Abstract
Myelinogenesis is a complex process that involves substantial and dynamic changes in plasma membrane architecture and myelin interaction with axons. Highly ramified processes of oligodendrocytes in the central nervous system (CNS) make axonal contact and then extrapolate to wrap around axons and form multilayer compact myelin sheathes. Currently, the mechanisms governing myelin sheath assembly and axon selection by myelinating cells are not fully understood. Here, we generated a transgenic mouse line expressing the membrane-anchored green fluorescent protein (mEGFP) in myelinating cells, which allow live imaging of details of myelinogenesis and cellular behaviors in the nervous systems. mEGFP expression is driven by the promoter of 2′-3′-cyclic nucleotide 3′-phosphodiesterase (CNP) that is expressed in the myelinating cell lineage. Robust mEGFP signals appear in the membrane processes of oligodendrocytes in the CNS and Schwann cells in the peripheral nervous system (PNS), wherein mEGFP expression defines the inner layers of myelin sheaths and Schmidt-Lanterman incisures in adult sciatic nerves. In addition, mEGFP expression can be used to track the extent of remyelination after demyelinating injury in a toxin-induced demyelination animal model. Taken together, the membrane-anchored mEGFP expression in the new transgenic line would facilitate direct visualization of dynamic myelin membrane formation and assembly during development and process remodeling during remyelination after various demyelinating injuries.
Keywords: myelinogenesis, in vivo imaging, spatiotemporal expression, transgenic reporter, developmental dynamics, mammalian nervous system
Results and discussion
Myelinating cells in the central and peripheral nervous systems, oligodendrocytes and Schwann cells, respectively, produce a unique, lipid-rich electrically insulating myelin sheath that wraps spirally around axons to facilitate saltatory nerve conduction. Formation of the myelin sheath, an extension of the plasma membrane, is a highly dynamic process. In rodents, during early postnatal stages, oligodendrocytes sample the axons, extent their processes and produce robust myelin sheathes. The myelinating process peaks around postnatal week 3, and continue throughout the adulthood via de novo myelin synthesis or remodeling of existing myelinated axons by newly differentiating oligodendrocytes (Miller et al., 2012; Simons and Lyons, 2013; Young et al., 2013). Upon injury, oligodendrocytes undergo myelin sheath breakdown and regeneration, however, they fail to remyelinate axons under certain pathological conditions such as multiple sclerosis. Currently, the genetic tools for in vivo tracking the membrane dynamics of myelin sheath generation in the CNS and PNS during development or after injury have not been fully developed.
The gene encoding 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (CNP) is expressed in oligodendrocytes in the CNS and Schwann cells in the PNS. Its expression increases in parallel with those encoding the major myelin structural proteins (Knapp et al., 1988; Scherer et al., 1994) and is sustained throughout development (Gravel et al., 1998). Several transgenic mouse lines based on CNP promoter/reporter gene constructs have been developed to visualize the expression pattern of CNP transcriptionally active cells, to identify mouse oligodendrocytes and Schwann cells (Chandross et al., 1999; Gravel et al., 1998; Yuan et al., 2002). However, the transgenic mice that express reporter specifically in the membrane process of myelinating cells have not been generated.
To directly visualize the dynamics of myelin membrane formation, we generate a transgenic line carrying myelinating cells-expressing CNP promoter upstream of the EGFP reporter gene with a membrane location signal (mEGFP). Our data indicate that the new transgenic line with membrane-anchored EGFP will be a helpful tool to directly analyzing myelin sheath assembly and remyelination processes in the CNS and PNS.
To generate a transgenic line that labels membrane structures of myelinating cells in nervous systems, we placed a 3.9-kb CNP enhancer, which was previously shown to direct transgene expression specifically in oligodendrocytes and Schwann cells (Gravel et al., 1998), before a membrane-anchored form of EGFP (mEGFP) which carry a Ras membrane localization (CAAX) sequence in the carboxyl terminus of EGFP (Chong et al., 2012) (Fig. 1a). The transgenic construct was injected into fertilized mouse eggs to produce different transgenic founders. Essentially each transgenic founder displays similar GFP expression patterns in the nervous systems. In this study, we mainly focus on a founder line CNP-mGFP, which produces the strongest GFP signal in myelinating processes.
