Abstract
Chemosignals play a crucial role in social and sexual communication among inter- and intra-species. Chemical cues are bound with protein that is present in the pheromones irrespective of sex are commonly called as pheromone binding protein (PBP). In rats, the pheromone compounds are bound with low molecular lipocalin protein α2u-globulin (α2u). We reported farnesol is a natural endogenous ligand (compound) present in rat preputial gland as a bound volatile compound. In the present study, an attempt has been made through computational method to evaluating the binding efficiency of α2u with the natural ligand (farnesol) and standard fluorescent molecule (2-naphthol). The docking analysis revealed that the binding energy of farnesol and 2-naphthol was almost equal and likely to share some binding pocket of protein. Further, to extrapolate the results generated through computational approach, the α2u protein was purified and subjected to fluorescence titration and binding assay. The results showed that the farnesol is replaced by 2-naphthol with high hydrophobicity of TYR120 in binding sites of α2u providing an acceptable dissociation constant indicating the binding efficiency of α2u. The obtained results are in corroboration with the data made through computational approach.
Chemical communication, a universal process of life, occurs at all levels of biological organization including the process of regulation of cells and organs within the body; they significantly influence the level of social behavior and ecological interactions among individuals1. The role of chemical molecules in mediating species-specific pheromonal communication is well documented in mammals including mouse2, rat3, bovine4, buffalo5, and human6. The major functions of mammalian pheromones include sexual attraction, mounting7, mother-young interactions8, aggression and so on. Though a number of scent glands produce pheromones, the preputial gland is reported to be a major source of pheromone production9.
In mice, the pheromone binding protein synthesized in the liver and released into the blood plasma and excreted through urine are called to major urinary protein (MUPs) or pheromone binding protein (PBP), while its equivalent protein in rats is referred as α2u2. This is also expressed in the urine and preputial gland of the rat. In male rats, the preputial glands are often better developed; in female similar gland is the clitoral gland. The preputial glandular activity has been documented through production of volatile compounds and sex associated proteins (18 kDa- α2u) which are testosterone dependent10. Among the exhaustively identified male pheromonal compounds in house mice, two sesquiterpenes from the preputial gland, (i.e.,) E-b-farnesene and E, E-a-farnesene, are excreted in the urine in order to communicate information about sex and dominance. In addition, these compounds also influence on estrus induction in grouped females11. Therefore, they would seem to carry information that leads to aggression when perceived by male mouse and to an attraction when perceived by females12. Farnesol has been identified as the major volatile compound in the preputial gland of albino rat10,13 and detected the occurrence of α2u with its bound putative pheromones (i.e., Farnesol1 and 2) in the preputial gland of house rat14. The pheromonal compound farnesol identified in the preputial gland of a rat is found to attract the opposite sex very efficiently15. We have also recently demonstrated through calcium image analysis that an extract of rat preputial gland is capable of activating the olfactory receptor neuron (ORN), confirming that preputial gland and so as expound with farnesol has shown to be a potent source of pheromone property16.
Bocskei et al.3 have reported the X-ray structure of a monoclinic crystal form of α2u (Protein data bank code [PDB]: 2a2u). The protein is a member of the lipocalin superfamily which is distinguished by an unusual tertiary structure motif that features an eight-stranded antiparallel β-barrel shape forming a hydrophobic pocket in the protein17. Many of these proteins, such as the retinol binding proteins, the odorant-binding proteins18, the major urinary protein (MUP)19 and the retinol epididymal acid binding protein20 contain a hydrophobic-binding site for small lipophilic ligands. It is significant to note that the mouse MUP which is identical to α2u, binds with the major signaling ligands such as 2-sec-butyl-4,5-dihydrothiazole and 3,4-dehydro-exo-brevicomin21. Subsequently, it is demonstrated that the fluorescent molecule 2-naphthol binds to the 2-Isobutyl-3-methoxypyrazine (iBMP) binding site of major urinary proteins with strong affinity22. Overall, these proteins can react with various volatile constituents of the male mouse urine and induce puberty acceleration in female23. In common house rat (Rattus rattus), the α2u also can binds with urinary pheromone compound in which 1-chlorodecane, a sex attractant compound, has been detected as the predominant bound compound15. The size of the ligand and its degree of flexibility can have a considerable influence on the ligand binding success24. The binding efficiency of α2u and its interaction with the natural ligand are not yet investigated.
In the study of macromolecules, an in silico approach like molecular docking may through more light on the molecular interaction between α2u with its natural ligand and the binding affinity score, interacting residues, intermolecular distance analysis which would ultimately lead to a better understating of receptor-ligand interaction. In support of such bioinformatics approach, fluorescence measurements would provide information about emission, excitation state and dissociation constant for bound α2u with the fluorescent probe (2-naphthol). The study of α2u represents photo-physical properties of fluorescence molecules in a hydrophobic environment. Hence in the present study, fluorescence emission spectrum of 2-naphthol characterized by the two major states (emission and excitation) was fully exploited at buffer pH 7.2.
Therefore, it is important to evaluate the interaction between farnesol and 2-naphthol for α2u in order to obtain the binding affinity through docking analysis. Further, it is also necessary to investigate the binding affinity of 2-naphthol with bound α2u by fluorescence titration assay. The long-term objective is that the assessment of α2u and molecular interaction with ligand would enhance the longevity of α2u by protein engineering tool.
