Abstract
B cell lineage ALL represents by far the most frequent malignancy in children and is also common in adults. Despite significant advances over the past four decades, cytotoxic treatment strategies have recently reached a plateau with cure rates at 80 percent for children and 55 percent for adults. Relapse after cytotoxic drug treatment, initial drug-resistance and dose-limiting toxicity are among the most frequent complications of current therapy approaches. For this reason, pathway-specific treatment strategies in addition to cytotoxic drug treatment seem promising to further improve therapy options for ALL patients.
In a recent study on 111 cases of pre-B cell-derived human ALL, we found that ALL cells carrying a BCR-ABL1-gene rearrangement lack expression of a functional pre-B cell receptor in virtually all cases. In a proof-of-principle experiment, we studied pre-B cell receptor function during progressive leukemic transformation of pre-B cells in BCR-ABL1-transgenic mice: Interestingly, signaling from the pre-B cell receptor and the oncogenic BCR-ABL1 kinase are mutually exclusive and only “crippled” pre-B cells that fail to express a functional pre-B cell receptor are permissive to transformation by BCR-ABL1.
Keywords: BCR-ABL1, pre-B cell receptor, acute lymphoblastic leukemia, ikaros, cell cycle arrest
Introduction
B cell precursors within the bone marrow represent the normal counterpart of ~85 percent of acute lymphoblastic leukemia (ALL) cases. Pre-B cells are destined to die by apoptosis within the bone marrow unless they are rescued through survival signals from a successfully assembled pre-B cell receptor.1 During normal early B cell development, the pre-B cell receptor has a dual function—it first promotes survival and proliferation of large cycling pre-B cells and subsequently induces differentiation in small resting pre-B cells.2–4 It consists of an immunoglobulin μ heavy chain (μ chain; IGHM) coupled to the surrogate light chain with its two components VpreB (VPREB1) and λ5 (IGLL1).5,6 Productive rearrangement of immunoglobulin VH to DJH gene segments is a prerequisite for the expression of a functional μ chain and hence the transition from the pro-B to pre-B cell stage.1 This extracellular part of the pre-B cell receptor is linked to the Igα and Igβ transmembrane signaling chains, which contain an immunoreceptor tyrosine-based activation motif (ITAM) in their intracellular tail.7 The cytoplasmic ITAM-bearing signaling chains mainly serve as a docking sites to assemble and activate the igniting components of the pre-B cell receptor signaling cascade, namely SYK (spleen tyrosine kinase), the SRC family kinases LYN, FYN and BLK, and Bruton’s tyrosine kinase (BTK).8 BTK binds to and activates PLCγ2 (PLCG2), a key enzyme for hydrolysis of PI(4,5)P2. Thereby, PLCγ2 generates diacylglycerol and IP3, which serve as second messengers for activation of PKCs and Ca2+ release from cytoplasmic stores, respectively. SLP65 (or BLNK, BASH),9 is a major linker protein to assemble the proximal signaling components of pre-B cell receptor. It has spe-cific docking sites for BTK and PLCγ2 (Fig. 1).10 In the absence of SLP65, the function of the pre-B cell receptor is compromised and SLP65-deficient B cell precursors are arrested at the pre-B cell stage.11 Once rearrangement of immunoglobulin V H to DJH gene segments is successfully completed, the pre-B cell receptor is assembled, expressed on the cell surface and delivers a strong proliferation signal to the developing pre-B cells, which then drastically expand within the bone marrow. Subsequently, the pre-B cell receptor initiates immunoglobulin κ light chain gene rearrangements via signals through SLP65 indicating the importance of SLP65 in pre-B cell differentiation.12,13 After successful rearrangement at the light chain locus, the B cell receptor carrying the definitive κ chain instead of surrogate light chains replaces the pre-B cell receptor.14 At this time, IgMκ+ immature B cells leave the bone marrow.