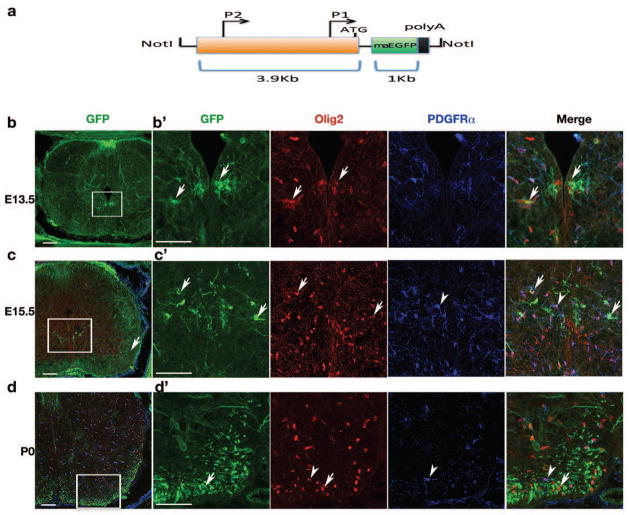
Fig. 1. CNP-mEGFP transgene expression during early development.
a) Schematic diagram depicts the transgenic construct CNP-mEGFP. mEGFP is directed by 3.9 kb CNP promoter consisting of CNP1 and CNP2 promoter elements. The black box indicates the polyadenylation sequence from human beta-globin gene, exons 2 and 3. The relative size of the individual sequences is indicated. b–d) CNP-mEGFP expression in the embryonic spinal cord of transgenic mice. Transverse sections of embryonic mouse spinal cord from E13.5 to P0 as indicated. Sections were immunostained for Olig2 and PDGFRα. Boxed areas are shown at higher magnification at right panels, respectively. Arrows and arrowheads indicate mEGFP-positive oligodendrocytes and mEGFP-negative PDGFRα+ OPCs, respectively.
Scale bars: 100 μm.
In the developing spinal cord, CNP-promoter directed mEGFP expression appeared at pMN domain (Lu et al., 2000; Zhou et al., 2000) commencing at E13.5 (Fig. 1b). These immature oligodendrocytes express Olig2 but not oligodendrocyte precursor (OPC) marker PDGFRα (Fig. 1b–b′;c–c′), suggesting they are postmitotic immature oligodendrocytes, or represent a late stage of OPCs with PDGFRα down-regulation. At E15.5, mEGFP-positive differentiating oligodendrocytes (Olig2+ PDGFRα−) showed strong GFP signals in cellular membrane process near the ventral pMN domain (Fig. 1c′). Strikingly, both ventral (the anterior corticospinal tract) and dorsal (the gracile fasciculus) white matter regions were positive for mEGFP (Fig. 1c), indicating the initiation of the differentiation process of OPCs at this stage. At later developmental stages e.g. P0, mEGFP+ cells mainly appeared in the white matter and were hardly detectable in the gray matter (Fig. 1d). The pattern of CNP-driven mEGFP expression indicates that CNP expression in the developing spinal cord is detected in early differentiating oligodendrocytes but not in OPCs during embryogenesis.
