Abstract
The most important extra-renal manifestation of autosomal dominant polycystic kidney disease (ADPKD) in terms of debilitating injury and premature death is the development of intracranial aneurysms (IAs) and other vascular complications, resulting in subarachnoid hemorrhage (SAH). IAs are found at a rate approximately five times higher in ADPKD patients than in the general population and in patients with a family history of SAH/IAs the frequency is elevated further three to five times, indicating the importance of genetic factors in its etiology. Expression of the ADPKD gene products, polycystin-1 (PKD1) and polycystin-2 (PKD2), in vascular smooth muscle and the endothelium, and evidence that reduced levels of these proteins leads to IA development in mouse models, suggests a direct role of these proteins in the vascular disease. PKD1 and PKD2 patients seem equally likely to develop IAs, while patients with mutations to the 5’ half of PKD1 may more likely have vascular complications. Genome wide association and candidate studies of multiplex families with IAs without ADPKD have identified a number of genes/proteins that may be risk factors for the development of IAs. These candidate proteins largely have roles in the maintenance and remodeling of the arterial wall of small brain arteries. The development of the genetic methodologies of massively parallel sequencing mean it is now possible to test these and other candidates in ADPKD families with multiplex and singleton IA cases. Identifying strong modifiers of this phenotype will be important for prioritizing patients for presymptomatic screening and interventions.
Keywords: ADPKD, intracranial aneurysms, genetic modifiers, genotype/phenotype correlations
THE ADPKD DISEASE PHENOTYPE IS PLEIOTROPIC AND COMPLEX
The impressive advancements in human genetic methodologies in the last decade has led to the identification of a large number of Mendelian disease genes and pathogenic mutations that has enabled precise correlations with the clinically defined phenotypes. As a consequence, there has been a muddying of the paradigm of mutation to a single gene causing a single clinical phenotype. Rather, a model has emerged where the Mendelian phenotype is modified allelically, by variants at other loci, and by environmental influences such that a rigid distinction between classical single gene conditions and complex traits has gradually shifted to one characterized by a continuum between the two extremes [1–3]. In reality, Mendelian conditions are complex in nature, with mutations to a single gene causative, but the precise clinical phenotypic spectrum and degree of severity modulated by modifying loci.
ADPKD is a striking example of a Mendelian condition characterized by phenotypic variability [4, 5]. This phenotypic variability includes the degree of kidney disease severity, ranging from severe in utero cases [6, 7] to mild phenotypes characterized by adequate kidney function into late life [8, 9]. The complexity of the phenotype is also evident by the presence of extra-renal clinical features [10] making ADPKD a truly pleiotropic condition, with vascular complications the one most associated with morbidity and mortality. Genetic heterogeneity, with two recognized loci, PKD1 on chromosome 16 and PKD2 on chromosome 4 [11,12], strongly influences renal disease severity; average age at end stage renal disease (ESRD) is ~54y for PKD1 and ~74y for PKD2 [5]. In addition, significant renal phenotypic variability is observed within each genic population indicating a role for the specific mutation (allelic effects) [13,14], as well as within families (intra-familial variability) that is presumed to be due to the influence of modifier loci and the environment [15,16]. The degree to which these factors influence the vascular phenotype is discussed below and the likely important of the various potential modifiers illustrated in Fig. (1).