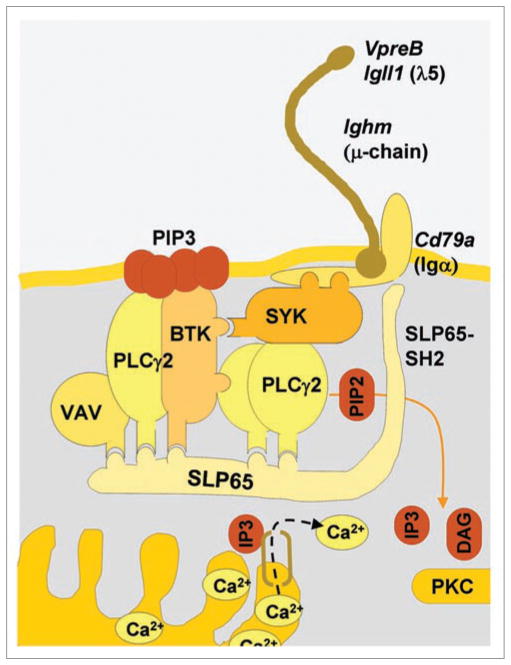
Figure 1.
The pre-B cell receptor complex consisting of the μ-heavy chain (Ighm), the surrogate light chain components VpreB and λ5 (Igll1) and the Igα signaling chain (Cd79a) are shown. Proximal pre-B cell receptor signaling involves the tyrosine kinases SYK, BTK and PLCγ2 (PLCG2), which are assembled through specific docking sites to the linker molecule SLP65. BTK and PLCγ2 are anchored to the membrane by binding to PIP3. PLCγ2 hydrolyzes PIP2 to IP3 and DAG. The latter activates PKC, whereas the former induces release of Ca2+ from stores within the endoplasmic reticulum.
Development of Acute Lymphoblastic Leukemia from Pre-B Cell Receptor-Independent Stages of B Cell Development
B cell lineage acute lymphoblastic leukemia (ALL) is virtually in all cases derived from pro-B or pre-B cells that are arrested in their development. Lack of SLP65 (BLNK) function in a substantial fraction of cases of ALL15–18 represents one explanation for this differentiation block. Also expression of BTK, which in addition to SLP65 is required for pre-B cell receptor function, is aberrant or missing in many cases of ALL.19 Recent data on the absence of functional pre-B cell receptor signaling in Philadelphia chromosome-positive (Ph+) ALL21 highlights the role of the pre-B cell receptor as tumor suppressor. Reconstitution of pre-B cell receptor signaling effectively suppresses leukemic growth in Ph+ ALL. How the pre-B cell receptor and its downstream signaling molecules (e.g., SLP65) mediate this tumor suppression is still a subject of ongoing studies. One mechanism could be inhibition of active kinases like JAK3.22 We found Ikaros to be one essential factor downstream of the pre-B cell receptor required for this tumor suppression. Ikaros family transcription factors—especially Ikaros and Aiolos are required for B cell development to progress.23 B and T cell development is arrested in Ikaros-deficient mice, and they also have abnormal myelopoiesis.24 Deficiency of Aiolos too, is shown to cause aberrant B cell development.25 Of note, deletions of the Ikzf1 gene encoding Ikaros represent a near-obligatory lesion in Ph+ ALL, which is found in more than 80% of the cases.26 Interestingly, small deletions within the Ikzf1 gene lead to expression of a dominant-negative Ikaros protein (IK6), which can also be expressed as a result of aberrant pre-mRNA splicing.27 Expression of dominant negative Ikaros splice variant IK6 blocked the tumor-suppressive effect of the pre-B cell receptor.21 Further mechanistic studies are still ongoing to delineate how the pre-B cell receptor and its downstream targets SLP65 and Ikaros cooperate in this tumor-suppressive effect.
Distinct Functions of the Pre-B Cell Receptor in Cytogenetic ALL Subtypes?
