Abstract
Nicotine alters appetite and energy expenditure, leading to changes in body weight. While the exact mechanisms underlying these effects are not fully established, both central and peripheral involvement of the alpha-7 nicotinic acetylcholine receptor (α7nAChR) has been suggested. Centrally, the α7nAChR modulates activity of hypothalamic neurons involved in food intake regulation, including proopiomelanocortin and neuropeptide Y. α7nAChRs also modulate glutamatergic and dopaminergic systems controlling reward processes that affect food intake. Additionally, α7nAChRs are important peripheral mediators of chronic inflammation, a key contributor to health problems in obesity. This review focuses on nicotinic cholinergic effects on eating behaviors, specifically those involving the α7nAChR, with the hypothesis that α7nAChR agonism leads to appetite suppression. Recent studies are highlighted that identify links between α7nAChR expression and obesity, insulin resistance, and diabetes and describe early findings showing an α7nAChR agonist to be associated with reduced weight gain in a mouse model of diabetes. Given these effects, the α7nAChR may be a useful therapeutic target for strategies to treat and manage obesity.
Keywords: α7 nicotinic receptor, nicotine, obesity, eating behaviors, food intake
INTRODUCTION
Nicotine has long been known to affect energy balance and weight. Smokers, for example, weigh less than age- and sex-matched non-smokers (Albanes et al., 1987), while smoking cessation is associated with increased food intake and weight gain (Stamford et al., 1986; Williamson et al., 1991; Filozof et al., 2004). Given the strong link between smoking and reduced weight, many report using smoking for weight control, or avoid cessation due to fear of weight gain (Camp et al., 1993; Wiseman et al., 1998; Fulkerson and French, 2003). Experimentally, nicotine has been shown to suppress appetite, increase energy expenditure, and alter feeding patterns, which can lead to weight loss (Jo et al., 2002; Zoli and Picciotto, 2012). Despite these known effects, however, the mechanisms underlying nicotine’s effects on eating behaviors and obesity remain unclear. Nicotine acts on both high-affinity nicotinic cholinergic receptors, such as the α4-β2 receptor, and low-affinity receptors, such as the α7 receptor, both centrally and peripherally. Recent studies suggest that the alpha-7 nicotinic acetylcholine receptor (α7nAChR) may play a particularly prominent role in nicotinic effects on eating behaviors. As such, this review focuses on neuronal effects of nicotinic agents, especially those involving the α7nAChR, how stimulation of this receptor influences eating behaviors and weight, and the potential utility of α7nAChR agonists as a novel treatment strategy for obesity.
ALPHA-7 NICOTINIC ACETYLCHOLINE RECEPTORS
Neuronal nicotinic acetylcholine receptors consist of ligand-gated ion channels that are activated by acetylcholine, but also respond to nicotine and similar compounds. These receptors are comprised of five transmembrane subunits arranged around a central pore (Paterson and Nordberg, 2000; Dani and Bertrand, 2007). These subunits include αβ combinations (α2–α6 and β2–β4), homomeric nAChRs (α7–α9), and a heteromer α combination (α9 with α10) (McGehee et al., 1995; Jones et al., 1999; Dani and Bertrand, 2007). The two main types of nAChRs found in the brain are α4–β2 receptors and α7 receptors (Jensen et al., 2005; Changeux, 2010). While different nAChR subtypes may affect circuits involved in feeding behavior (Jo et al., 2002; Mineur et al., 2011a,b; Zoli and Picciotto, 2012), this review will focus on α7nAChRs, which are receiving increased research attention for their involvement in eating behaviors and food intake.
CENTRAL EFFECTS OF α7nAChRs ON EATING BEHAVIORS
Previous reviews have described peripheral effects of nicotine and other α7nAChR agonists on obesity and eating behaviors (Bencherif et al., 2011; Lakhan and Kirchgessner, 2011). As such, while recent evidence for peripheral effects will be briefly examined, the primary focus of this review will be on central effects. Overall, nicotine and other α7nAChR agonists appear to suppress appetite through numerous complex, interacting central pathways, particularly those in the hypothalamus, which plays a fundamental role in energy balance. When various interactions are jointly considered, activation of hypothalamic α7nAChRs is thought to result in overall increased inhibition of appetite circuits, resulting in decreased food intake (Jo et al., 2002). Stimulation of α7nAChRs may also reduce food intake via effects on reward pathways or cortical networks involved in eating behaviors.
α7nAChR EFFECTS ON HYPOTHALAMIC NEUROPEPTIDES
Hypothalamic nuclei most associated with energy balance and feeding regulation include the lateral hypothalamus (LH), ventromedial hypothalamus (VMH), arcuate nucleus (ARC), and paraventricular nucleus (PVN). The LH is often simplistically described as the “hunger center” and the VMH the “satiety center” (Schwartz et al., 2000; Zoli and Picciotto, 2012). The ARC is a primary center for peripheral feeding signal integration (e.g., leptin, insulin) and contains neurons that stimulate feeding and those that inhibit feeding when activated, with projections to the PVN and LH (Schwartz et al., 2000; Kageyama et al., 2012; Zoli and Picciotto, 2012).