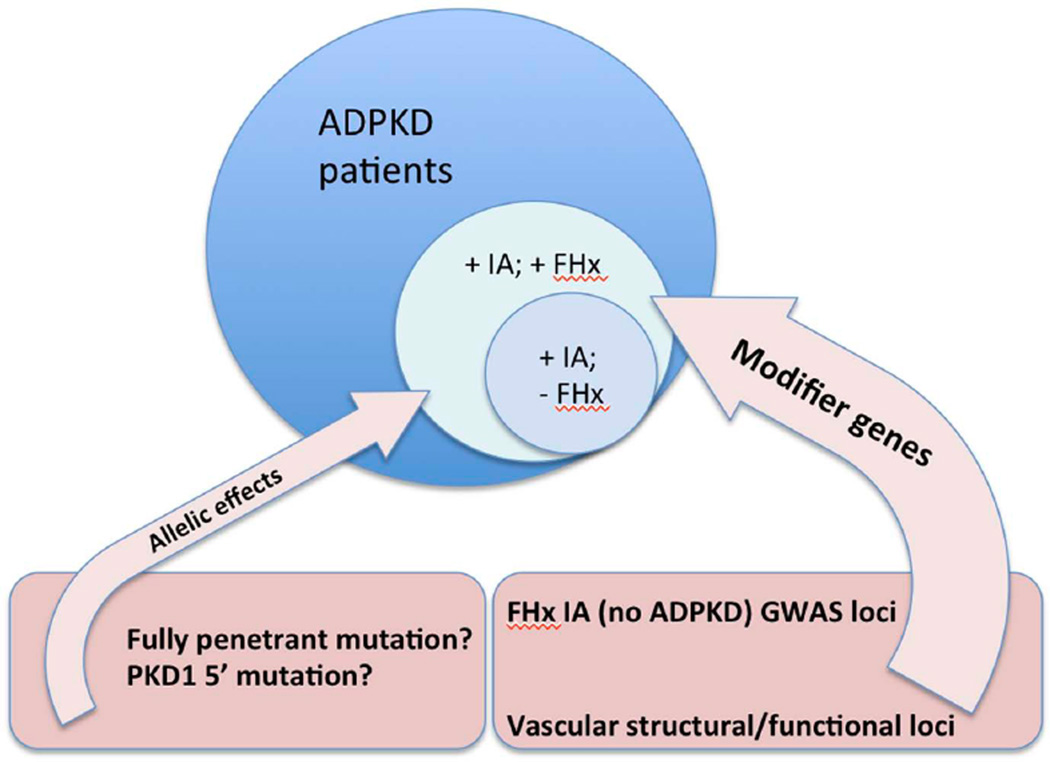
Fig. (1).
Factors influencing the development of IAs in ADPKD patients. Patients with IAs with (+) or without (−) a family history (FHx) are illustrated. Modifier genes are likely the major determinant whether a patient develops vascular complications and may include variants in genes encoding vascular proteins, with some candidates already identified by GWAS in multiplex families with IA, without ADPKD. PKD1 and PKD2 allelic effects are probably less important, with the changes most likely to be associated with IA development illustrated.
CLINICAL SPECTRUM AND SCREENING RECOMMENDATIONS FOR VASCULAR COMPLICATIONS IN ADPKD
A wide spectrum of vascular abnormalities is seen in ADPKD patients. These include intracranial aneurysms (IAs) and dolichoectasias, thoracic aorta and cervicocephalic artery dissections and coronary artery aneurysms [17–21]. IAs are the most life-threatening phenotypic feature in ADPKD. In a recent study of 407 ADPKD patients presymptomatically screened by magnetic resonance angiography (MRA) [22], a prevalence of 9.3% was estimated for asymptomatic IAs. The estimated prevalence of unruptured IAs in ADPKD patients with a family history of IAs and/or subarachnoid hemorrhage was 21.2%, while the estimated prevalence of unruptured IAs in ADPKD patients without such a history was 6.3%. Another recent study of 355 patients estimated a prevalence of 12.4% of IAs in the general ADPKD population, with an age dependent increase of prevalence, with a peak of 23.3% in patients older than 60 years [23]. Familial clustering is observed [22, 24, 25], with the IA frequency five times higher in ADPKD pedigrees with a positive family history of ruptured IAs than in those without such a history [26].
Rupture of an IA is associated with high morbidity and mortality, suggesting pre-symptomatic screening in ADPKD pedigrees as a preventive approach. However, the utility of pre-symptomatic screening depends on the tendency of IAs to grow and rupture and must take into consideration the not insignificant risk associated with clipping and other interventional procedures. The natural history of ADPKD-associated IAs was recently studied in a follow-up of 38 ADPKD patients (36 pedigrees), where 45 saccular aneurysms were detected by presymptomatic screening by MRA [22]. Most of the IAs were small (less than 3.5mm in diameter) and 84% of them were in the anterior brain circulation. In this cohort, minimal change was observed in the evolution of the vascular lesions during the 243 years of cumulative follow-up. Only one de novo IA appeared, in two other patients the pre-existent IAs grew in size, but in the remainder no changes were observed and no IAs ruptured. This study, performed in a relatively young population of patients, suggested that the risk of IA enlargement and rupture is low. Other studies found that the only strong risk predictor for IAs enlargement [27–30] and rupture [29,31] is the IA size: an IA larger than 5mm has a higher risk of undergoing size increase, while the risk of rupture is higher for diameters larger than 7 [24] or 10 mm [31]. Hence, due to the significant mortality and morbidity associated with surgical intervention, screening for asymptomatic IAs is not recommended in ADPKD patients without a family history of SAH or aneurysmal rupture, unless undergoing major elective surgeries or in high-risk occupations, such as airline pilot or bus driver [22].