While a differentiation block at the pro-B or pre-B cell stage represents a uniform feature for ALL, there are heterogeneous cytogenetic subtypes of the disease. In human ALL, four major oncogenic gene rearrangements are recurrently found, namely E2A-PBX1, BCR-ABL1 (resulting from the Philadelphia chromosome), MLL-AF4 and TEL-AML1. Additional cytogenetic subgroups of ALL include cases with sporadic gene rearrangements and cases with hyper- and hypodiploid or normal karyotype.28 Whereas E2A-PBX1, MLL-AF4 and TEL-AML1 encode oncogenic transcription factors, the BCR-ABL1 fusion gene codes for a constitutively active tyrosine kinase.
The t(1;19)(q23;p13) chromosomal translocation leading to expression of the chimeric E2A-PBX1 transcription factor is found in approximately 23% of cases with childhood ALL. While the E2A factors E12 and E47 encoded by the TCF3 gene (19q13.3) have a critical function during B lymphopoiesis, PBX1 is not expressed in hematopoietic cells.29 Interestingly, the chimeric E2A-PBX1 transcription factor induces aberrant expression of WNT16,30 which could lead to autocrine stimulation of the LEF1/WNT/β-catenin pathway in these cells.
The t(4;11)(q21;q23) translocation leading to the expression of the chimeric MLL-AF4 transcription factors is associated with a particularly unfavorable prognosis and found in ~50% of cases with infant leukemia.31 Owing to aberrant MLL-AF4 transcription factor activity, MLL-AF4-expressing leukemia cells typically exhibit a mixed B cell/myeloid cell lineage phenotype, which led to the designation of the MLL gene typically rearranged in this type of leukemia as “mixed lineage leukemia” gene.32 In addition, the oncogenic MLL-AF4 transcription factor also induces upregulation of the stem cell antigen Prominin1 (CD133),33 which is aberrantly expressed on cancer stem cells in a variety of malignancies.
The t(9;22)(q34;q11) chromosomal rearrangement leading to the so-called Philadelphia chromosome (Ph)34 and expression of the oncogenic BCR-ABL1 tyrosine kinase,35 represents the most frequent cytogenetic abnormality in adult ALL (about 25–30% of cases)36 and also occurs in childhood ALL (4–5%).37 Unlike the normal ABL1 kinase, BCR-ABL1 is constitutively active and previous work by our group showed that BCR-ABL1 mimics survival signals from a constitutively active pre-B cell receptor, mainly through tyrosine phosphorylation of BTK.17,20 Unlike other oncogenic gene rearrangements in ALL, the BCR-ABL1 fusion gene is required and sufficient for malignant transformation of B cell precursors.38 Among all cytogenetic subtypes of ALL, the BCR-ABL1 fusion gene defines the subgroup of ALL with the worst clinical prognosis.31 The main reason for the unfavorable clinical outcome of BCR-ABL1 ALL is genetic instability, likely owing to aberrant expression of the mutator enzyme AID in this subtype of ALL.39
The high frequency of defects in the pre-B cell receptor-related signaling molecules in ALL cells identified by others16–19 and us suggests that the pre-B cell receptor may counteract malignant transformation especially in Ph+ ALL. On the other hand, the pre-B cell receptor also delivers critical survival and proliferation signals in early B cell precursors and its expression is required for abnormal lymphop-roliferation.15 In addition, previous work demonstrated that the pre-B cell receptor and the pre-B cell receptor-related tyrosine kinase Syk are required for Myc-mediated transformation of pre-B cells.40 Our group recently demonstrated that the pre-B cell receptor-related signaling molecule BTK plays a central role in the oncogenic signaling complex activated by BCR-ABL1.20 Based on these findings, it is currently unclear whether pre-B cell receptor signaling is required to enable malignant outgrowth in ALL or functions to suppress it.
