Summary
NOD like receptors (NLRs) areincreasingly implicated in control of pathogen sensing pathways. In this issue of immunity Zhang et al. describe a role for NLRC3 in regulating STING and the inflammatory response to cytosolicDNA.
Intracellular sensing of nucleic acids is integral for the initiation of antimicrobial responses. The protein Stimulator of Interferon inducible Genes (STING) has emerged as a non-redundant molecule in the DNA sensing pathway that drives both pro-inflammatory cytokine and type I interferon production(Barber, 2014). Cells lacking STING fail to mount a robust immune response to diverse variants of cytosolic DNA, including viral DNA, plasmid DNA and DNA released from apoptotic or necrotic cells.Furthermore, mice lacking STING fail to control the DNA virusHerpes Simplex Virus -1 (HSV-1)due to a deficiency in type I interferon generation(Barber, 2014).
STING directly sensesvarious types of cyclic dinucleotides delivered by bacteria. In addition, cytosolic double-stranded DNA activatescyclic GMP– AMP synthase (cGAS) to produce a unique isomer of cyclic GMP-AMP (cGAMP) from GTP and ATP. The nucleotidyltransferasecGASthereby generates an endogenousligand to activate STING(Barber, 2014).
Given that STING plays a central role in DNA recognition and the sensing of intracellular bacteria,it is not surprising that its activity must be tightly controlled. Indeed, previous work has shown that STING is regulated by both ubiquitination and phosphorylation, suggesting that it is finely tuned by multiple feedback mechanisms(Barber, 2014).
In this issue of Immunity, Zhang and colleagues demonstrate a role for a NOD-like receptor (NLR) in regulating the activity of the STING (Lu, 2014). NLRs are best knownfor their ability to form large signaling complexes, termed inflammasomes, whichactivate capase-1, leading to activation and release of theinterleukin-1β(IL-1β) family ofcytokines(Latz et al., 2013). In addition to these pro-inflammatory functions, increasing evidence indicates that certain NLRs can alsoregulate diverse inflammatory pathways. The NF-κB signaling pathway for example is tuned down by NLRs including NLRP2, NLRC3, NLRP6 and NLR12(Lupfer and Kanneganti, 2013). [AU: please note it is journal house style to remove claims of novelty]
Zhang and colleagues demonstrate that deficiency of NLRC3 results in complete protection from an otherwise lethal infection of Herpes Simplex virus-1 (HSV-1)(Lu, 2014). Further in vitrostudies established that NLRC3 also controls STING activation by cytosolic dsDNA.In addition, inhibition of cellular responses by NLRC3 extended to the bacterial derivedcyclic di-nucleotides, suggesting that NLRC3 may control STINGdirectly rather than a DNA sensor such as cGAS.Theinhibitory role of NLRC3 is intriguing given that itis also implicated inlimiting NF-κB activation after LPS stimulation by binding TRAF6, resulting in TRAF6 degradation(Schneider et al., 2012). These dual roles performed by NLRC3 in response to diverse immune stimuli suggest that NLRC3 behaves as awatchdog to prevent an overshooting onset of immune responses. TLR activation also results in decreased transcription of NLRC3,essentially releasing the brake on NF-κB activationmediated by MyD88-dependent receptors(Schneider et al., 2012). This suggests that NLRC3 is part of a finely tuned feedback system that regulates the strength and timing of inflammatory signaling. It would thus be interesting to investigate whether pre-priming WT macrophages with TLR agonists would accelerateSTING responses to cytosolic dsDNA.
Further biochemical investigation of NLRC3-mediated inhibition of STING revealed that the two proteins interact directly via the nucleotide binding domain (NBD) of NLRC3.Notably,expression of aconstruct containing theNBD and LRR domains inhibited the interaction with STING. It is therefore possible that the LRR domain regulates the NBD-STING interaction. It remains unclear however, whether this direct interaction is required for NLRC3-mediated inhibition of STING signaling.The NBD of NLRC3 also mediated its interaction with TRAF6, which also occurs through a TRAF binding motif(Ser-Leu-Gln-Glu) in the NBD(Schneider et al., 2012). Whether STING and TRAF6 share this interaction site on NLRC3 or whether both TRAF6 and STING could potentially interact with the NBD of NLRC3 through distinct sites remains to be investigated.
The authors also sought to ascertain how NLRC3 mediates inhibition of STING by investigating the effect of NLRC3on the interaction between STING and TBK-1. In the absence of NLRC3, the kinetics of the STING−TBK-1 interaction was accelerated, indicating that NLRC3 may delay the interaction between the two, but notentirely prevent it.Furthermore, NLRC3 inhibited the translocation of STING from the endoplasmic reticulum (ER) to perinuclearpuncta, a feature required for full TBK-1 activation(Barber, 2014). Whether NLRC3 restricts the STING−TBK-1 interaction by preventing STING translocation to the perinuclearpunctaor by interfering with the interaction site between STING and TBK-1has not been addressed.