PATHOPHYSIOLOGY OF IAS IN ADPKD
Both the PKD1 and PKD2 proteins, polycystin-1 (PC-1) and polycystin-2 (PC-2), respectively, are expressed in the vasculature, particularly in smooth muscle cells of the tunica media and myofibroblasts [32, 33], but also in the endothelium [34]. A role has been suggested for the polycystin complex as a pressure sensor in the vasculature [35]. While the expression of PC-1 appears to be developmentally regulated, the expression of PC-2 is more constant [36]. Immunostaining of ruptured intracranial aneurysms in ADPKD patients shows expression of PC-1 and PC-2 in spindle-shaped cells on the wall of the aneurysm as well as in the smooth muscle cells of the tunica media of the parental arteries [32, 33, 36], suggesting a direct involvement of the two proteins in the maintenance of the arterial wall of the small arteries in the brain and the development of IAs in ADPKD patients. A similar pattern is observed in the arterial wall of ADPKD patients with aortic dissections or dolichoectatic intracranial arteries [32]. The localization of PC-1 and PC-2 in the proximity of smooth muscle cell dense plaques (structures similar to focal adhesions in epithelial cells) supports the involvement of these two proteins in the development and maintenance of the myoelastic wall of arteries.
The finding of lower levels of PC-2 expression in smooth muscle cells of Pkd2+/− mice suggests a direct involvement of PC-2 (a TRP-like calcium channel) in the regulation of intracellular calcium, and that Pkd2 haploinsufficiency may be related to the development of the vascular phenotype in humans [37]. Furthermore, Pkd2+/− mice induced to develop hypertension form IAs at twice the level of wildtype controls [37]. Meanwhile, in the Pkd1nl/nl hypomorphic mouse model that expresses PC-1 at 26% of the normal level in the aorta, dissecting aortic aneurysms are common [38]. These were characterized by accumulation of matrix components between elastin lamella and a tear in the intima resulting in an intramural bleed. This data further emphasizes the importance of the level of PC-1 expression to maintain vascular integrity.
GENETICS OF IAs IN ADPKD: GENIC AND ALLELIC INFLUENCE
The association of IAs with PKD1- [39] or PKD2- [40] linked pedigrees has been known since the mapping of the two disease genes. However, it was only after the identification of the PKD1 and PKD2 genes [11,12], and the development of comprehensive assays [41–44] that genotype-phenotype correlations could be investigated.
In a preliminary analysis of 35 ADPKD pedigrees [45] that included individuals with IAs and/or early onset disease, the PKD1 region encompassing exons 11–21 was sequenced using locus-specific amplicons to overcome the problem of genomic duplication of the PKD1 locus [11]. Five different frameshifting (c.3009_3012delCAAC, c.5014_5015delAG, c.5572delG, c.5813_5814insC, 7186_7187insTTGCCTCAATT) and two missense mutations (Arg2329Pro Ser2423Phe) [46] were found in eight pedigrees with ruptured or unruptured IAs. Notably, the frameshifting change c.5014_5015delAG was common to three pedigrees showing a vascular phenotype or early onset disease [45]. This suggested that this mutation could be a predisposing factor for developing more severe disease/vascular phenotype. However, this initial observation was not supported by mutation analysis on larger populations, and c.5014_5015delAG is now known to be the most common PKD1 mutation (1–2% of total) [46, 47] and associated with a wide range of phenotypes. Recently the co-inheritance of an inactivating and hypomorphic PKD1 allele has been associated with early onset ADPKD due to reduction in the level of functional PC-1 [13,14]. No evidence from human populations has yet shown if unusually low levels of functional PC-1 are associated with IA development, but studies from mouse models (see above) suggest that as a possibility (Fig. 1) [37,38].