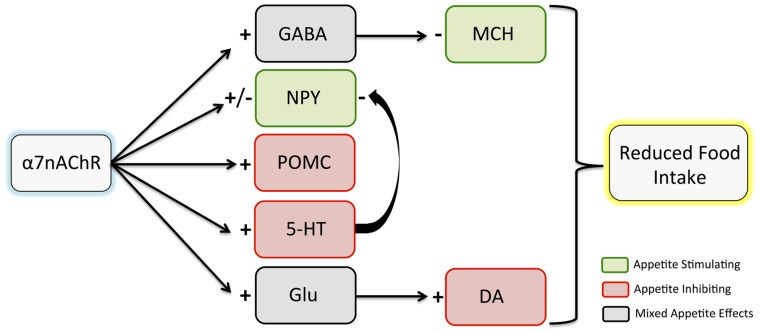
A primary potential pathway for α7nAChR mediation of eating behaviors involves hypothalamic cholinergic input. The hypothalamus contains rich cholinergic innervation and some of the highest levels of α7nAChR expression in the brain (Sargent, 1993). Appetite-related circuits within the hypothalamus can be modulated by nAChR activation, with a complex network of hormone and neuropeptide signals exerting neuronal effects to regulate eating behaviors. A number of studies have demonstrated effects of nicotine on these signals. Here, we will discuss α7nAChR involvement in cholinergic effects on proopiomelanocortin (POMC), neuropeptide Y (NPY), and melanin-concentrating hormone (MCH), all of which are involved in feeding regulation (Figure 1).
FIGURE 1.
Effects of α7nAChR stimulation on hypothalamic neuropeptide and neurotransmitter release. Although there are complex interactions among pathways, it is hypothesized that the net effect of α7nAChR stimulation leads to reduced food intake. Inhibitory effects are indicated with a minus sign, while excitatory effects are indicated with a plus sign. Note that both inhibitory and excitatory effects of α7nAChR stimulation have been observed on NPY. While appetite effects of dopamine elsewhere in the brain are mixed, studies suggest that hypothalamic dopamine release contributes to appetite inhibition. α7nAChR, α7 nicotinic acetylcholine receptor; GABA, gamma aminobutyric acid; NPY, neuropeptide Y; POMC, proopiomelanocortin; 5-HT, serotonin; Glu, glutamate; MCH, melanin-concentrating hormone; DA, dopamine.
POMC AND NPY
Nicotine may suppress appetite via activation of POMC neurons. POMC is produced in the hypothalamus (Huang et al., 2011; Zoli and Picciotto, 2012) and is a precursor for melanocortins, such as α-melanocyte-stimulating hormone (α-MSH), associated with suppressed food intake (Schwartz et al., 2000; Zoli and Picciotto, 2012). Electrophysiologically, Huang et al. (2011) demonstrated that nicotine excites mouse hypothalamic POMC neurons and that α7nAChRs are present on these neurons. Nicotine effects were reduced by the α7nAChR antagonist methyllycaconitine (MLA), suggesting at least partial mediation by α7nAChRs. As such, POMC stimulation is a potential mechanism through which α7nAChR agonism may suppress appetite. It should be noted, however, that MLA is not as selective an antagonist for α7nAChRs as α-bungarotoxin (Klink et al., 2001; Mogg et al., 2002), which should be considered when MLA is used to assess α7nAChR effects.
Neuropeptide Y, also produced in the hypothalamus, is associated with increased food intake (Schwartz et al., 2000). NPY neurons in the ARC project to the PVN to stimulate feeding (Morris, 1989; Kageyama et al., 2012). Thus, POMC and NPY have opposing effects on food intake. Smokers show reduced NPY levels compared to non-smokers, and smoking cessation is associated with increased NPY (Hussain et al., 2012), suggesting NPY inhibition as a mechanism for appetite suppression. However, nicotine effects on NPY are complex. As with POMC, NPY neurons in the hypothalamus are stimulated by nicotine and express α7nAChRs. Excitation of NPY neurons by nicotine is partially mediated by α7nAChRs, as MLA reduces excitation. Although nicotine reduces hypothalamic NPY mRNA in rats acutely (Frankish et al., 1995), NPY mRNA increases with chronic administration (Frankish et al., 1995; Li et al., 2000), which is accompanied by decreased food intake (Li et al., 2000). This is counterintuitive, as NPY stimulates food intake. However, nicotine also reduces hypothalamic NPY receptor density (Kane et al., 2001), which could explain the decreased intake. Another explanation for the net appetite-inhibiting effect of nicotine is that the depolarizing effect of nicotine on POMC neurons (anorexigenic) is significantly greater than that on NPY neurons (orexigenic). Furthermore, in addition to NPY neuron excitation by nicotine, inhibition of excitatory synaptic activity (glutamate release) on NPY neurons was also observed, an effect not seen in POMC neurons (Huang et al., 2011). Thus, although nicotine can excite NPY neurons, the greater direct excitation of appetite-inhibiting POMC neurons compared to appetite-stimulating NPY neurons, in addition to indirect inhibition of NPY neurons (via reduced glutamate release) may contribute to the net effect of appetite inhibition by nicotine and other α7nAChR agonists.
MELANIN-CONCENTRATING HORMONE
Melanin-concentrating hormone (MCH) neurons are primarily located in the LH (Zamir et al., 1986) and also stimulate food intake (Qu et al., 1996). MCH may have a particular role in reward-related aspects of food, as MCH neurons project to the nucleus accumbens (NAC) and the ventral tegmental area (VTA), brain areas involved in reward processes (Schilstrom et al., 1998; Jo et al., 2005). MCH knockout mice are excessively lean and demonstrate reduced food intake (Shimada et al., 1998; Marsh et al., 2002). α7nAChRs may mediate gamma aminobutyric acid (GABA)-related inhibition of MCH neurons in the LH, leading to this appetite suppression (Jo et al., 2005).