Results
Computational analysis
Computed atlas of surface topography of proteins analysis (CASTp)
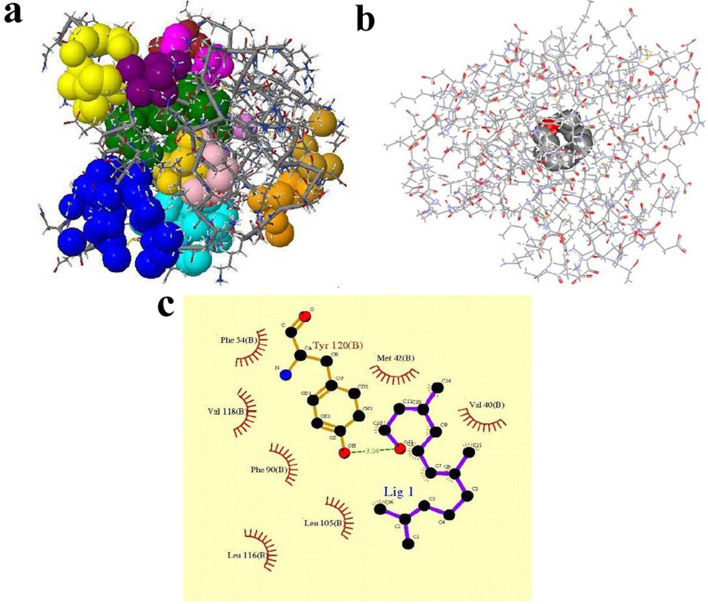
CASTp is a program performs automatic searching and measuring the surface binding pockets of desired proteins. This program was used to identify thirteen possible binding pockets in α2u (Fig. 1a). The identified α2u pockets are extremely hydrophobic, with only a handful of polar atoms at the surface of the pocket. It includes a ten-stranded antiparallel β-sheet, which forms an eight-stranded β-barrel structure with both N-terminal and C-terminal extensions. Furthermore, the cavity within α2u is roughly spherical in shape. Pocket one; being the largest is shown with green colour (Fig. 1a) contains the most residues (Table 1). Hence, our docking study focused on Pocket 1 of the chain B of 2a2u.
Figure 1. Binding sites interaction was predicted using docking study in α2u.
(a) The binding sites of the α2u colored by several indications. (b) Docking study of α2u with the farnesol. (c) The Ligplot analysis for protein and ligand interactions.
Table 1. CASTp analysis confirmed of α2u– globulin binding pockets. The table shows the area and volumes of the 13 pockets found by CASTp. Locations of pockets with significant volumes are indicated.
| aPocket | bArea,Å2 | cVolume, Å3 | dColor | eAmino acid residues with position |
|---|---|---|---|---|
| 1 | 361.7 | 461 | green | Met38, Val40, Met42, Phe54,Phe56, Leu69, Ala71, Val82, Tyr84, Asn88, phe90, Phe103, Leu105, Met117,Val118, Tyr120 |
| 2 | 214 | 185.6 | blue | Glu34, Asn35, Gly36, Arg39, Phe41,Ile58, Lys59, Gly62, Thr154,Asp155 |
| 3 | 127.1 | 23.8 | cyan | Phe20, Ser21, Phe41, Met42, Gln43, Arg57, Arg156, Leu158 |
| 4 | 92.7 | 91.3 | yellow | Lys28, Lys31, Asp85, Asn107, Lys109, Glu112, Thr113, Phe114 |
| 5 | 40.1 | 33.4 | magenta | Thr89, Phe90, Thr91, His104, Ile106 |
| 6 | 41.5 | 24.1 | pink | Ser21, Ile22, Val23, Val24, Val118, Leu119, Tyr120 |
| 7 | 32.5 | 22 | purple | Asn27, Asn27, Lys28, Phe114, Gln115 |
| 9 | 25.5 | 13.9 | brown | Thr74, Glu79, Glu79, Tyr80, Thr89 |
| 10 | 39.2 | 22.3 | gold | Ser21, Val24, Val40, Val118, Tyr120 |
| 11 | 32.4 | 17.1 | violet | Leu15, Ile45, Leu52, Phe03 |