The relationship between the germline mutation and the risk of developing a vascular phenotype was further analyzed in a larger population of 58 ADPKD families where at least one individual developed a vascular phenotype, which was clinically well characterized [48]. Both the PKD1 and PKD2 genes were screened for mutation over the entire coding sequence of the two genes. Of the 58 pedigrees, 51 (88%) were PKD1 and seven PKD2 (12%), similar to their ratio in a typical ADPKD population (85% PKD1, 15% PKD2) [43], indicating that IAs are not less common in PKD2 populations, although they have milder kidney disease [5]. These 58 families contained 85 members with a vascular finding (46% males and 54% females) and 79% of the subjects had a ruptured IA, leading to death in half of them. The population was classed according to disease severity, including: individuals with rupture, individuals with rupture before 40y, and cases with familial clustering of IAs. No association was found with the mutation type in PKD1, truncating or in-frame change (including missense). However, a significant association was found with the mutation position in PKD1; the median position of the mutations was further 5’ (or N-terminal) in the vascular cohort as compared to the control cohort, which was most evident in the subgroups with rupture, early rupture and familial clustering (Fig. 1). A receiver operating characteristics (ROC) analysis indicated an increased predictive ability from 7.5% in the control cohort to 12.6% in the cohort with mutation 5’ to the median position, and an increase from 2.5% to 7.1% in the sub-group with familial clustering. It is not clear why 5’ mutations may be more associated with IA development but it could be due to different roles of N-terminal and C-terminal GPS cleavage products in the vasculature [49, 50].
In a recent study of pre-symptomatic screening using MRA, a prevalence of 9.3% was found with a much higher prevalence in pedigrees with known IA history (21.1%) [22]. Molecular analysis of 26 pedigrees showed that 21 had a PKD1 mutation (84%) and four a PKD2 mutation (16%), while in the remaining family no mutation was identified. In this relatively small group the authors did not observe any specific genotype-phenotype correlation as to the size or location of the IAs, development of multiple IAs or presence of family history of rupture. As described above [48], PKD1 and PKD2 individuals appeared to be at equal risk of developing IAs.
GENETICS OF IAS IN THE GENERAL POPULATION
IAs occur in the general population with a prevalence between 0.5 and 1% [19, 51, 52], they are mainly located in the anterior circulation (80–85%), and are a common finding in post-mortem autopsies (1–6% in adults) [53–55]. Usually multiple IAs are found per patient, as many as 2–3 in 20–30% of the cases, and SAH is a major clinical problem associated with high morbidity and mortality [56–61]. Several lines of evidence suggest that genetics is an important risk factor for IAs in the general population, including the familial clustering observed in IAs pedigrees [62] and the association with Mendelian syndromes, particularly connective tissue disorders [63].
Familial clustering of IAs (not in association with a Mendelian syndrome) has been documented in hundreds of pedigrees [64]. It has been estimated that 7–20% of patients with IAs/SAH have a first or second degree relative with a confirmed IA [65–69]. Furthermore, the risk of IA rupture for a first-degree relative of an index patient is four times higher than for the general population [68–70]. However, no clear pattern of inheritance has been identified and so a high level of genetic heterogeneity with a low level of penetrance and environment input has been hypothesized [62]; it is a complex trait [71–74].
Two recent genome wide association studies (GWAS) were conducted on large cohorts with familial clustering of IAs and identified several critical intervals and a reproducible group of candidates. Study of a cohort of 2,100 cases and 8,000 controls from Finland, the Netherlands and Japan [75] identified loci on chromosomes 2q, 8q and 9p. The second larger study utilized 5,891 cases and 14,181 controls from Europe and Japan [76] and confirmed the two loci on 8q and 9p and identified three new loci on chromosomes 18q, 13q and 10q. Analysis in these intervals identified potential candidate genes: RBBP8 at 18q (encoding the retinoblastoma binding protein 8); STARD13 at 13q (the StAR-related lipid transfer (START) domain containing 13), with KL (klotho) close to this interval; CNNM2 at 10q (encoding cyclin M2); BOLL and PLCL1 at 2q (an RNA binding protein and a protein with high homology to phospholipase C, respectively); SOX17 at 8q and CDKN2A, CDKN2B and ANRIL (a non-protein-coding transcript) at 9p. All these genes code for proteins putatively involved in cell cycle regulation and progression, cell proliferation and senescence of progenitor cells or regulate a balance between progenitor cells and terminally differentiated cells in the arterial wall [76]. In fact, SOX17 is required for both endothelial formation and maintenance [77–80]; CDKN2A and CDKN2B encode for a cyclin-dependent kinase inhibitor and a regulator of p53 activity [81]; RBBP8 influences the cell cycle by interacting with BRCA1 [82]; STARD13 overexpression causes suppression of cell proliferation [83]; and KL modulates the fibroblast growth factor receptor specificity [84] and its absence causes accelerated aging in mouse models [85]. All these functions merge into a unified model that defines the risk to develop IAs in the general population as a defect in the maintenance and remodeling of the arterial wall of the small brain arteries.