Hypothesis
Congenital defects in pre-B cell receptor-related signaling molecules cause a severe block of early B cell development in humans.41 For instance, inherited mutations of the IGLL1 (λ5),42 CD79A (Igα),43 CD79B (Igβ),44 SLP65 (BLNK)45 and IGHM genes (μ-chain)46 all lead to compromised pre-B cell receptor function and all lead to a severe B cell differentiation block at or before the pre-B cell stage. Likewise, in acute lym-phoblastic leukemia (ALL), a malignancy derived from B cell precursors in most cases, cells are arrested at early stages of B cell development. In previous studies from our group, we found defective expression of IGLL1, CD79B, IGHM and SLP65,17,18,21 as a frequent feature in Ph+ ALL. In addition, recent genomic studies in various subtypes of ALL identified multiple genetic lesions within the pre-B cell receptor signaling pathway.26,47 Future work will test the hypothesis that the developmental arrest in B cell lineage ALL predominantly reflects aberrant pre-B cell receptor function.
Acknowledgments
R.N. is supported by a scholarship award from the Saban Research Institute, Childrens Hospital Los Angeles; M.M. is supported by grants from the Leukemia and Lymphoma Society and the NIH (R01CA137606 and R01CA139032).
References
- 1.LeBien TW. Fates of human B-cell precursors. Blood. 2000;96:9–23. [PubMed] [Google Scholar]
- 2.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 3.Rolink AG, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J Exp Med. 2000;191:23–32. doi: 10.1084/jem.191.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolink AG, Schaniel C, Busslinger M, Nutt SL, Melchers F. Fidelity and infidelity in commitment to B-lymphocyte lineage development. Immunol Rev. 2000;175:104–11. [PubMed] [Google Scholar]
- 5.Karasuyama H, Rolink A, Shinkai Y, Young F, Alt FW, Melchers F. The expression of Vpre-B/lambda 5 surrogate light chain in early bone marrow precursor B cells of normal and B cell-deficient mutant mice. Cell. 1994;77:133–43. doi: 10.1016/0092-8674(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T, Mundt C, Licence S, Melchers F, Martensson IL. VpreB1/VpreB2/lambda5 triple-deficient mice show impaired B cell development but functional allelic exclusion of the IgH locus. J Immunol. 2002;168:6286–93. doi: 10.4049/jimmunol.168.12.6286. [DOI] [PubMed] [Google Scholar]
- 7.Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WW, Zurn C, et al. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell. 2002;10:1057–69. doi: 10.1016/s1097-2765(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 8.Guo B, Kato RM, Garcia-Lloret M, Wahl MI, Rawlings DJ. Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity. 2000;13:243–53. doi: 10.1016/s1074-7613(00)00024-8. [DOI] [PubMed] [Google Scholar]
- 9.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 10.Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, et al. BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells. Immunity. 1999;10:117–25. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 11.Jumaa H, Wollscheid B, Mitterer M, Wienands J, Reth M, Nielsen PJ. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity. 1999;11:547–54. doi: 10.1016/s1074-7613(00)80130-2. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Hayashi K, Nojima T, Matsuzaki Y, Kawano Y, Karasuyama H, et al. BASH-novel PKC-Raf1 pathway of pre-BCR induces kappa chain rearrangement. Blood. 2006;108:2703–11. doi: 10.1182/blood-2006-05-024968. [DOI] [PubMed] [Google Scholar]
- 13.Herzog S, Hug E, Meixlsperger S, Paik JH, DePinho RA, Reth M, Jumaa H. SLP65 regulates immuno-globulin light chain recombination through the PI(3) K-PKB-Foxo pathway. Nat Immunol. 2008;9:623–31. doi: 10.1038/ni.1616. [DOI] [PubMed] [Google Scholar]