α7nAChR MODULATION OF NEUROTRANSMITTERS INVOLVED IN FOOD INTAKE BEHAVIORS
In addition to hypothalamic neuropeptides, nicotine modulates effects of multiple other neurotransmitter systems in the brain. The following section describes the impact of nicotine on GABA, glutamate, dopamine (DA), and serotonin, focusing on how α7nAChRs may inhibit appetite by modulating these neurotransmitter systems.
GAMMA AMINOBUTYRIC ACID
Release of GABA, the main inhibitory neurotransmitter in the brain, is influenced by nAChRs (McGehee et al., 1995; Jones et al., 1999). Nicotine effects on appetite reduction may be associated with decreased excitability of MCH neurons in the LH via increased GABAergic inhibitory tone. Jo et al. (2005) found nicotine administration to facilitate GABAergic transmission in adult mice, and prenatal nicotine exposure to enhance postnatal GABAergic transmission. Specific involvement of α7nAChRs was also demonstrated, as an α7nAChR-specific antagonist (α-bungarotoxin) blocked these effects. As such, activation of α7nAChRs on GABAergic terminals in the hypothalamus may contribute to the anorexigenic effects of nicotine.
GLUTAMATE AND DOPAMINE
Glutamate is the main excitatory neurotransmitter in the brain and plays a role in rewarding effects of nicotine, as nicotine increases glutamate release in the VTA and NAC, brain regions central to reward mechanisms (McGehee et al., 1995; Reid et al., 2000; Schilstrom et al., 2000). High concentrations of α7nAChRs are observed in the VTA (Clarke and Pert, 1985; Dominguez del Toro et al., 1994; Schilstrom et al., 1998; Jones and Wonnacott, 2004) and are thought to mediate nicotine-associated glutamate release (McGehee et al., 1995; Schilstrom et al., 2000). α7nAChR-mediated glutamate release plays a large role in nicotine’s effects on DA, a neurotransmitter critical in the reinforcing effects of nicotine (Schilstrom et al., 1998; Fowler et al., 2008). α4-β2nAChRs are sufficient for these reinforcing effects (Besson et al., 2012), likely via direct effects on DA neurons (Wooltorton et al., 2003; Besson et al., 2012). However, stimulation of α7nAChRs activates DA neurons via glutamatergic inputs (Yoshida et al., 1992; Schilstrom et al., 2000, 2003; Garzon et al., 2013). Thus, α7nAChR activation ultimately increases DA, but this is largely mediated via glutamatergic effects. Additionally, α7nAChRs may be important in dopaminergic function following long-term nicotine exposure, as they are more resistant to desensitization at usual levels for smokers than nAChR subunits containing β2 receptors, and may prevent dopaminergic hypoactivation resulting from chronic β2 desensitization (Besson et al., 2007, 2012).
The role of α7nAChR-mediated glutamate release in food consumption remains unclear. Administration of a glutamate antagonist has been found to increase food intake in rats (Maldonado-Irizarry et al., 1995; Stratford et al., 1998). As such, glutamate release stimulated by an α7nAChR agonist could decrease food intake. Increased DA release, amplified by α7nAChR-mediated glutamate release, increases the reward value of food (Yoshida et al., 1992; Schilstrom et al., 1998). Quarta et al. (2009) observed striatal DA release in mice following administration of an α7nAChR agonist (choline), an effect not observed in mice lacking α7nAChRs. Food-induced DA release is attenuated by an α7nAChR antagonist (MLA), implicating α7nAChRs in eating-related reward (Schilstrom et al., 1998). However, the role of DA in feeding behaviors is complex and varies by brain region. Although DA contributes to rewarding aspects of food intake in areas such as the VTA and NAC, hypothalamic DA release is though to contribute to nicotine-related reductions in food intake (Meguid et al., 2000; Schwartz et al., 2000). Thus, further study is needed to determine if effects of α7nAChRs on DA lead to overall increased or decreased consumption.
SEROTONIN
Serotonin inhibits food intake (Waldbillig et al., 1981; Jo et al., 2002), likely by promoting satiety (i.e., meal stopping; Shor-Posner et al., 1986). One mechanism may be via NPY, as evidence suggests serotonin inhibits NPY release (Dryden et al., 1995, 1996a,b). Nicotine-induced nAChR activation can increase serotonin release, contributing to appetite suppression (Summers and Giacobini, 1995; Jo et al., 2002). Activation of α7nAChRs is thought to influence serotonin release, as α7nAChRs have been identified on serotonergic neurons (Galindo-Charles et al., 2008) and α7nAChR stimulation increases serotonin release in the dorsal raphe nucleus (Li et al., 1998).
CORTICAL α7nAChR INVOLVEMENT IN FOOD INTAKE BEHAVIORS
Cortically, α7nAChR activation may affect limbic and paralimbic brain systems such as the insula and cingulate cortex, which also play a role in reward aspects of eating behaviors (Volkow et al., 2010) and contain rich cholinergic innervation (Nyback et al., 1989).