| 12 | 26.4 | 12.5 | hotpink | His44, Asp46, Gly53, Tyr68 |
| 13 | 10.8 | 6.8 | goldenrod | Arg99, Tyr100, Ile130, Lys133 |
Molecular docking
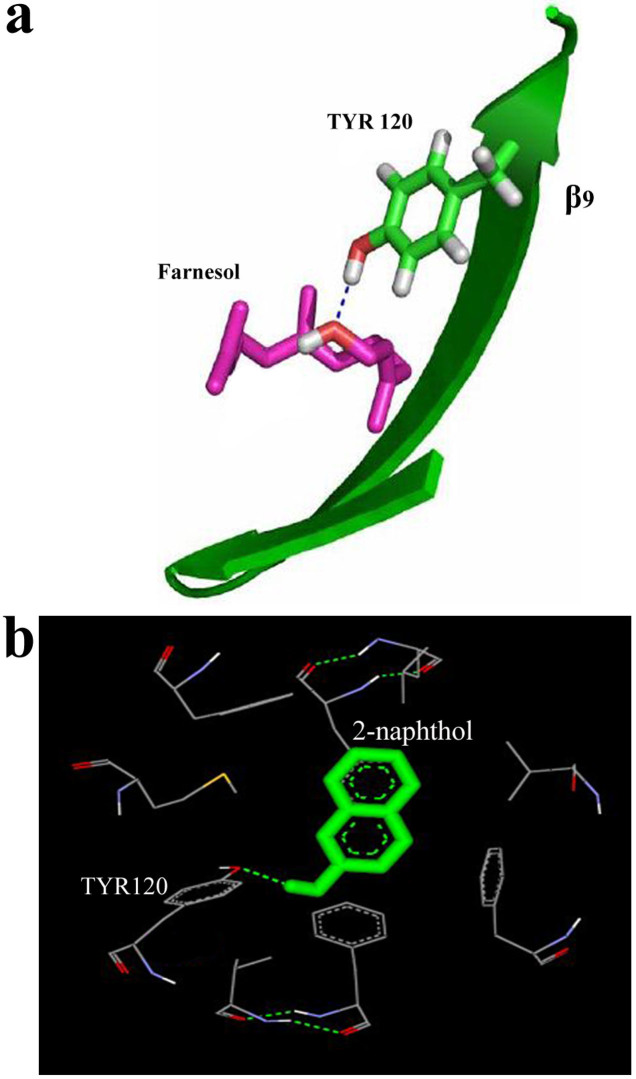
We first docked farnesol with Pocket one of the protein shown as compound one in Fig. 1b. The conformation of the docked farnesol with the best fitness score was displayed as a ball-and-stick model. The detailed binding mode of farnesol with the protein distal pocket was composed by a number of hydrophobic residues including Val40, Met42, Phe54, Leu105, Phe90, Leu116, Val118 and Tyr120 (Fig. 1c). The docking study of the protein-ligand complex, when clustering of the conformations was performed, gave a low binding energy of −6.42 kcal/mol with a Cluster RMS score value of 1.26. A hydrogen bond interaction could be observed between farnesol and hydroxyl group of TYR120 (Fig. 2a). Also, we docked 2-naphthol with α2u; the conformation of docked 2-naphthol got almost equal to farnesol. Indeed the 2-naphthol had −6.04 kcal/mol; a more efficient hydrogen bond interaction occurred between 2-naphthol with TYR120 residue of α2u (Fig. 2b). Since, the preliminary computational results provided a strong binding interaction between α2u with farnesol and 2-naphthol. Further the experimental study is required to confirm the findings of this study.
Figure 2. Hydrogen bond interactions of farnesol and 2-naphthol are towards α2u.
(a) The location of the pheromone (farnesol) within the β-barrel suggests that the β9 intervening loop may function as an entrance to the ligand binding site at TYR120. (b) 2-naphthol interaction with the same binding pocket of α2u at TYR120.
Experimental analysis
Protein Purification
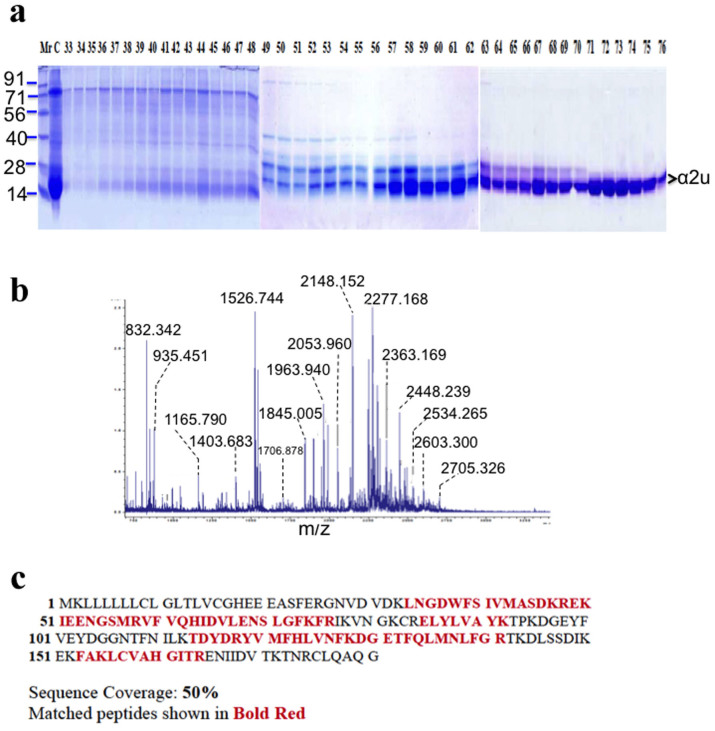
The 12% sodium dodecyl sulphate- polyacrylamide gel electrophoresis (SDS-PAGE) was used to analyze the purity of the glandular protein. The rat preputial gland protein profiles revealed several proteins, in which low molecular mass of 18.54 kDa proteins was expressed predominantly and the molecular mass of the α2u commensurate with that of deduced PBPs (Fig. 3a). Purified protein was used for further proteomics analysis.
