456. doi: 10.1093/sleep/33.11.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, Dolan LM. Dietary intake following experimentally restricted sleep in adolescents. Sleep. 2013;36:827–834. doi: 10.5665/sleep.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2(4):453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep: Journal of Sleep Research and Sleep Medicine. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. The Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cousins JC, Whalen DJ, Dahl RE, Forbes EE, Olino TM, Ryan ND, Silk JS. The bidirectional association between daytime affect and nighttime sleep in youth with anxiety and depression. Journal of Pediatric Psychology. 2011;36(9):969–979. doi: 10.1093/jpepsy/jsr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Affect regulation, brain development, and behavioral/emotional health in adolescence. CNS Spectrums. 2001;6(1):60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of opportunities and vulnerabilities. Annals of the New York Academy of Science. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Biological, developmental, and neurobehavioral factors relevant to adolescent driving risks. American Journal of Preventative Medicine. 2008;35(3 Suppl):S278–S284. doi: 10.1016/j.amepre.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responseiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Lewin DS. Pathways to adolescent health: Sleep regulation and behavior. Journal of Adolescent Health. 2002;31:175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Dagys N, McGlinchey EL, Talbot LS, Kaplan KA, Dahl RE, Harvey AG. Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. Journal of Child Psychology and Psychiatry. 2012;53(6):660–667. doi: 10.1111/j.1469-7610.2011.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner F, Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. Journal of Clinical Sleep Medicine. 2008;4(6):533–535. [PMC free article] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Wiliams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–267. [PubMed] [Google Scholar]
- Drake CL, Roehrs TA, Burduvali E, Bonahoom A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38:979–987. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- Eide E, Showalter MH. Sleep and student achievement. Eastern Economic Journal. 2012;38(4):512–524. [Google Scholar]
- Epstein R, Chillag N, Lavie P. Starting times of school: Effects on daytime functioning of fifth-grade children in Israel. Sleep. 1998;21:250–256. doi: 10.1093/sleep/21.3.250. [DOI] [PubMed] [Google Scholar]
- Everson C. Physiological consequences of sleep deprivation. Journal of Musculoskeletal Pain. 1998;6:93–101. [Google Scholar]
- Fallone G, Acebo C, Seifer R, Carskadon MA. Experimental restriction of sleep opportunity in children: Effects on teacher ratings. Sleep. 2005;28(12):1561–1567. doi: 10.1093/sleep/28.12.1561. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17(1):34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. BRIEF – Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Gregory AM, O’Connor T. Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(8):964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Guy SC, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function-Self Report version. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition. Bloomington, MN: Pearson, Inc; 2004. [Google Scholar]
- Magid V, Colder CR, Stroud LR, Nichter M, Nichter M TERN Members. Negative affect, stress, and smoking in college students: unique associations independent of alcohol and marijuana use. Addictive Behaviors. 2009;34(11):973–975. doi: 10.1016/j.addbeh.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: A brief history of sleep recommendations for children. Pediatrics. 2012;129(3):548–556. doi: 10.1542/peds.2011-2039. [DOI] [PubMed] [Google Scholar]
- McGlinchey EL, Talbot LS, Chang K, Kaplan KA, Dahl RE, Harvey AG. The effect of sleep deprivation on vocal expression of emotion in adolescents and adults. Sleep. 2011;34(9):1233–1241. doi: 10.5665/SLEEP.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EITS manual for the profile of mood states. San Diego: Educational and Industrial Test Services; 1971. [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JDE, Ochsner KN. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents, and young adults. Social Cognitive and Affectiuve Neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. Summary of Findings: 2006 Sleep In America Poll. Washington, DC: National Sleep Foundation; 2006. [Google Scholar]
- Nixon GM, Thompson JM, Han DY, Becroft DM, Clark PM, Robinson E, Waldie KE, Wild CJ, Black PN, Mitchell EA. Short sleep duration in middle childhood: Risk factors and consequences. Sleep. 2008;31(1):71–78. doi: 10.1093/sleep/31.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen EJ, Porkka-Heiskanen E, Lahikainen AR. Sleep quality, duration, and behavioral symptoms among 5–6 year-old children. European Child & Adolescent Psychiatry. 2009;18:747–754. doi: 10.1007/s00787-009-0033-8. [DOI] [PubMed] [Google Scholar]
- Paterson JL, Dorrian J, Ferguson SA, Jay SM, Lamond N, Murphy PJ, Campbell SS, Dawson D. Changes in structural aspects of mood during 39–66 h of sleep loss using matched controls. Applied Ergonomics. 2011;(42):196–201. doi: 10.1016/j.apergo.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Pilchur JJ, Huffcutt AI. Effects of sleep deprivation on performance: A meta-analysis. Sleep. 1996;19(4):318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Rubin R. How much sleep do teens really need? Maybe less than you think. 2012 Feb 19; Retrieved from http://www.today.com/health/how-much-sleep-do-teens-really-need-maybe-less-you-1C9381965.
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep. 1994;17(3):201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: Changes in affect. Emotion. 2010;10(6):831–841. doi: 10.1037/a0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E, Healey D, Gray AR, Galland BC. Sleep hygiene intervention for youth aged 10 to 18 years with problematic sleep: a before-after pilot study. BMC Pediatrics. 2012;12(1):189. doi: 10.1186/1471-2431-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorona RD, Szklo-Coxe M, Wu A, Dubik M, Zhao Y, Ware JC. Dissimilar teen crash rates in two neighboring southeastern Virginia cities with different high school start times. Journal of Clinical Sleep Medicine. 2011;15(7):145–51. [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom K. Changing times: findings from the first longitudinal study of later high school start times. NAASP Bulletin. 2002;86:3–21. [Google Scholar]
- Walker EF. Adolescent neurodevelopment and psychopathology. Current Directions in Psychological Science. 2002;11(1):24–28. [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. [PubMed] [Google Scholar]
- Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. Journal of Pediatric Psychology. 2003;28:559–567. doi: 10.1093/jpepsy/jsg046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.