INSULA/SALIENCE NETWORK
The insula, containing primary taste cortex, is involved in eating behavior regulation, including involvement in rewarding aspects of food and food-related arousal (Tataranni et al., 1999; Hinton et al., 2004; Cornier et al., 2009). The insula is also a central component of the salience network, an intrinsic brain network involved in assessing relevance of internal and external stimuli (Seeley et al., 2007; Bressler and Menon, 2010), in which altered response has been observed in obese, compared to lean, individuals (Garcia-Garcia et al., 2012; Kullmann et al., 2013). The insula is associated with urges and cravings related to both food and drugs of abuse (Pelchat et al., 2004; Naqvi and Bechara, 2009; Forget et al., 2010). Indeed, smokers sustaining insula damage following a stroke showed little subsequent difficulty quitting smoking, suggesting a role for the insula in effects of nicotine (Naqvi et al., 2007). However, the role of α7nAChRs in the insula is not yet known. Via α-bungarotoxin binding, studies have found α7nAChRs in the insula in both rats (Fuchs, 1989) and monkeys (Han et al., 2003). Presence of α7nAChRs in the human insula has been suggested by detection of α7nAChR mRNA (Wevers, 2011), but insular α7nAChR protein levels have not yet been studied in humans. As such, further study of α7nAChRs in the insula, and how activation of these receptors relates to eating behaviors, is needed.
POSTERIOR CINGULATE/DEFAULT MODE NETWORK
The posterior cingulate cortex may also be involved in eating behaviors, having been associated with neuronal responses to visual food cues and taste (Tataranni et al., 1999; DelParigi et al., 2005; Cornier et al., 2009). The posterior cingulate is also a key component of the default mode network (DMN), an intrinsic brain network involved in self-referential thoughts and attention to internal stimuli (Buckner et al., 2008). DMN activity may play a role in eating behaviors, as overactivity of this network has been observed in obese, compared to lean, individuals (Tregellas et al., 2011a). Furthermore, this activity, which was associated with measures of appetite, was shown to change in response to feeding in lean, but not obese individuals. Nicotine can reduce resting-state DMN activity, including the posterior cingulate (Tanabe et al., 2011). α7nAChRs are present in high concentrations in the cingulate cortex, as assessed by α-bungarotoxin binding (Breese et al., 1997; Marutle et al., 2001). A study of DMN activity in schizophrenia patients observed reduced response following treatment with an α7nAChR partial agonist [3-2,4-dimethoxybenzylidene anabaseine (DMXB-A)], specifically in the posterior cingulate (Tregellas et al., 2011b). As with non-mentally ill obese individuals, DMN overactivity has been observed in schizophrenia patients (Garrity et al., 2007; Whitfield-Gabrieli et al., 2009), who are obese are rates twice those observed in the general population. Given these findings, it is possible that activation of α7nAChRs could be a mechanism to normalize DMN hyperactivity in obesity.
α7nAChRs AND PERIPHERAL FACTORS INVOLVED IN EATING BEHAVIORS AND OBESITY
Recent studies have discovered a key role for α7nAChRs in peripheral factors related to obesity. In a mouse model of diabetes, Marrero et al. (2010) found that an α7nAChR-selective agonist (TC-7020) reduced weight gain and food intake, as well as glucose and triglyceride levels and expression of proinflammatory cytokines. These effects were reversed by an α7nAChR antagonist (MLA), supporting α7nAChR involvement. In humans, Cancello et al. (2012) have also found evidence supporting α7nAChR involvement in obesity. In addition to identifying α7nAChR expression in human mature adipocytes, they found that expression was downregulated in obese compared to lean adults, and that weight loss partially restored α7nAChR expression.
A potential mechanism through which peripheral α7nAChRs may exert weight and food intake effects is by mediating anti-inflammatory effects. Inflammation is a key feature of obesity, associated with increased proinflammatory cytokine production, insulin resistance, and development of type 2 diabetes (Marrero et al., 2010; Wang et al., 2011). Activation of α7nAChRs on cytokine-producing cells, such as macrophages, mediates this inflammatory response by inhibiting inflammatory cytokine production (Wang et al., 2011). A number of studies have demonstrated anti-inflammatory effects of nicotine (Wang et al., 2003; Lakhan and Kirchgessner, 2011) and smokers may have a reduced risk of some inflammatory diseases such as ulcerative colitis (Lakhan and Kirchgessner, 2011). The “cholinergic anti-inflammatory pathway” can be activated by α7nAChR agonists (Cheng et al., 2007). Supporting this, nicotine-induced cytokine inhibition can be blocked by α7nAChR-specific antagonists (Cheng et al., 2007), and α7nAChR knockout mice show increased LPS-induced proinflammatory cytokine production, including TNFα and IL-1β (Wang et al., 2003). Wang et al. (2011) found adipose tissue and macrophages in mice to express α7nAChRs, and while nicotine suppressed proinflammatory cytokine production, this effect was not observed in α7nAChR knockout mice. Additionally, nicotine reduced adipose tissue inflammation and improved insulin sensitivity in obese mice. Xu et al. (2012) observed improved insulin sensitivity in rodents following treatment with either nicotine or an α7nAChR agonist (PNU-282987), an effect not observed in α7nAChR knockout animals. These studies suggest that α7nAChRs are critical in anti-inflammatory effects of nicotine. Given this, therapeutics targeting α7nAChRs are increasingly being explored for diseases involving inflammation, such as diabetes, arthritis, and ulcerative colitis (Wang et al., 2003; Marrero et al., 2010; Bencherif et al., 2011; Lakhan and Kirchgessner, 2011).
CONCLUSION
The α7nAChR plays an important role in both central and peripheral mechanisms involved in eating behaviors and energy balance. Studies have found links between α7nAChR expression and obesity, insulin resistance, and diabetes. Centrally, α7nAChRs modulate hypothalamic neuropeptides and neurotransmitters involved in feeding regulation and play a role in cortical processes affecting intake behavior. Overall, although the circuits involved are complex, it appears that net effects of nicotine and other α7nAChR agonists result in appetite suppression, which could lead to weight loss. Peripherally, and perhaps also centrally, α7nAChRs are also an important mediator of inflammation, a key contributor to health problems in obesity.