Figure 3.
Proteomics analysis of preputial gland of Indian commensal rat: (a) Proteins were extracted from the preputial gland and 40 μg of protein was separated with the aid of 12% SDS-PAGE and the total band from the whole cell extract was subjected into gel filtration chromatography (GFC) lanes from 33 to 76 shows the fraction of protein lysates from GFC. The pure form of α2u eluted from 72 to 76 fractions. (b) MALDI-TOF/MS spectra showed lowed molecular mass protein resemblance to α2u. (c) The matched peptides are shown as underlined and sequence coverage 50% of α2u based on the database analysis.
MALDI-TOF/MS experiment
The purified protein appeared as a single band with a low molecular mass of 18.54 kDa. The highly expressed α2u proteins were excised and subjected to in-gel tryptic digestion. The high-quality of MALDI spectra was obtained for the 18.54 kDa protein (Fig. 3b); the observed and expected masses of spectrum are shown in Table 2 and the protein were determined as α2u by MALDI-TOF/MS. The α2u was confirmed by the maximum resemblance of this protein (accession number: Q9JJI2) with 50% sequence coverage (Fig. 3c).
Table 2. Tryptic digested 18.54 kDa (α2u): The observed and expected masses [M + H]+ of α2u by mass spectroscopy.
| Start | End | Observed masses (m/z) | Expected masses (m/z) | Sequence |
|---|---|---|---|---|
| 34 | 48 | 1738.84 | 1737.83 | K.LNGDWFSIVMASDKR.E |
| 49 | 58 | 1192.56 | 1191.56 | R.EKIEENGSMR.V |
| 51 | 58 | 935.45 | 934.44 | K.IEENGSMR.V |
| 59 | 74 | 1844.99 | 1843.99 | R.VFVQHIDVLENSLGFK.F |
| 59 | 76 | 2148.14 | 2147.13 | R.VFVQHIDVLENSLGFKFR.I |
| 85 | 92 | 999.53 | 998.52 | R.ELYLVAYK.T |
| 114 | 128 | 1947.93 | 1946.92 | K.TDYDRYVMFHLVNFK.D |
| 129 | 141 | 1526.73 | 1525.73 | K.DGETFQLMNLFGR.T |
| 153 | 164 | 1373.69 | 1372.69 | K.FAKLCVAHGITR.E |
| 156 | 164 | 1026.56 | 1025.55 | K.LCVAHGITR.E |
Fluorescence titration study
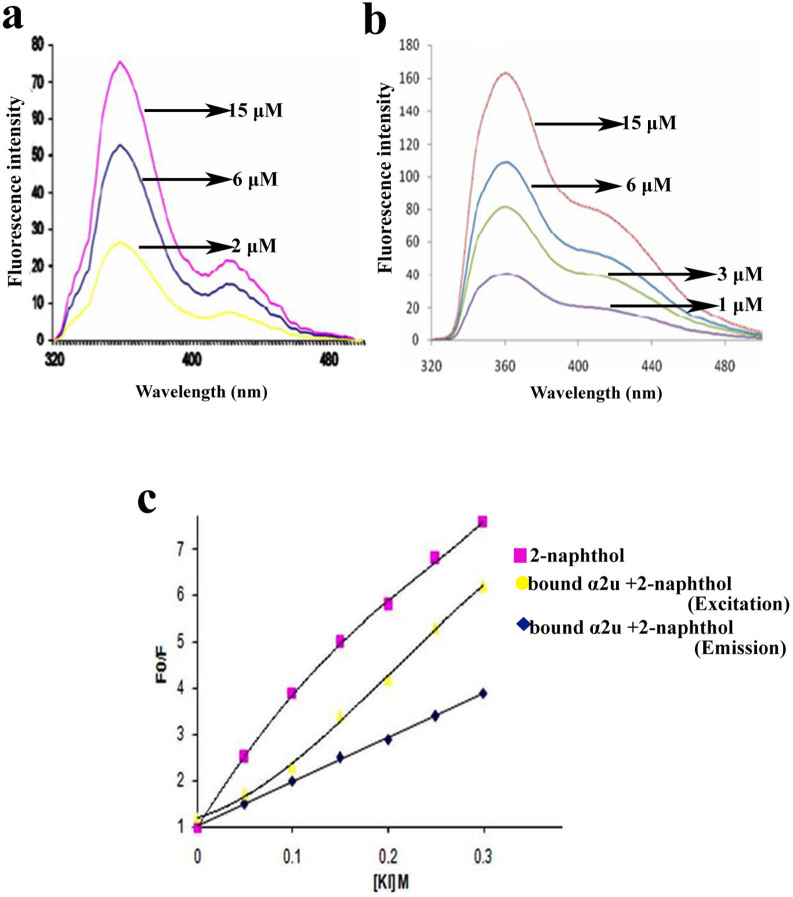
The emission and excitation states appeared in fluorescence titration assay in different concentration of protein ligand fluorescence titration. Two types of titration experiments (Inversed and Direct) were used to measure the concentration of the protein ligand fluorescence. The inverse titration meant a presence of standard ligand concentration to measure the function of protein concentration in the ligand fluorescence. The direct titration defines a standard protein concentration used to measure the various functions of ligand concentration. Both titrations exhibited emission, excitation peaks at 350 nm and 420 nm respectively. In the fluorescence titration study, the fluorescence intensity was measured in different concentrations of α2u (Viz., 2 μM,6 μM and 15 μM) against standard 25 μM 2-naphthol. The α2u concentration level decreased in inverse titration. The fluorescence emission also decreased at 360 nm and this was accompanied by an increase in fluorescence excitation at 420 nm (Fig. 4a). During direct titration, the fluorescence intensity was analyzed in different concentrations (Viz., 1 μm, 3 μM, 6 μM and15 μM) of 2-naphthol with standard 24 μM α2u. The undissociated event was noticed in the emission state at 360 nm and the molecules dissociated into the excited state at 420 nm. The fluorescence intensity was vertically increased in the initial part of the titration at 350 nm, while was absent at 420 nm (Fig. 4b). In addition, the fluorescence intensity ratio F/F0 measured (F means fluorescence intensity in the presence of farnesol and F0 represents the fluorescence intensity in the absence of farnesol)22. The results revealed that, there is an affinity between α2u and 2-naphthol as determined through the fluorescence titration confirming the binding of 2-naphthol in the same active sites of natural endogenous ligand. Therefore, 2-naphthol was displaced from the binding cavity of bound α2u while increasing the protein concentration at 360 nm in inverse titration.