- 14.Klein F, Feldhahn N, Mooster JL, Sprangers M, Hofmann WK, Wernet P, et al. Tracing the pre-B to immature B cell transition in human leukemia cells reveals a coordinated sequence of primary and secondary IGK gene rearrangement, IGK deletion, and IGL gene rearrangement. J Immunol. 2005;174:367–75. doi: 10.4049/jimmunol.174.1.367. [DOI] [PubMed] [Google Scholar]
- 15.Flemming A, Brummer T, Reth M, Jumaa H. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nat Immunol. 2003;4:38–43. doi: 10.1038/ni862. [DOI] [PubMed] [Google Scholar]
- 16.Jumaa H, Bossaller L, Portugal K, Storch B, Lotz M, Flemming A, et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature. 2003;423:452–6. doi: 10.1038/nature01608. [DOI] [PubMed] [Google Scholar]
- 17.Klein F, Feldhahn N, Harder L, Wang H, Wartenberg M, Hofmann WK, et al. The BCR-ABL1 kinase bypasses selection for the expression of a pre-B cell receptor in pre-B acute lymphoblastic leukemia cells. J Exp Med. 2004;199:673–85. doi: 10.1084/jem.20031637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprangers M, Feldhahn N, Liedtke S, Jumaa H, Siebert R, Müschen M. SLP65 deficiency results in perpetual V(D)J recombinase activity in pre-B-lymphoblastic leukemia and B-cell lymphoma cells. Oncogene. 2006;25:5180–6. doi: 10.1038/sj.onc.1209520. [DOI] [PubMed] [Google Scholar]
- 19.Feldhahn N, Rio P, Soh BN, Liedtke S, Sprangers M, Klein F, et al. Deficiency of Bruton’s tyrosine kinase in B cell precursor leukemia cells. Proc Natl Acad Sci USA. 2005;102:13266–71. doi: 10.1073/pnas.0505196102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldhahn N, Klein F, Mooster JL, Hadweh P, Sprangers M, Wartenberg M, et al. Mimicry of a constitutively active pre-B cell receptor in acute lymphoblastic leukemia cells. J Exp Med. 2005;201:1837–52. doi: 10.1084/jem.20042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trageser D, Iacobucci I, Nahar R, Duy C, von Levetzow G, Klemm L, et al. Pre-B cell receptor-mediated cell cycle arrest in Philadelphia chromosome-positive acute lymphoblastic leukemia requires IKAROS function. J Exp Med. 2009;206:1739–53. doi: 10.1084/jem.20090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama J, Yamamoto M, Hayashi K, Satoh H, Bundo K, Kubo M, et al. BLNK suppresses pre-B cell leukemogenesis through inhibition of JAK3. Blood. 2009;113:1483–92. doi: 10.1182/blood-2008-07-166355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson EC, Cobb BS, Sabbattini P, Meixlsperger S, Parelho V, Liberg D, et al. Ikaros DNA-Binding proteins as intergral components of B cell developmental stage specific regulatory circuits. Immunity. 2007;26:335–44. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Wang JH, Nichogiannopoplou A, Wu L, Sun L, Sharpe AH, Bigby M, et al. Selective defects in the development of fetal and adult lymphoid system in mice with Ikaros null mutation. Immunity. 1996;5:537–49. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang JH, Avitahi N, Cariappa A, Friedrich C, Ikeda T, Renold A, et al. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9:543–53. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- 26.Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–4. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 27.Klein F, Feldhahn N, Herzog S, Sprangers M, Mooster JL, Jumaa H, et al. BCR-ABL1 induces aberrant splicing of IKAROS and lineage infidelity in pre-B lymphoblastic leukemia cells. Oncogene. 2006;25:1118–24. doi: 10.1038/sj.onc.1209133. [DOI] [PubMed] [Google Scholar]
- 28.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–64. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 29.Dedera DA, Waller EK, LeBrun DP, Sen-Majumdar A, Stevens ME, Barsh GS, et al. Chimeric homeobox gene E2A-PBX1 induces proliferation, apoptosis and malignant lymphomas in transgenic mice. Cell. 1993;74:833–43. doi: 10.1016/0092-8674(93)90463-z. [DOI] [PubMed] [Google Scholar]