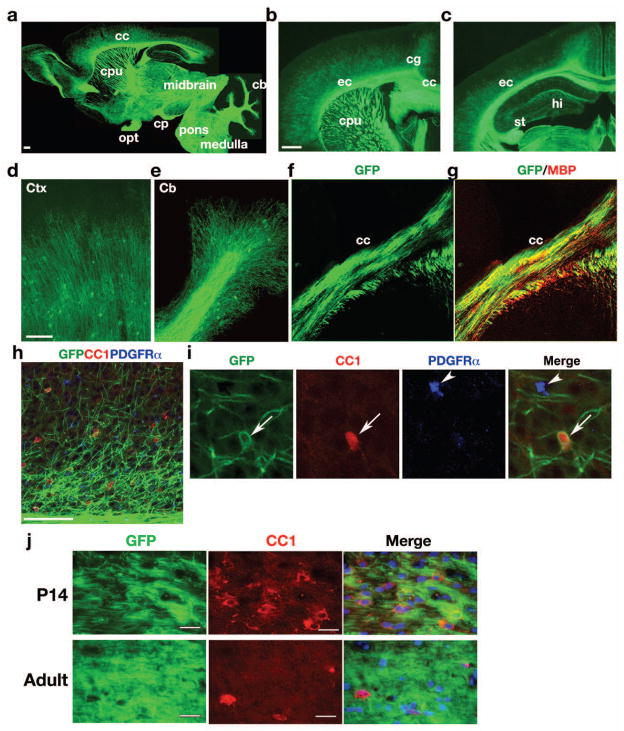
At P28, high levels of mEGFP expression were notable in the corpus callosum, external capsule, cingulum, caudate putamen, anterior commissure, medial forebrain bundle and cerebellar white matter, all major myelinating tracts in the brain (Fig. 2a–c). mEGFP is mainly detected in the processes of myelinating oligodendrocytes in the cortex (Fig. 2d) and cerebellum (Fig. 2e). mEGFP+ cell processes were co-stained with MBP in white matter regions, such as the corpus callosum (Fig. 2f, g). To determine the stages of mEGFP+ cells in oligodendrocyte lineage, we performed immunostaining with the markers CC1 and PDGFRα for mature oligodendrocytes (Fig. 2h) and OPCs, respectively, in the cortex (Fig. 2h, i). mEGFP expression co-labels cells expressing CC1 but not PDGFRα, suggesting that CNP-mGFP+ cells are mainly confined to differentiated oligodendrocytes but not their precursors in the postnatal brain. We detected CNP-mEGFP+ cell bodies sparsely distributed in the developing cortex. At P28, we observed approximately 34 ± 5% of detectable mGFP+ cell bodies among CC1+ oligodendrocytes (320 CC1+ cell counts, n = 3 animals), and all detectable mEGFP+ cell bodies were co-labeled with CC1. In the developing and adult white matter, essentially no distinct cell bodies of GFP+ cells can be identified (Figure 2j). Thus, the predominant mEGFP signal on myelin processes in the white matter underscores the advantage of the mEGFP line, which preferably labels the myelin structure rather than the oligodendrocyte cell body.
Fig. 2. CNP-mEGFP expression in the postnatal brain.
a–e) Sagittal (a) and coronal sections (b, c) of transgenic mouse brains at P28. Abundant EGFP expression was confined to white matter tracts. Panels d, e show higher magnification of cortex and cerebellum, respectively. f–i) Brain sections from transgenic mice at P28 were immunostained with antibodies to MBP, CC1 or PDGFRα in corpus callosum (f, g) and cortex (h, i). Arrows and arrowheads in panel indicate CC1+ mature oligodendrocytes and OPCs, respectively. j) The corpus callosum from P14 and 8-weeks old adult mice was immunostained with antibody to CC1 and visualized with immunofluorescence.
cc: corpus callosum, cpu: caudate putamen, ec: external capsule, cg: cingulum, hi: hilus; opt: optic tract; cp: cerebral peduncle; cb: cerebellum; st: striatum.
Scale bars in a, b, 200 μm; in d and h; 100 μm; j, 25 μm.