Figure 4. Fluorescence titrations study.
(a) The inverse titration experiments showed spectra of 25 μM 2-naphthol at different α2u concentrations shows 2 μM (yellow line), 6 μM (blue line), and 15 μM (pink line). (b) The direct titration experiments show spectra of 24 μM α2u at different 2-naphthol increasing concentrations (1 μM, 3 μM, 6 μM and 15 μM). (c) Stern-Volmer plots of 2-naphthol with potassium iodide (KI) fluorescence quenching experiments.
Stern-volmer plot using KI quenching
The quenching experiments was applied to analyze the fluorescence intensity of bound α2u with 2-naphthol in emission excited state at 350 nm and 420 nm respectively. The potassium iodide (KI), quenching experiments, showed that the quenching became prominently more effective during concentration increased through Stern-Volmer plot. During, Free 2-naphthol emission peak and emission, an excitation peak was observed for bound α2u with 2-naphthol. An emission peak represented a dynamic quenching and it was decreased during increasing concentration of the quencher at 350 nm based on inverse titration; the emission peak of Free 2-naphthol increases while increasing quencher at 350 nm (Fig. 4c). Hence, the maximum displacement of 2-naphthol was noticed instead of farnesol occupied the α2u binding sites.
Discussion
In molecular docking study, α2u has the largest pocket on a protein that has the most likely active site25. It exists inside the protein and occupies an area of 361.7Å2 and a volume of 461Å3. The side chains Val and Tyr account for the hydrophobic nature of this pocket, while the hydroxyl groups from a Ser residue contributes to its hydrophobicity. All the listed amino acids with the corresponding residue number (Table 1) and the detailed binding sites of α2u have been depicted (Fig. 1a).
During the docking process it was observed that the farnesol docked to α2u with the lowest energy. The occurrence of lowest energy within the protein-ligand complex is mainly due to stabilization by maximum hydrogen bonding interactions (Fig. 1b). Protein-ligand interaction calculated by Ligplot program showed interacting residues in the docking process. The recognition and affinity of protein towards the ligand were interpreted based on the atomic distances lesser than 3.04 Å, implying that the interactions between the protein and ligand occur only within the active site pocket. A hydrogen bonding interaction could be observed between the ligand and the hydroxyl group of TYR120 (Fig. 1c). However, this can only function as a weak hydrogen-bond acceptor and would be able to interact only with the open diol form of the ligand, which is not very physiologically relevant. The β-barrel structure encloses a cup like structure and allows the non-covalent binding of volatile semio chemicals; these properties have given rise to PBP or Pheromone Carrying Proteins (PCPs)21,26. The characterizations of glandular α2u observed in this study are consistent with previous reports on urinary PCPs in male rats and mice acting as pheromone carriers2,15. The location of farnesol within the β-barrel suggests that the β9 intervening loop may function as an entrance to the ligand-binding site (Fig. 2a). Further it is important to note that the 2-naphthol interacts with the same binding pocket residue of TYR120 as that of farnesol (Fig. 2b). A previous report proposed that the loop a/b function as the entrance to the interior of the β-barrel27.