- 30.McWhirter JR, Neuteboom ST, Wancewicz EV, Monia BP, Downing JR, Murre C. Oncogenic home-odomain transcription factor E2A-Pbx1 activates a novel WNT gene in pre-B acute lymphoblastoid leukemia. Proc Natl Acad Sci USA. 1999;96:11464–9. doi: 10.1073/pnas.96.20.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) Blood. 2007;109:926–35. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–7. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 33.Thomas M, Gessner A, Vornlocher HP, Hadwiger P, Greil J, Heidenreich O. Targeting MLL-AF4 with short interfering RNAs inhibits clonogenicity and engraftment of t(4;11)-positive human leukemic cells. Blood. 2005;106:3559–66. doi: 10.1182/blood-2005-03-1283. [DOI] [PubMed] [Google Scholar]
- 34.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–3. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 35.Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Ph’ translocation. Nature. 1985;315:758–61. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- 36.Mancini M, Scappaticci D, Cimino G, Nanni M, Derme V, Elia L, et al. A comprehensive genetic classification of adult acute lymphoblastic leukemia (ALL): analysis of the GIMEMA 0496 protocol. Blood. 2005;105:3434–41. doi: 10.1182/blood-2004-07-2922. [DOI] [PubMed] [Google Scholar]
- 37.Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–43. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 38.Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet. 2000;24:57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- 39.Feldhahn N, Henke N, Melchior K, Duy C, Soh BN, Klein F, et al. Activation-induced cytidine deaminase acts as a mutator in BCR-ABL1-transformed acute lymphoblastic leukemia cells. J Exp Med. 2007;204:1157–66. doi: 10.1084/jem.20062662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wossning T, Herzog S, Köhler F, Meixlsperger S, Kulathu Y, Mittler G, et al. Deregulated Syk inhibits differentiation and induces growth factor-independent proliferation of pre-B cells. J Exp Med. 2006;203:2829–40. doi: 10.1084/jem.20060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conley ME, Rohrer J, Rapalus L, Boylin EC, Minegishi Y. Defects in early B-cell development: comparing the consequences of abnormalities in pre-BCR signaling in the human and the mouse. Immunol Rev. 2000;178:75–90. doi: 10.1034/j.1600-065x.2000.17809.x. [DOI] [PubMed] [Google Scholar]
- 42.Minegishi Y, Coustan-Smith E, Wang YH, Cooper MD, Campana D, Conley ME. Mutations in the human lambda5/14. 1 gene result in B cell deficiency and agammaglobulinemia. J Exp Med. 1998;187:71–7. doi: 10.1084/jem.187.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minegishi Y, Coustan-Smith E, Rapalus L, Ersoy F, Campana D, Conley ME. Mutations in Igα (CD79a) result in a complete block in B-cell development. J Clin Invest. 1999;104:1115–21. doi: 10.1172/JCI7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobbs AK, Yang T, Farmer D, Kager L, Parolini O, Conley ME. A hypomorphic mutation in Igβ in a patient with immunodeficiency and a leaky defect in B cell development. J Immunol. 2007;179:2055–9. doi: 10.4049/jimmunol.179.4.2055. [DOI] [PubMed] [Google Scholar]
- 45.Minegishi Y, Rohrer J, Coustan-Smith E, Lederman HM, Pappu R, Campana D, et al. An essential role for BLNK in human B cell development. Science. 1999;286:1954–7. doi: 10.1126/science.286.5446.1954. [DOI] [PubMed] [Google Scholar]
- 46.Lopez Granados E, Porpiglia AS, Hogan MB, Matamoros N, Krasovec S, Pignata C, et al. Clinical and molecular analysis of patients with defects in μ-heavy chain gene. J Clin Invest. 2002;110:1029–35. doi: 10.1172/JCI15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–64. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]