Although α7nAChR agonists have not yet been investigated for eating behavior effects in humans, preliminary animal work supports this idea, finding peripheral effects such as improved insulin sensitivity (Wang et al., 2011; Xu et al., 2012) and reduced weight gain and metabolic changes in a model of diabetes (Marrero et al., 2010). Further support for extending α7nAChR studies to humans lies in the observation that α7nAChRs are downregulated in human obesity, but normalize with weight loss (Cancello et al., 2012). In conclusion, given nicotine’s effects in humans, experimental support for α7nAChR involvement in eating behavior regulation, and early evidence of α7nAChR agonist effects in animal studies, the α7nAChR may represent a promising new therapeutic target for weight management and the treatment of obesity in humans.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Work on this manuscript was supported by NIH/NIDDK grant R01DK089095.
REFERENCES
- Albanes D., Jones D. Y., Micozzi M. S., Mattson M. E. (1987). Associations between smoking and body weight in the US population: analysis of NHANES II. Am. J. Public Health 77 439–444 10.2105/AJPH.77.4.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif M., Lippiello P. M., Lucas R., Marrero M. B. (2011). Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell. Mol. Life Sci. 68 931–949 10.1007/s00018-010-0525-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M., David V., Baudonnat M., Cazala P., Guilloux J. P., Reperant C., et al. (2012). Alpha7-nicotinic receptors modulate nicotine-induced reinforcement and extracellular dopamine outflow in the mesolimbic system in mice. Psychopharmacology (Berl.) 220 1–14 10.1007/s00213-011-2422-1 [DOI] [PubMed] [Google Scholar]
- Besson M., Granon S., Mameli-Engvall M., Cloez-Tayarani I., Maubourguet N., Cormier A., et al. (2007). Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc. Natl. Acad. Sci. U.S.A. 104 8155–8160 10.1073/pnas.0702698104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese C. R., Adams C., Logel J., Drebing C., Rollins Y., Barnhart M., et al. (1997). Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J. Comp. Neurol. 387 385–398 [DOI] [PubMed] [Google Scholar]
- Bressler S. L., Menon V. (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 14 277–290 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Buckner R. L., Andrews-Hanna J. R., Schacter D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124 1–38 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Camp D. E., Klesges R. C., Relyea G. (1993). The relationship between body weight concerns and adolescent smoking. Health Psychol. 12 24–32 10.1037/0278-6133.12.1.24 [DOI] [PubMed] [Google Scholar]
- Cancello R., Zulian A., Maestrini S., Mencarelli M., Della Barba A., Invitti C., et al. (2012). The nicotinic acetylcholine receptor alpha7 in subcutaneous mature adipocytes: downregulation in human obesity and modulation by diet-induced weight loss. Int. J. Obes. (Lond.) 36 1552–1557 10.1038/ijo.2011.275 [DOI] [PubMed] [Google Scholar]
- Changeux J. P. (2010). Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat. Rev. Neurosci. 11 389–401 10.1038/nrn2849 [DOI] [PubMed] [Google Scholar]
- Cheng P. Y., Lee Y. M., Law K. K., Lin C. W., Yen M. H. (2007). The involvement of AMP-activated protein kinases in the anti-inflammatory effect of nicotine in vivo and in vitro. Biochem. Pharmacol. 74 1758–1765 10.1016/j.bcp.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Clarke P. B., Pert A. (1985). Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 348 355–358 10.1016/0006-8993(85)90456-1 [DOI] [PubMed] [Google Scholar]
- Cornier M. A., Salzberg A. K., Endly D. C., Bessesen D. H., Rojas D. C., Tregellas J. R. (2009). The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE 4:e6310. 10.1371/journal.pone.0006310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Bertrand D. (2007). Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 47 699–729 10.1146/annurev.pharmtox.47.120505.105214 [DOI] [PubMed] [Google Scholar]
- DelParigi A., Chen K., Salbe A. D., Reiman E. M., Tataranni P. A. (2005). Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage 24 436–443 10.1016/j.neuroimage.2004.08.035 [DOI] [PubMed] [Google Scholar]
- Dominguez del Toro E., Juiz J. M., Peng X., Lindstrom J., Criado M. (1994). Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J. Comp. Neurol. 349 325–342 10.1002/cne.903490302 [DOI] [PubMed] [Google Scholar]
- Dryden S., Frankish H. M., Wang Q., Pickavance L., Williams G. (1996a). The serotonergic agent fluoxetine reduces neuropeptide Y levels and neuropeptide Y secretion in the hypothalamus of lean and obese rats. Neuroscience 72 557–566 10.1016/0306-4522(95)00566-8 [DOI] [PubMed] [Google Scholar]
- Dryden S., Frankish H. M., Wang Q., Williams G. (1996b). Increased feeding and neuropeptide Y (NPY) but not NPY mRNA levels in the hypothalamus of the rat following central administration of the serotonin synthesis inhibitor p-chlorophenylalanine. Brain Res. 724 232–237 10.1016/0006-8993(96)00329-0 [DOI] [PubMed] [Google Scholar]
- Dryden S., Wang Q., Frankish H. M., Pickavance L., Williams G. (1995). The serotonin (5-HT) antagonist methysergide increases neuropeptide Y (NPY) synthesis and secretion in the hypothalamus of the rat. Brain Res. 699 12–18 10.1016/0006-8993(95)00841-D [DOI] [PubMed] [Google Scholar]