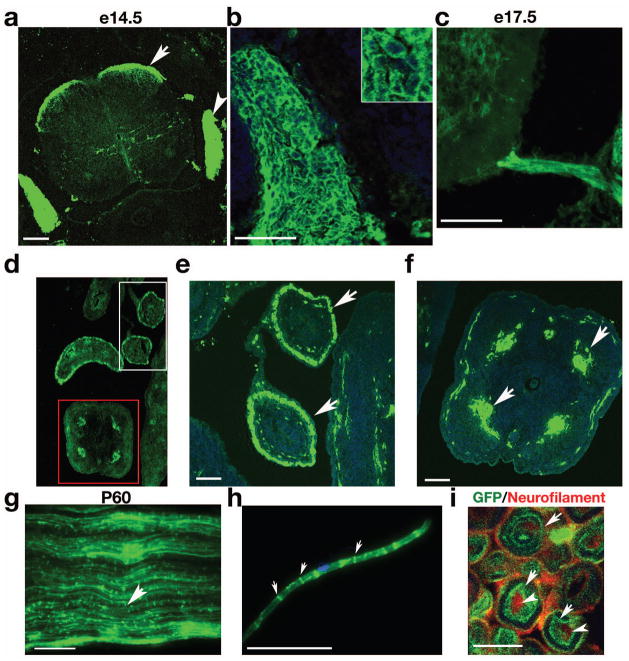
The CNP gene is also known to be actively transcribed in Schwann cells in the PNS (Gravel et al., 1998; Yuan et al., 2002). In dorsal root ganglion (DRG) at E14.5, strong signals for mEGFP were abundant in satellite cells, the non-myelinating glial cells associated with the cell bodies of peripheral ganglia neurons (Fig. 3a, b) (Toma et al., 2007). Sympathetic ganglia tracts serving the gastrointestinal tract in the dorsal root entry zone were also positive for mEGFP (Fig. 3a). The ventral nerve root was also labeled by mEGFP in the embryonic stage, e.g. at E17.5 (Fig. 3c). In addition, mEGFP was detected in the nerves along guts e.g. in the midgut (Fig. 3d, e) and the tail (Fig. 3f). Intact sciatic nerves isolated from P60 transgenic mice exhibited mEGFP expression along the axons, particularly in the Schmidt-Lanterman incisures, indicating mEGFP labels myelinating Schwann cells (Fig. 3g). The teased fibers of sciatic nerves confirmed that mEGFP was present mainly in the Schmidt-Lanterman incisures of adult myelinated axons (Fig. 3h). A high-power image of cross-section of sciatic nerves show CNP-mEGFP specifically labels myelin sheaths at the inmost layers (Fig. 3i, arrows) that wrapped closely around the axons (Fig. 3i, arrowheads). This is consistent with recent findings that CNP is associated with extension of the innermost tongue of myelin membrane sheath adjacent to axons (Snaidero et al., 2014). Low amounts CNP-like phosphodiesterase activity was reported in other organs, for instance, the spleen (Moller et al., 1992). However, in the CNP-mEGFP transgenic line, we did not observe the notable GFP signal in the spleen (Supplementary Fig. 1).
Fig. 3. Expression of mEGFP in the PNS of transgenic mice.
a) Cross-section of embryonic spinal cord examined at E14.5. Arrow and arrowhead indicate the sympathetic ganglia and DRG, respectively. b) Strong mEGFP signal was detected in satellite cells in DRG. Insert in b shows a higher magnification of satellite cells. c) mEGFP signal in the ventral root of spinal cord at E17.5. d–f) mEGFP expression in the cross-section of midguts (d, e) and the tail (d, f). g) Whole-mount longitudinal preparations of intact sciatic nerves from P60 transgenic mice. h) mEGFP expression in a teased nerve fiber showing signals in Schmidt-Lanterman incisures (arrows) of myelinating SCs. Dapi; blue. i) Cross-section of the sciatic nerve at P60 were immunostained with a neurofilament antibody SMI31 to label axons (red, arrowheads) and visualized with the mEGFP signal (arrows).
Scale bars: a–h, 100 μm; i, 5 μm.