An overarching question from these studies ishow a single NLR can perform different roles. This study demonstrates that NLRC3 can regulate two seemingly unconnected signaling pathways, TRAF-6 signaling and STING signaling. Other NLRs, such as NLRP6 and NLRP12, can form inflammasomes leading to the activation of caspase-1 dependent cytokines, and in additioncan suppress NF-κB, ERK- and MAP-kinase dependent immune cell activation (Anand et al., 2012; Zaki et al., 2014). It is conceivablethat such NLRs are constitutively expressed, and in the absence of certain immune stimuli act to repress the signaling pathways leading to pro- inflammatory factors. However, upon recognition of a specific stimulus some of the NLRswill form an inflammasome topromote cleavage and release of IL-1β(Lupfer and Kanneganti, 2013). Therefore, the immune down-regulatory function of these NLRs may be controlled by their transcription,while the pro-inflammatory activities, which are typically are regulated by additionalmeans, will only occur in response to a specific stimulus.In the work by Zhang and colleagues it was shown thatthe LRR region appears to reduce binding of the NBD domain to STING. This would suggest the exciting possibility thatan unidentified activation signal or scaffold may be required to expose the NBD domain and thus allow its regulatory function to occur.
So far it is unclear if and when the negative regulatory activity of NLRs is needed to promote homeostatic conditions and prevent immune-driven pathology. It is intriguing that both NLRP6- and NLRC3-deficient mice, for example, appear to have a survival advantage during pathogenic infection(Anand et al., 2012; Lu, 2014). For NLRP6, this protection seems to occur at the cost of reduced control of the gut microbiome and increased susceptibility for inflammatory pathologies upon dietary challenge(Elinav et al., 2011). It remains to be determined whether the lack of NLRC3 has other -as of yet - undefined liabilities. Further investigation must be made into how these negatively regulating proteins act under normal conditions, as currently it is not clear how expression of these proteins is advantageous to the immune system.
NLRC3 joins the growing list of NLRs involved in negative regulation of immune responses, though it is the first implicated in control of the cytosolic dsDNA response.The STING pathway can contribute to inflammatory pathologies and the development of autoimmune diseases under conditions of increased appearance or oxidative modifications of endogenous DNA(Ahn et al., 2012; Gehrke et al., 2013). Hence, it would be interesting to test whether NLRC3 functions to suppress inflammation and auto-immune responses in models of systemic lupus erythematosis. Moreover,it is possible that inactivating mutations in NLRC3 might be associated with autoimmune disorders involving detection of self-DNA.
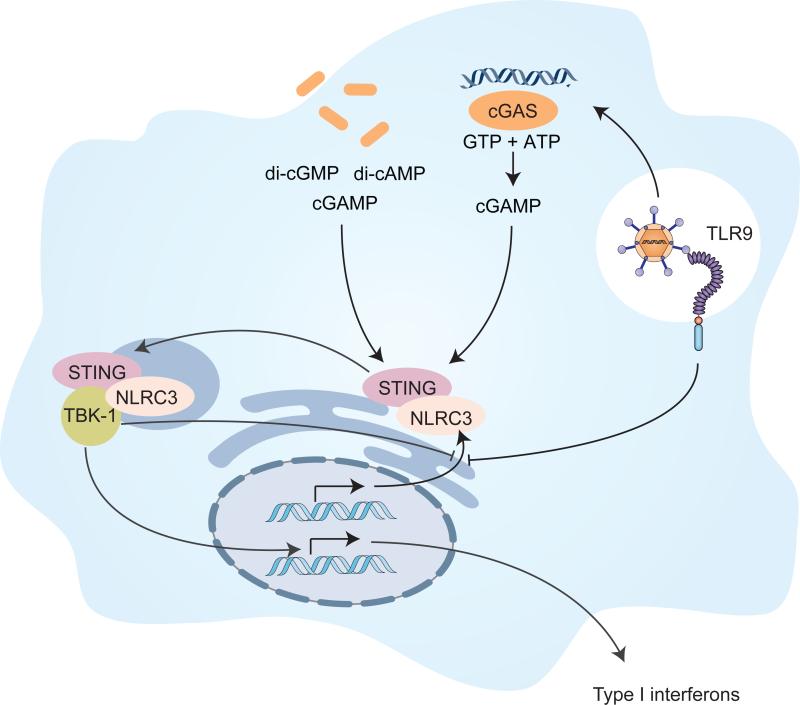
Figure 1. NLRC3 controls STING activation and type 1 interferon secretion.
STING is triggered by cyclic dinucleotides released from bacteria or generated by cGAS upon recognition of cytosolic dsDNA. Zhang et al. demonstrate that NLRC3 binds to STING, sequestering it at the ER and preventing it from interacting with TBK-1. Afterviral infection, TLR9 and STING activate NF-κB, whichlowersNLRC3 expression. Hence, it is possible that these pathways commence a positive feedback loop, resulting in increased type 1 interferon production and secretion through enhanced STING activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand PK, Malireddi RKS, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti T-D. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends in Immunology. 2014;35:88–93. doi: 10.1016/j.it.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau Andrew L., Henao-Mejia J, Thaiss Christoph A., Booth Carmen J., Peaper David R., Bertin J, Eisenbarth Stephanie C., Gordon Jeffrey I., Flavell Richard A. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, Tuting T, Hartmann G, Barchet W. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity. 2014 doi: 10.1016/j.immuni.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupfer C, Kanneganti T-D. Unsolved mysteries in NLR biology. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Zimmermann AG, Roberts RA, Zhang L, Swanson KV, Wen H, Davis BK, Allen IC, Holl EK, Ye Z, et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-[kappa]B. Nat Immunol. 2012;13:823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Man SM, Vogel P, Lamkanfi M, Kanneganti TD. Salmonella exploits NLRP12-dependent innate immune signaling to suppress host defenses during infection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:385–390. doi: 10.1073/pnas.1317643111. [DOI] [PMC free article] [PubMed] [Google Scholar]