- Filozof C., Fernandez Pinilla M. C., Fernandez-Cruz A. (2004). Smoking cessation and weight gain. Obes. Rev. 5 95–103 10.1111/j.1467-789X.2004.00131.x [DOI] [PubMed] [Google Scholar]
- Forget B., Pushparaj A, Le Foll B. (2010). Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol. Psychiatry 68 265–271 10.1016/j.biopsych.2010.01.029 [DOI] [PubMed] [Google Scholar]
- Fowler C. D., Arends M. A., Kenny P. J. (2008). Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav. Pharmacol. 19 461–484 10.1097/FBP.0b013e32830c360e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankish H. M., Dryden S., Wang Q., Bing C., Macfarlane I. A., Williams G. (1995). Nicotine administration reduces neuropeptide Y and neuropeptide Y mRNA concentrations in the rat hypothalamus: NPY may mediate nicotine’s effects on energy balance. Brain Res. 694 139–146 10.1016/0006-8993(95)00834-D [DOI] [PubMed] [Google Scholar]
- Fuchs J. L. (1989). [125I]alpha-bungarotoxin binding marks primary sensory area developing rat neocortex. Brain Res. 501 223–234 10.1016/0006-8993(89)90640-9 [DOI] [PubMed] [Google Scholar]
- Fulkerson J. A., French S. A. (2003). Cigarette smoking for weight loss or control among adolescents: gender and racial/ethnic differences. J. Adolesc. Health 32 306–313 10.1016/S1054-139X(02)00566-9 [DOI] [PubMed] [Google Scholar]
- Galindo-Charles L., Hernandez-Lopez S., Galarraga E., Tapia D., Bargas J., Garduno J., et al. (2008). Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse 62 601–615 10.1002/syn.20526 [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia I., Jurado M. A., Garolera M., Segura B., Sala-Llonch R., Marques-Iturria I., et al. (2012). Alterations of the salience network in obesity: a resting-state fMRI study. Hum. Brain Mapp. 34 2786–2797 10.1002/hbm.22104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity A. G., Pearlson G. D., Mckiernan K., Lloyd D., Kiehl K. A., Calhoun V. D. (2007). Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry 164 450–457 10.1176/appi.ajp.164.3.450 [DOI] [PubMed] [Google Scholar]
- Garzon M., Duffy A. M., Chan J., Lynch M. K., Mackie K., Pickel V. M. (2013). Dopamine D(2) and acetylcholine alpha7 nicotinic receptors have subcellular distributions favoring mediation of convergent signaling in the mouse ventral tegmental area. Neuroscience 252 126–143 10.1016/j.neuroscience.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z. Y., Zoli M., Cardona A., Bourgeois J. P., Changeux J. P, Le Novere N. (2003). Localization of [3H]nicotine, [3H]cytisine, [3H]epibatidine, and [125I]alpha-bungarotoxin binding sites in the brain of Macaca mulatta. J. Comp. Neurol. 461 49–60 10.1002/cne.10659 [DOI] [PubMed] [Google Scholar]
- Hinton E. C., Parkinson J. A., Holland A. J., Arana F. S., Roberts A. C., Owen A. M. (2004). Neural contributions to the motivational control of appetite in humans. Eur. J. Neurosci. 20 1411–1418 10.1111/j.1460-9568.2004.03589.x [DOI] [PubMed] [Google Scholar]
- Huang H., Xu Y, Van Den Pol A. N. (2011). Nicotine excites hypothalamic arcuate anorexigenic proopiomelanocortin neurons and orexigenic neuropeptide Y neurons: similarities and differences. J. Neurophysiol. 106 1191–1202 10.1152/jn.00740.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T., Al-Daghri N. M., Al-Attas O. S., Draz H. M., Abd Al-Rahman S. H., Yakout S. M. (2012). Plasma neuropeptide Y levels relate cigarette smoking and smoking cessation to body weight regulation. Regul. Pept. 176 22–27 10.1016/j.regpep.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Jensen A. A., Frolund B., Liljefors T., Krogsgaard-Larsen P. (2005). Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 48 4705–4745 10.1021/jm040219e [DOI] [PubMed] [Google Scholar]
- Jones I. W., Wonnacott S. (2004). Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J. Neurosci. 24 11244–11252 10.1523/JNEUROSCI.3009-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Sudweeks S., Yakel J. L. (1999). Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 22 555–561 10.1016/S0166-2236(99)01471-X [DOI] [PubMed] [Google Scholar]
- Jo Y. H., Talmage D. A., Role L. W. (2002). Nicotinic receptor-mediated effects on appetite and food intake. J. Neurobiol. 53 618–632 10.1002/neu.10147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y. H., Wiedl D., Role L. W. (2005). Cholinergic modulation of appetite-related synapses in mouse lateral hypothalamic slice. J. Neurosci. 25 11133–11144 10.1523/JNEUROSCI.3638-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama H., Takenoya F., Hirako S., Wada N., Kintaka Y., Inoue S., et al. (2012). Neuronal circuits involving neuropeptide Y in hypothalamic arcuate nucleus-mediated feeding regulation. Neuropeptides 46 285–289 10.1016/j.npep.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Kane J. K., Parker S. L., Li M. D. (2001). Hypothalamic orexin-A binding sites are downregulated by chronic nicotine treatment in the rat. Neurosci. Lett. 298 1–4 10.1016/S0304-3940(00)01730-4 [DOI] [PubMed] [Google Scholar]
- Klink R., De Kerchove D’Exaerde A., Zoli M., Changeux J. P. (2001). Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci. 21 1452–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S., Pape A. A., Heni M., Ketterer C., Schick F., Haring H. U., et al. (2013). Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb. Cortex 23 1247–1256 10.1093/cercor/bhs124 [DOI] [PubMed] [Google Scholar]