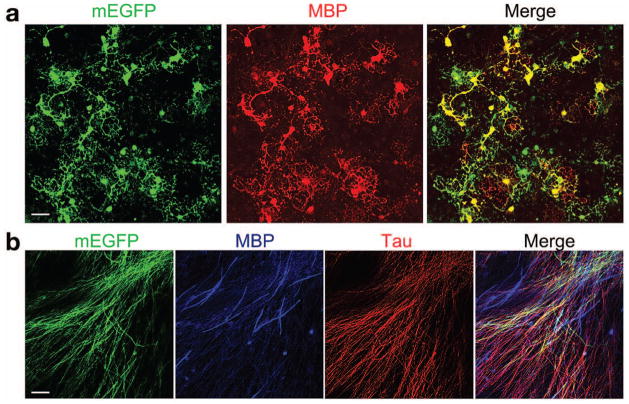
In cultured oligodendrocytes (Chen et al., 2007), mEGFP positive cells were exclusively expressed in MBP+ oligodendrocytes and the mEGFP signal co-labeled with MBP localized on the cell membrane and processes (Fig. 4a). Neither GFAP+ astrocytes nor Tuj1+ neurons were positive for EGFP (data not shown).
Fig. 4. mEGFP expression in oligodendrocyte-enriched culture and in DRG explant myelinating culture.
a) Cortical progenitors from CNP-mEGFP embryos at E15.5 were cultured in oligodendrocyte differentiation media. Cells were immunostained with antibody to MBP (red). b) DRG explant cultures were induced to form myelin and immunostained with MBP and Tau antibody.
Scale bars: 25 μm.
To examine mEGFP expression in Schwann cells-neuron co-culture, we carried out DRG explant cultures, and induced Schwann cells to form myelin. Robust and extensive axon ensheathment and myelination were detected with expression of CNP-mEGFP (Fig. 4b). mEGFP signals were observed along many axons labeled with a neurofilament marker Tau (Fig. 4b). Strikingly, mEGFP-labelled membrane processes covered more extensive than those of MBP+ membrane structures, suggesting that axon ensheathment by CNP-mEGFP+ processes precedes MBP-associated myelin sheath compaction. This is consistent with observation that CNP is highly enriched in newly synthesized layers at inner tongues adjacent to the axon, which is followed by MBP-mediated compaction of myelin sheaths (Snaidero et al., 2014).
Since CNP-mEGFP expression mainly marks the differentiating stage of oligodendrocytes, we then examined whether it could be utilized to directly visualize remyelination after lysolecithin-induced demyelinating injury, an animal model of multiple sclerosis (Franklin, 2002). This remyelination model exhibits a well-defined and predictable pattern of remyelination (Franklin, 2002; Jeffery and Blakemore, 1995). The remyelination process can be distinctly divided into a proliferation/migration phase within the first week after injury, and a remyelination phase starting week two after injury, when OPCs become differentiated to form myelinating oligodendrocytes (Franklin, 2002).
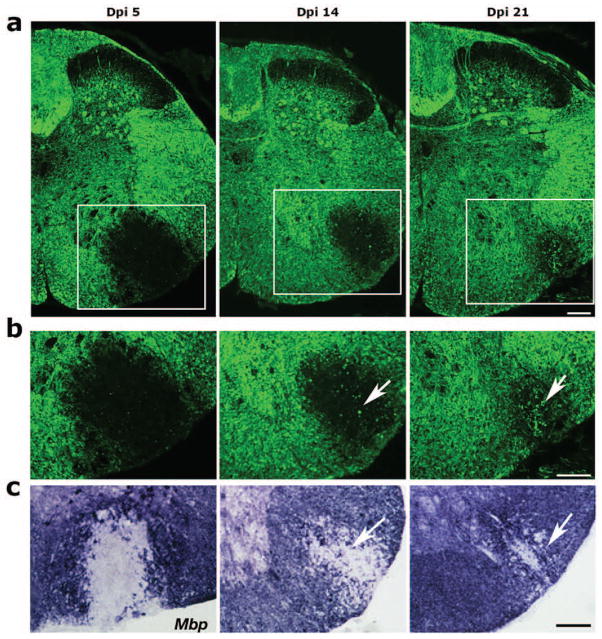
Lysolecithin was injected into the lateral white matter region of the ventral spinal cord in the 8-week old adult transgenic mice. In the uninjured mice, mEGFP labeled myelinated axons throughout the spinal cord. Five days post injury (Dpi 5), mEGFP expression was hardly detectable in the demyelinated lesion. During OPC differentiation phase at Dpi 14 when spontaneous repair process occurred, a subset of mEGFP+ processes was clearly visible in the lesion. During the remyelination phase at Dpi 21, substantial mEGFP signals appeared in the lesion, and the perimeter of lesion was much smaller compared to that at Dpi 5 (Fig. 5b). The appearance of mEGFP in the lesion was correlated to that of Mbp mRNA expression detected by in situ hybridization (Fig. 5c). These observations suggest that mEGFP expression can effectively track oligodendrocyte maturation and the remyelination process in an animal model of multiple sclerosis.