After generating results from computational analysis, the outcome was extrapolated and confirmed by an in vitro study. The analysis of SDS-PAGE protein profiles also revealed that the rat preputial gland comprised 18.5 kDa band from lane 72–76th fractions (Fig. 3a). These results are consistent with the previous reports on the presence of 17 kDa lipocalin protein in male urine of mouse and the X-ray crystal structure form of α2u at 2.5 Å resolution3. In the present study, the presence of 18.54 kDa protein was confirmed as α2u with the aid of MALDI-TOF/MS (Fig. 3b). The spectra represented monoisotopic masses that were pooled together and further analyzed with MASCOT search28. The results showed the highest resemblance to α2u (accession number: Q9JJI2), and provide 50% of sequence coverage (Fig. 3c). The matched and expected mass values of peptides are listed (Table 2). Subsequently, the fluorescence titration analysis provided an adequate evidence to understand the binding efficiency of α2u with farnesol and 2-naphthol. By inverse titration, the presence of 2-naphthol with 2 μM, 6 μM and 15 μM was found to increase the concentration of α2u fluorescence on emission at 350 nm. On the other hand, to the presence of α2u at 420 nm on excitation state the 2-naphthol was dissociated, that would represent to originate only from the unbound fluorescent molecules remaining in solution (Fig. 4a). In contrast to direct titration, the equivalent fluorescence intensity emission of 2-naphthol increased at 360 nm and decreased the level of concentration, as well as dissociated event occurred at 420 nm (Fig. 4b). Hence, the hypothesis put forward is that the 2-naphthol is displaced from the protein binding site by farnesol.
In order to identify the interaction of 2-naphthol with bound α2u, fluorescence quenching experiments were carried out using potassium iodide as quencher. The fluorescence intensity decreases with an increase the concentration of the quencher. Based on the inverse titration, the fluorescence emission between the quencher and bound α2u with 2-naphthol showed a linear and non-linear plots through Stern-Volmer method (Fig. 4c). In addition, the maximum emission for the calculated fluorescence spectrum of the bound species is blue-shifted and more intense29. The dissociation constant of α2u with 2-naphthol was measured as kd = 0.46 μM. Hence, we presume through the docking study that 2-naphthol interact same binding pocket as that of farnesol. It may be postulated that the farnesol might possess the same kd as that of 2-naphthol. This is the first report in reference to the target of farnesol and 2-naphthol is interacting on the same binding pockets of α2u. Since, the farnesol has a good binding efficiency with α2u. It can be considered as an ideal candidate in the development of a pheromone trap or biotrap for the rodent pest management program. Finally, we found trends in the level of clustering and predicted binding energies that are able to distinguish natural volatile ligands from non-binding molecules. This approach would be useful to design ligand based engineered protein active sites that can be created and in designing the biotrap based on pheromone compounds for rodent pest control programs.
Methods
Molecular structure and optimization
The 3D structure of α2u B chain (PDB code: 2a2u) was obtained from the RCSB-PDB (http://www.rcsb.org/pdb). The crystal structure of the protein deposited in the PDB was recorded at a resolution of 2.5 Å. The chemical compound farnesol has been detected in the preputial gland of the Indian common house rat by gas chromatography coupled to mass spectrometry. The 2D structure of the farnesol was obtained from the Pubchem compound module (http://pubchem.ncbi.nlm.nih.gov/) and the compound id (CID) are 1549109 (farnesol) and 8663 (2-naphthol). The AMBER force field and Gasteiger charges were assigned and fixed successfully for farnesol using VEGA ZZ software30. The 2D structure of the compound was then converted into the corresponding 3D coordinates using the Babel server (http://openbabel.sf.net).
Molecular docking study
The active site of α2u was analyzed using the CASTp program to identify and characterize the surface accessible pockets of the protein. The pockets were characterized by two parameters, namely the solvent accessible surface (SA, Richards' surface) and the molecular surface (MS, Connolly's surface)31. The AutoDock4 program used to calculate the interaction between the farnesol and α2u using the lamarckian genetic algorithm (LGA). The purpose to calculate the possible conformations for farnesol and 2-naphthol as it binds to the protein32. During docking analysis, a maximum of ten best conformers with lowest binding energy were considered for farnesol. All calculations were performed on an SGI FUEL workstation. The resulting structural files were visualized and analyzed using Pymol software and the LIGPLOT 4.2.2 program.
Experimental animals
Male house rats (Rattus rattus) were collected from the nearby village of Bharathidasan University and were acclimatized to laboratory condition for 2 weeks prior to experiments. They were housed in a cage, in the laboratory, reared on pelleted food (Sai Durag Ltd, Bangalore) and water ad libitum. Male and female rats were housed separately in poly propylene cages (40 × 25 × 15 cm) with 2 cm of rice husk lining the bottom as bedding material. The bedding material was changed once in 3 or 4 days to maintain the hygienic condition of the animals. All animals were maintained in L & D cycle (12:12) throughout the experimental period. The procedures were strictly adhered to following the guidelines for animal care by Institutional Animal Ethics Committee (IAEC), India.
Preparation of preputial gland extract
The sexually matured and reproductively active normal male house rats (Rattus rattus) were used for the collection of samples and animals were sacrificed through cervical dislocation and preputial glands were collected. The glands were then homogenized with the PBS (pH 7.2) and centrifuged at 4°C for 15 min at 10,000 rpm. After centrifugation, the clear supernatant was collected and separated by size exclusion chromatography.
Protein purification by size exclusion chromatography
The α2u was identified and purified from preputial gland of Rattus rattus. Protein purified by Sephadex G-50 (gel-filtration chromatography). The column (length: 28 cm; i.d.: 0.7) was packed with Sephadex G-50, and equilibrated with 10 mM phosphate buffer (pH 7.8). After the equilibration, the concentrated glandular homogenates were poured onto the top of the column in a single motion and eluted with the same buffer at a flow rate of 0.5 mL/h. The fractions were analyzed for optical absorbance at 280 nm with a UV-visible spectrophotometer and the purity was checked once again by 12% SDS-PAGE.