- Lakhan S. E., Kirchgessner A. (2011). Anti-inflammatory effects of nicotine in obesity and ulcerative colitis. J. Transl. Med. 9:129. 10.1186/1479-5876-9-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. D., Kane J. K., Parker S. L., Mcallen K., Matta S. G., Sharp B. M. (2000). Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res. 867 157–164 10.1016/S0006-8993(00)02283-6 [DOI] [PubMed] [Google Scholar]
- Li X., Rainnie D. G., Mccarley R. W., Greene R. W. (1998). Presynaptic nicotinic receptors facilitate monoaminergic transmission. J. Neurosci. 18 1904–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Irizarry C. S., Swanson C. J., Kelley A. E. (1995). Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J. Neurosci. 15 6779–6788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero M. B., Lucas R., Salet C., Hauser T. A., Mazurov A., Lippiello P. M., et al. (2010). An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J. Pharmacol. Exp. Ther. 332 173–180 10.1124/jpet.109.154633 [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Weingarth D. T., Novi D. E., Chen H. Y., Trumbauer M. E., Chen A. S., et al. (2002). Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc. Natl. Acad. Sci. U.S.A. 99 3240–3245 10.1073/pnas.052706899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutle A., Zhang X., Court J., Piggott M., Johnson M., Perry R., et al. (2001). Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J. Chem. Neuroanat. 22 115–126 10.1016/S0891-0618(01)00117-X [DOI] [PubMed] [Google Scholar]
- McGehee D. S., Heath M. J., Gelber S., Devay P., Role L. W. (1995). Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269 1692–1696 10.1126/science.7569895 [DOI] [PubMed] [Google Scholar]
- Meguid M. M., Fetissov S. O., Varma M., Sato T., Zhang L., Laviano A., et al. (2000). Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 16 843–857 10.1016/S0899-9007(00)00449-4 [DOI] [PubMed] [Google Scholar]
- Mineur Y. S., Abizaid A., Rao Y., Salas R., Dileone R. J., Gundisch D., et al. (2011a). Nicotine decreases food intake through activation of POMC neurons. Science 332 1330–1332 10.1126/science.1201889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur Y. S., Einstein E. B., Seymour P. A., Coe J. W., O’Neill B. T., Rollema H., et al. (2011b). alpha4beta2 nicotinic acetylcholine receptor partial agonists with low intrinsic efficacy have antidepressant-like properties. Behav. Pharmacol. 22 291–299 10.1097/FBP.0b013e328347546d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg A. J., Whiteaker P., Mcintosh J. M., Marks M., Collins A. C., Wonnacott S. (2002). Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J. Pharmacol. Exp. Ther. 302 197–204 10.1124/jpet.302.1.197 [DOI] [PubMed] [Google Scholar]
- Morris B. J. (1989). Neuronal localisation of neuropeptide Y gene expression in rat brain. J. Comp. Neurol. 290 358–368 10.1002/cne.902900305 [DOI] [PubMed] [Google Scholar]
- Naqvi N. H., Bechara A. (2009). The hidden island of addiction: the insula. Trends Neurosci. 32 56–67 10.1016/j.tins.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N. H., Rudrauf D., Damasio H., Bechara A. (2007). Damage to the insula disrupts addiction to cigarette smoking. Science 315 531–534 10.1126/science.1135926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyback H., Nordberg A., Langstrom B., Halldin C., Hartvig P., Ahlin A., et al. (1989). Attempts to visualize nicotinic receptors in the brain of monkey and man by positron emission tomography. Prog. Brain Res. 79 313–319 10.1016/S0079-6123(08)62490-5 [DOI] [PubMed] [Google Scholar]
- Paterson D., Nordberg A. (2000). Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 61 75–111 10.1016/S0301-0082(99)00045-3 [DOI] [PubMed] [Google Scholar]
- Pelchat M. L., Johnson A., Chan R., Valdez J., Ragland J. D. (2004). Images of desire: food-craving activation during fMRI. Neuroimage 23 1486–1493 10.1016/j.neuroimage.2004.08.023 [DOI] [PubMed] [Google Scholar]
- Quarta D., Naylor C. G., Barik J., Fernandes C., Wonnacott S., Stolerman I. P. (2009). Drug discrimination and neurochemical studies in alpha7 null mutant mice: tests for the role of nicotinic alpha7 receptors in dopamine release. Psychopharmacology (Berl.) 203 399–410 10.1007/s00213-008-1281-x [DOI] [PubMed] [Google Scholar]
- Qu D., Ludwig D. S., Gammeltoft S., Piper M., Pelleymounter M. A., Cullen M. J., et al. (1996). A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380 243–247 10.1038/380243a0 [DOI] [PubMed] [Google Scholar]
- Reid M. S., Fox L., Ho L. B., Berger S. P. (2000). Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse 35 129–136 [DOI] [PubMed] [Google Scholar]
- Sargent P. B. (1993). The diversity of neuronal nicotinic acetylcholine receptors. Annu. Rev. Neurosci. 16 403–443 10.1146/annurev.ne.16.030193.002155 [DOI] [PubMed] [Google Scholar]
- Schilstrom B., Fagerquist M. V., Zhang X., Hertel P., Panagis G., Nomikos G. G., et al. (2000). Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse 38 375–383 [DOI] [PubMed] [Google Scholar]
- Schilstrom B., Rawal N., Mameli-Engvall M., Nomikos G. G., Svensson T. H. (2003). Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. Int. J. Neuropsychopharmacol. 6 1–11 10.1017/S1461145702003188 [DOI] [PubMed] [Google Scholar]