Fig. 5. mEGFP expression during remyelination after lysolecithin-induced demyelination in CNP-mEGFP transgenic mice.
a) mEGFP expression in the cross-sections of spinal cord from eight-week old CNP-mEGFP transgenic mice after injection of lysolecithin and harvested at Dpi5 (proliferation/migration phase), Dpi14 (differentiation phase) and Dpi 21 (remyelination phase). Arrows indicate the mEGFP+ cellular process in the lesion. b) The boxed areas in a were shown in the corresponding panels in b. c) Mbp expression (blue purple) on the corresponding sections of Dpi 5, Dpi 14 and Dpi 21 in the spinal cord lesions was examined by mRNA in situ hybridization with an antisense probe to mouse Mbp. Arrows indicate Mbp+ cells in the lesion.
Scale bars: 100 μm.
How highly motile membrane processes become stabilized, assembled and establish axon ensheathment and maintain the extent of myelin sheath formation is not fully understood. In this study, we generate a new transgenic line wherein the membrane-anchored GFP is targeted to the membrane processes of oligodendrocytes and Schwann cells. The appearance of EGFP+ cells from embryonic stages to adulthood allows us to track the temporal waves of myelinating cell maturation in vivo, and to define the axonal wrapping process by myelinating cells and the leading edge that initiates myelinogenesis. In the previous CNP-eGFP transgenic line (Yuan et al., 2002), GFP signals identified oligodendrocytes and Schwann cells, however, the eGFP signal is largely diffused throughout the cytoplasm, mainly detect cell bodies and, to some extent, cellular processes. In contrast, in the present CNP-mEGFP line, mEGFP is integrated into cell membrane and exhibits a predominant mEGFP signal in myelinating processes, particularly at the inner layers of myelin membrane adjacent to axons, rather than the cell body. Given the membrane-anchoring feature, the present CNP-mEGFP line may potentially present a valuable tool to track myelin sheath formation, assembly and remodeling of multilayered myelin structures during normal myelination and remyelination process after demyelinating injury. Detection of CNP-mEGFP+ membrane sheath formation prior to MBP expression in the Schwann cells-DRG co-culture study suggests that membrane ensheathment extends along the axon initially by newly synthesized, uncompacted membrane layers at inner tongues before increased expression of MBP, which mediate the compaction of the cytoplasm of the myelin lamellae, leading to myelin sheath compaction. In conclusion, we have generated a new transgenic mouse that enables direct visualization of the dynamics of oligodendrocyte and Schwann cell morphogenesis in particular the cell processes and myelin membrane architecture during development and under pathological conditions.