SDS-PAGE
The 12% of SDS-PAGE carried out using a slightly modified protocol33 with 30 μL of protein in each fraction; 4 μL of medium range molecular weight marker (Bangalore Genie cat. No. PMW-M) was used for the molecular weight reference. The entire electrophoresis was carried out at a constant voltage (50 V) and at room temperature for 4 hours and bands was detected by Coomassie brilliant blue stain.
Mass spectrometry analysis
α2u was prepared by the tryptic in-gel digestion and MALDI-TOF/MS methods according to Muthukumar et al.34. The collected mass spectra were processed with FLEX analysis software; the monoisotopic peptide masses were assigned and used in the database search. The protein identification was accomplished utilizing the MASCOT search (http//www.matrixscience.com). Scores > 65 were considered to be significant (p < 0.05) in the MASCOT search.
Fluorescence study with titrations
For fluorescence study, 2-naphthol was used without further purification methods and chemical obtained from Sigma (St. Louis, MO, USA). The α2u protein was purified by chromatography method15 and the determination of concentration was calculated through spectrophotometrically using the molar extinction coefficient €276 = 11450 cm−1 M−1 at 276 nm24. The following concentrations of purified protein (24 μM), and 2-naphthol (1 mM stock) was prepared in ice-cold 100% ethanol and they were stored in the dark at 4°C, and the final concentration of 25 μM diluted for each experiment. The pH 7.2 for 10 mM Tris and room temperature maintained in all experiments. Fluorescence measurements were carried out on an FP-6500 spectrofluorimeter (JASCO, Japan) equipped with a thermostat bath and 1.0 cm quartz cells, using 5 nm/5 nm slit widths. All pH values were measured by a pH-3c digital pH-meter (Shanghai Lei Ci Device Works, Shanghai, China) with a combined glass-calomel electrode. We used 25 μM 2-naphthol, and 24 μM α2u in a cuvette and then consecutively diluted by adding aliquots of a 25 μM of 2-naphthol for the inverse titration. Instead of 2-naphthol, buffer was prepared as a blank and added 3 ml of 24 μM α2u solution in a cuvette and aliquots of 1 μM 2-naphthol stock solution was added in the direct titration.
Fluorescence quenching experiment
Our previous study shows the bound form of farnesol with purified α2u, therefore, we were interested to explore the binding efficiency of farnesol with α2u. Fluorescence quenching analysis was applied using 2 M stock solution of potassium iodide (KI). The KI acts as a quencher and the fresh solution were prepared for the experiment.
Author Contributions
R.I., R.R., P.P. and G.A. designed the study; R.I., R.R. and A.R.M. performed research; R.I., R.R., A.R.M., D.R. and P.P. analyzed data; R.I., R.R., P.P. and G.A. wrote the manuscript.
Acknowledgments
We thank Dr. Chellam Balasundram, UGC-Emeritus Professor, Bharathidasan University for critical reading of this manuscript. The facility availed through UGC, UGC-SAP and DST-PURSE, Government of India is acknowledged. PP acknowledges the support from the LKC School of Medicine, NTU Singapore.
References
- Petrulis A. Chemosignals, hormones and mammalian reproduction. Horm. Behav. 63, 723–741 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaggioni A. & Mucignat-Caretta C. Major urinary proteins, alpha (2U)-globulins and aphrodisin. Biochim. Biophys. Acta. 1482, 218–228 (2000). [DOI] [PubMed] [Google Scholar]
- Bocskei Z. et al. Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography. Nature. 360, 186–188 (1992). [DOI] [PubMed] [Google Scholar]
- Sankar R. & Archunan G. Identification of putative pheromones in bovine (Bos taurus) faeces in relation to estrus detection. Anim. Reprod. Sci. 103, 149–153 (2008). [DOI] [PubMed] [Google Scholar]
- Rajanarayanan S. & Archunan G. Occurrence of flehmen in male buffaloes (Bubalus bubalis) with special reference to estrus. Theriogenology 61, 861–866 (2004). [DOI] [PubMed] [Google Scholar]
- Grammer K., Fink B. & Neave N. Human pheromones and sexual attraction. Eur. J. Obstet. Gynecol. Reprod. Biol. 118, 135–142 (2005). [DOI] [PubMed] [Google Scholar]
- Rekwot P. I., Ogwu D., Oyedipe E. O. & Sekoni V. O. The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 65, 157–170 (2001). [DOI] [PubMed] [Google Scholar]
- Leon M. [Chemical Communication in Mother Young Interactions]. Pheromone and Reproduction in Mammals. [Vandenbergh, J. G. (ed.)] [39–77] (Academic Press, New York, 1983). [Google Scholar]
- Agosta W. C. Chemical Communication: The Language of Pheremones. [Reilly, S. (ed.)] [23–25] (Scientific American Library, New York, 1992). [Google Scholar]