- Schilstrom B., Svensson H. M., Svensson T. H., Nomikos G. G. (1998). Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience 85 1005–1009 10.1016/S0306-4522(98)00114-6 [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Woods S. C., Porte D., Jr., Seeley R. J., Baskin D. G. (2000). Central nervous system control of food intake. Nature 404 661–671 10.1038/35007534 [DOI] [PubMed] [Google Scholar]
- Seeley W. W., Menon V., Schatzberg A. F., Keller J., Glover G. H., Kenna H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27 2349–2356 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M., Tritos N. A., Lowell B. B., Flier J. S., Maratos-Flier E. (1998). Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396 670–674 10.1038/25341 [DOI] [PubMed] [Google Scholar]
- Shor-Posner G., Grinker J. A., Marinescu C., Brown O., Leibowitz S. F. (1986). Hypothalamic serotonin in the control of meal patterns and macronutrient selection. Brain Res. Bull. 17 663–671 10.1016/0361-9230(86)90198-X [DOI] [PubMed] [Google Scholar]
- Stamford B. A., Matter S., Fell R. D., Papanek P. (1986). Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am. J. Clin. Nutr. 43 486–494 [DOI] [PubMed] [Google Scholar]
- Stratford T. R., Swanson C. J., Kelley A. (1998). Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav. Brain Res. 93 43–50 10.1016/S0166-4328(97)00140-X [DOI] [PubMed] [Google Scholar]
- Summers K. L., Giacobini E. (1995). Effects of local and repeated systemic administration of (-)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem. Res. 20 753–759 10.1007/BF01705545 [DOI] [PubMed] [Google Scholar]
- Tanabe J., Nyberg E., Martin L. F., Martin J., Cordes D., Kronberg E., et al. (2011). Nicotine effects on default mode network during resting state. Psychopharmacology (Berl.) 216 287–295 10.1007/s00213-011-2221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni P. A., Gautier J. F., Chen K., Uecker A., Bandy D., Salbe A. D., et al. (1999). Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl. Acad. Sci. U.S.A. 96 4569–4574 10.1073/pnas.96.8.4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas J. R., Wylie K. P., Rojas D. C., Tanabe J., Martin J., Kronberg E., et al. (2011a). Altered default network activity in obesity. Obesity (Silver Spring) 19 2316–2321 10.1038/oby.2011.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas J. R., Tanabe J., Rojas D. C., Shatti S., Olincy A., Johnson L., et al. (2011b). Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol. Psychiatry 69 7–11 10.1016/j.biopsych.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Fowler J. S., Tomasi D., Telang F., Baler R. (2010). Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays 32 748–755 10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbillig R. J., Bartness T. J., Stanley B. G. (1981). Increased food intake, body weight, and adiposity in rats after regional neurochemical depletion of serotonin. J. Comp. Physiol. Psychol. 95 391–405 10.1037/h0077790 [DOI] [PubMed] [Google Scholar]
- Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., et al. (2003). Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421 384–388 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- Wang X., Yang Z., Xue B., Shi H. (2011). Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology 152 836–846 10.1210/en.2010-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers A. (2011). Localisation of pre- and postsynaptic cholinergic markers in the human brain. Behav. Brain Res. 221 341–355 10.1016/j.bbr.2010.02.025 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H. W., Milanovic S., Tsuang M. T., Faraone S. V., Mccarley R. W., et al. (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 106 1279–1284 10.1073/pnas.0809141106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. F., Madans J., Anda R. F., Kleinman J. C., Giovino G. A., Byers T. (1991). Smoking cessation and severity of weight gain in a national cohort. N. Engl. J. Med. 324 739–745 10.1056/NEJM199103143241106 [DOI] [PubMed] [Google Scholar]
- Wiseman C. V., Turco R. M., Sunday S. R., Halmi K. A. (1998). Smoking and body image concerns in adolescent girls. Int. J. Eat. Disord. 24 429–433 [DOI] [PubMed] [Google Scholar]
- Wooltorton J. R., Pidoplichko V. I., Broide R. S., Dani J. A. (2003). Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 23 3176–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T. Y., Guo L. L., Wang P., Song J., Le Y. Y., Viollet B., et al. (2012). Chronic exposure to nicotine enhances insulin sensitivity through alpha7 nicotinic acetylcholine receptor-STAT3 pathway. PLoS ONE 7:e51217. 10.1371/journal.pone.0051217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Yokoo H., Mizoguchi K., Kawahara H., Tsuda A., Nishikawa T., et al. (1992). Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: measurement by in vivo microdialysis. Neurosci. Lett. 139 73–76 10.1016/0304-3940(92)90861-Z [DOI] [PubMed] [Google Scholar]
- Zamir N., Skofitsch G., Bannon M. J., Jacobowitz D. M. (1986). Melanin-concentrating hormone: unique peptide neuronal system in the rat brain and pituitary gland. Proc. Natl. Acad. Sci. U.S.A. 83 1528–1531 10.1073/pnas.83.5.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M., Picciotto M. R. (2012). Nicotinic regulation of energy homeostasis. Nicotine Tob. Res. 14 1270–1290 10.1093/ntr/nts159 [DOI] [PMC free article] [PubMed] [Google Scholar]