Methods
Generation of CNP-mEGFP transgenic mice
The CNP-mEGFP transgene used for generation of transgenic mice was constructed as follows. The MBP promoter segment in pMG2 (Gow et al., 1992) was replaced with a 3.9 kb XbaI-HindIII fragment containing mouse CNP promoter (Gravel et al., 1998). Membrane anchored EGFP coding sequence (Gift of Bruce Appel, Univ. Colorado) was then inserted after the CNP promoter. The pCNP-mEGFP vector contained a poly-A signal from human beta-globin gene, exons 2 and 3. A NotI fragment carrying the transgene was excised, purified by elute-tip D (Promega), and injected into fertilized oocytes from FVB/N genetic backgrounds. Founders were identified by polymerase chain reaction (PCR), using genomic DNAs extracted from tail biopsies. Primers for PCR were designed based on the CNP promoter and EGFP coding sequence: 5′-forward Cnp3740 5′-agggtgctttaagttaggcttggg-3′, reverse primer GFP-80: 5′-5′-acacgctgaacttgtggccgttta-3′. The five male founders were used to breed with FVB/N females. No gross behavioral differences were noticeable in the CNP-mEGFP mice compared with wild-type littermates. A preliminary analysis was carried out by visualizing the in vivo expression of mEGFP in brain sections of CNP-mEGFP mice. Transgene was transmitted to the offspring of all five lines (data not shown); each founder line displayed a similar expression pattern. We did not detect any phenotype(s) associated with homozygosity for the transgene insertion. All animal use and studies were approved by the Institutional Animal Care and Use Committee of the Cincinnati Children’s Hospital Medical Center, and the University of Texas Southwestern Medical Center and Texas A&M University, USA and the Sichuan University, Chengdu, China. The transgenic line will be available to the research community upon acceptance of the manuscript.
Immunohistochemistry and histology
Mice were anesthetized and perfused intracardially with PBS and then 4% paraformaldehyde. Brains were dissected out, postfixed with 4% paraformaldehyde at 4°C for 1h and processed for vibratome section (50 μm). Pregnant mice at indicated gestational days were anesthetized and perfused using PBS. Embryos were dissected out and fixed with 4% paraformaldehyde at 4°C overnight and processed for frozen sections (16 μm).
To visualize PNS myelin, freshly extracted mouse sciatic nerves segments (10 mm) were teased on poly-L-lysine coated microscope slides, using fine needles to gently separate the nerve fibers. The preparations were then dried overnight at room temperature, washed in PBS 3×5 min and finally mounted in 50% glycerol/PBS. Immunostaining with antibodies against Olig2 (Millipore), PDFGRα (BD Bioscience, 558774), Tau (Sigma T6402), CC1 (Oncogene Research, OP80) and MBP (Covance, SMI-94R) or Neurofilament (Covance, SMI-31R), which labels a phosphorylated epitope in extensively phosphorylated neurofilament H and, to a lesser extent, with neurofilament M in axons, was performed on tissue or cell cultures using standard protocols along with corresponding fluorescent secondary antibodies (Jackson lab). Topro3 (Molecular Probe) was included to label nuclei. In situ hybridization was carried out as previously described (Yu et al., 2013).
Oligodendrocyte-enriched cultures
For mouse oligodendrocyte enriched cultures, cortical precursors were isolated from CNP-mEGFP mouse embryos at E15.5 as described previously (Chen et al., 2007). Briefly, to obtain oligodendrocyte precursor cell (OPC)-enriched cultures, the cortical progenitors were grown in N2+bFGF growth medium for five days, then passaged by trypsinization and cultured in oligodendrocyte growth medium (N2 supplemented with bFGF and PDGFAA). Most of neurons did not survive after precursor cell passaging while cultured in the oligodendrocyte growth medium. To initiate oligodendrocyte differentiation, culture medium was changed to differentiation medium supplemented with CNTF and T3.
Dissociated DRG explants
DRGs were collected from E13.5 mouse embryos and dissociated using 0.25% trypsin for 45 min at 37°C. Dissociated DRGs were plated and maintained in M1 medium (MEM medium containing 10% FBS and 100 ng/ml NGF) on collagen-coated coverslips. Axonal processes and endogenous SCs were allowed to grow and establish themselves for approximately 7 days, and then ascorbic acid (50 μg/ml) was added to initiate myelin formation.
Lysolecithin-induced demyelinating injury
Mice were anesthetized by isoflurane. Dorsal laminectomies were performed on the upper thoracic (T11-T13) region of the spinal cord. A glass needle attached to a Hamilton syringe mounted on a stereotactic micromanipulator was used to inject 1 μl of a 1% PBS solution of lysolecithin (L-a-lysophosphatidylcholine) (Sigma, St. Louis, MO), pH 7.4. The needle was inserted into the lateral part of the spinal cord, lysolecithin solution was injected, and then the needle was slowly withdrawn. Spinal cord segments including the injection site in the center were harvested. Spinal cord sections from each time point (day after injury) dpi5, dpi14 and d28 were analyzed by immunohistochemistry or mRNA in situ hybridization.