- Ponmanickam P. et al. Identification of testosterone-dependent volatile compounds and proteins in the preputial gland of rat Rattus norvegicus. Gen. Comp. Endocrinol. 167, 35–43 (2010). [DOI] [PubMed] [Google Scholar]
- Ma W., Miao Z. & Novotny M. V. Induction of estrus in grouped female mice (Mus domesticus) by synthetic analogues of preputial gland constituents. Chem. Senses. 24, 289–293 (1999). [DOI] [PubMed] [Google Scholar]
- Bronson F. H. & Caroom D. Preputial gland of the male mouse; attractant function. J. Reprod. Fert. 25, 279–282 (1971). [DOI] [PubMed] [Google Scholar]
- Kannan S., Ramesh Kumar K. & Archunan G. Sex attractants in male preputial gland: Chemical identification and their role in reproductive behaviour of rats. Curr. Sci. 74, 689–691 (1998). [Google Scholar]
- Rajkumar R. et al. Detection of α2u-Globulin and its bound putative pheromones in the preputial gland of indian commensal rat (Rattus rattus): using mass spectrometry. Rapid Commun. Mass. Spectrom. 24, 721–728 (2010). [DOI] [PubMed] [Google Scholar]
- Liebich H. M., Zlatkis A., Bertsch W., Van Dahm R. & Whitten W. K. Identification of dihydrothiazoles in urine of male mice. Biomed. Mass Spectrom. 4, 69–72 (1977). [DOI] [PubMed] [Google Scholar]
- Ponmanickam P. et al. Preputial gland activates olfactory receptor neurons in rat: Calcium imaging study using laser scanning confocal microscopy. Indian J. Biochem. Biophy. 50, 242–246 (2013). [PubMed] [Google Scholar]
- Papiz M. Z. et al. The structure of beta-lactoglobulin and its similarity to plasma retinol- binding protein. Nature 324, 383–385 (1986). [DOI] [PubMed] [Google Scholar]
- Bianchet M. A. The three-dimensional structure of bovine odorant binding protein and its mechanism of odor recognition. Nat. Struct. Biol. 3, 934–949 (1996). [DOI] [PubMed] [Google Scholar]
- Rajkumar R., Prakash S., Archunan G. & Sowdhamini R. Primary structural documentation of the major urinary protein of the Indian commersal rat (Rattus rattus): using proteomic plotform. Protein Pept. Letters 17, 449–457 (2010). [DOI] [PubMed] [Google Scholar]
- Newcomer M. E. Structure of the epididymal retinoic acid binding protein at 2.1 A resolution. Structure 15, 7–18 (1993). [DOI] [PubMed] [Google Scholar]
- Bacchini A., Gaetani E. & Cavaggioni A. Pheromone binding proteins of the mouse, Mus musculus. Experientia. 48, 419–421 (1992). [DOI] [PubMed] [Google Scholar]
- Sartor G. et al. Determining the binding capability of the mouse major urinary proteins using 2-naphthol as a fluorescent probe. Anal Biochem. 292, 69–75 (2001). [DOI] [PubMed] [Google Scholar]
- Novotny M. V. et al. A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem. Biol. 6, 377–383 (1999). [DOI] [PubMed] [Google Scholar]
- Cavaggioni A., Findlay J. B. & Tirindelli R. Ligand binding characteristics of homologous rat and mouse urinary proteins and pyrazine-binding protein of calf. Comp. Biochem. Physiol. B. 96, 513–20 (1990). [DOI] [PubMed] [Google Scholar]
- Liang J., Edelsbrunner H., Fu P., Sudhakar P. V. & Subramaniam S. Analytical shape computing of macromolecules II: Identification and computation of inaccessible cavities inside proteins. Proteins 33, 18–29 (1998). [PubMed] [Google Scholar]
- Briand L., Trotier D. & Pernollet J. C. Aphrodisin: an aprodisiac lipocalins secreted in hamster vaginal secretions. Peptides 25, 1545–1552 (2004). [DOI] [PubMed] [Google Scholar]
- Flower D. R. A lipocalin protein family: A role in cell regulation. FEBS Lett. 354, 7–11 (1994). [DOI] [PubMed] [Google Scholar]
- Helsens K., Martens L., Vandekerckhove J. & Gevaert K. MascotDatfile: An open-source library to fully parse and analyse MASCOT MS/MS search results. Proteomics 7, 364–366 (2007). [DOI] [PubMed] [Google Scholar]
- Lucke C. et al. Solution structure of mouse recombinant major urinary protein. Eur. J. Biochem. 266, 1210–1218 (1999). [DOI] [PubMed] [Google Scholar]
- Pedretti A., Villa L. & Vistoli G. VEGA: A versatile program to convert, handle and visualize molecular structure on windows-based PCs. J. Mol. Graph. 21, 47–49 (2002). [DOI] [PubMed] [Google Scholar]
- Binkowski T. A., Naghibzadeh S. & Liang J. CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res. 31, 3352–3355 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. M., Goodsell D. S., Huey R. & Olson A. J. Distributed automated docking of flexible ligands to proteins: parallel applications of AutoDock 2.4. J. Comput. Aided Mol. Des. 10, 296–304 (1996). [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970). [DOI] [PubMed] [Google Scholar]
- Muthukumar S. et al. Urinary Lipocalin Protein in a Female Rodent with Correlation to Phases in the Estrous Cycle: An Experimental Study Accompanied by In Silico Analysis. PLoS ONE 8, e71357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]