Supplementary Material
The section of spleen tissues from CNP-mGFP mice at P14 was stained with tomato-lectin to stain spleen cells and nuclear counterstained with Hoechst 33258 as indicated. Scale bar: 50 μm.
Acknowledgments
Grant sponsors: The US National Institutes of Health (grant numbers: R01NS072427 and R01NS075243 to QRL, and R21NS077215 to QRL and JRL); The National Multiple Sclerosis Society (grant number: NMSS-4727 to QRL); The National Natural Science Foundation of China (grant number: 31271128 to XZ); The National Science Foundation of China (grant number: NSFC 81270757) and Program for Changjiang Scholars and Innovative Research Team in Sichuan University (grant number: IRT0935).
Authors would like to thank Dr. Michel Gravel for providing the CNP promoter segment and Dr. Alex Gow for the pMG2 vector and are grateful to Jie Cai and Guojiao Huang for their technical assistance.
References
- Chandross KJ, Cohen RI, Paras P, Jr, Gravel M, Braun PE, Hudson LD. Identification and characterization of early glial progenitors using a transgenic selection strategy. J Neurosci. 1999;19:759–774. doi: 10.1523/JNEUROSCI.19-02-00759.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044–1051. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, Rowitch DH, Franklin RJ, Lu QR, Chan JR. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109:1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Gow A, Friedrich VL, Lazzarini RA. Myelin basic protein gene contains separate enhancers for oligodendrocyte and Schwann cell expression. J Cell Biol. 1992;119:605–616. doi: 10.1083/jcb.119.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel M, Di Polo A, Valera PB, Braun PE. Four-kilobase sequence of the mouse CNP gene directs spatial and temporal expression of lacZ in transgenic mice. J Neurosci Res. 1998;53:393–404. doi: 10.1002/(SICI)1097-4547(19980815)53:4<393::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Blakemore WF. Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J Neurocytol. 1995;24:775–781. doi: 10.1007/BF01191213. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Skoff RP, Sprinkle TJ. Differential expression of galactocerebroside, myelin basic protein, and 2′,3′-cyclic nucleotide 3′-phosphohydrolase during development of oligodendrocytes in vitro. J Neurosci Res. 1988;21:249–259. doi: 10.1002/jnr.490210217. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JR, Ramaswamy SG, Jacobowitz DM, Quarles RH. A rabbit autoantibody specific for the 46-kDa form of 2′,3′-cyclic nucleotide 3′-phosphodiesterase. J Neurochem. 1992;58:1829–1835. doi: 10.1111/j.1471-4159.1992.tb10059.x. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J. Differential regulation of the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene during oligodendrocyte development. Neuron. 1994;12:1363–1375. doi: 10.1016/0896-6273(94)90451-0. [DOI] [PubMed] [Google Scholar]
- Simons M, Lyons DA. Axonal selection and myelin sheath generation in the central nervous system. Curr Opin Cell Biol. 2013;25:512–519. doi: 10.1016/j.ceb.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Snaidero N, Mobius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave KA, Simons M. Myelin Membrane Wrapping of CNS Axons by PI(3,4,5)P3-Dependent Polarized Growth at the Inner Tongue. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma JS, McPhail LT, Ramer MS. Differential RIP antigen (CNPase) expression in peripheral ensheathing glia. Brain Res. 2007;1137:1–10. doi: 10.1016/j.brainres.2006.12.053. [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chen Y, Kim B, Wang H, Zhao C, He X, Liu L, Liu W, Wu LM, Mao M, Chan JR, Wu J, Lu QR. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell. 2013;152:248–261. doi: 10.1016/j.cell.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. J Neurosci Res. 2002;70:529–545. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The section of spleen tissues from CNP-mGFP mice at P14 was stained with tomato-lectin to stain spleen cells and nuclear counterstained with Hoechst 33258 as indicated. Scale bar: 50 μm.