Interestingly, the 9p interval has also been associated with abdominal aortic aneurysms [86], myocardial infarction, abdominal aortic aneurysms and IAs [87], suggesting a wider influence for CDKN2A, CDKN2B and/or ANRIL [88]. Conversely, DAB2IP, an inhibitor of cell growth and survival, was identified as a candidate gene conferring susceptibility to abdominal aortic aneurysm [89] but not IAs, suggesting that in some cases different specific genetic susceptibility may exist for aneurysms in different arterial locations.
An alternative approach to identify susceptibility genes for IAs has utilized linkage analysis on large, multiplex families [90,91], a candidate approach [92–94]; or expression analysis of human arterial wall-associated genes [95]. Candidates have been tested in IA pedigrees that play a critical role in arterial wall maintenance and repair or are involved in Mendelian syndromes that include IAs as part of the phenotypic spectrum. A group of functional candidates has been defined based on knowledge of the structure and function of the arterial wall [91,96]. These include genes involved in the remodeling of the extracellular matrix (alpha 1 antitrypsin, elastases, metalloproteinases); basal membrane constituents (collagens, laminins, nidogen); and constituents of the tunica media (collagens, vitronectin, laminin, fibrillins and elastin). Some other obvious candidates include genes mutated in syndromic disorders that include the development of vascular aneurysms, or pathways known to influence vasculature remodeling. These include the TGF beta pathway [97–101] and collagen genes [102–107]. Overall, meta-analysis of all the published studies suggests a high level of genetic heterogeneity and polygenic inheritance [71,74,108].
GENETICS OF IAS IN ADPKD: GENETIC MODIFIERS
Although some genotype-phenotype correlation have been observed in ADPKD for the development of a vascular phenotype (see above) [22,48], strong intra-familial variability is observed, suggesting that other genetic variants are involved besides the PKD1 or PKD2 mutation (Fig. 1). The task ahead is to identify and functionally evaluate genetic modifiers associated with the development of IAs and other vascular complications in ADPKD patients. The populations are too small to consider GWAS but the availability of next-generation sequencing technologies makes it possible to deeply scan the human genome, particularly the coding portion (exome), for disease-causing variants. A likely design of such studies will employ whole exome analysis in ADPKD pedigrees with multiple vascular cases with follow-up in singleton families. The other option, and one that might be attractive at this time, is employing a candidate-based approach analyzing genes identified from the familial IA studies and other candidates suggested from their involvement in the maintenance, integrity and function of the arterial wall of the small brain arteries (see above) in the multiplex and singleton vascular ADPKD pedigrees (Fig. 1) [109]. However, segregation analysis and comprehensive comparison to an ADPKD population without IAs will be required, plus functional validation using in vivo testing and animal models, to definitely prove the disease-association of identified genes and variants.
ACKNOWLEDGEMENTS
This study was supported by NIDDK funding to SR (DK83669) and PCH (DK58816).
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Badano JL, Katsanis N. Beyond Mendel: an evolving view of human genetic disease transmission. Nat Rev Genet. 2002;3:779–789. doi: 10.1038/nrg910. [DOI] [PubMed] [Google Scholar]
- 2.Nadeau JH. Modifier genes in mice and humans. Nat Rev Genet. 2001;2:165–174. doi: 10.1038/35056009. [DOI] [PubMed] [Google Scholar]
- 3.Cutting GR. Modifier genetics: cystic fibrosis. Annu Rev Genomics Hum Genet. 2005;6:237–260. doi: 10.1146/annurev.genom.6.080604.162254. [DOI] [PubMed] [Google Scholar]
- 4.Hateboer N, Lazarou LP, Williams AJ, Holmans P, Ravine D. Familial phenotype differences in PKD1. Kidney Int. 1999;56:34–40. doi: 10.1046/j.1523-1755.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- 5.Hateboer N, van Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353:103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 6.Zerres K, Rudnik-Schöneborn S, Deget F German working group on paediatric nephrology. Childhood onset autosomal dominant polycystic kidney disease in sibs: clinical picture and recurrence risk. J Med Genet. 1993;30:583–588. doi: 10.1136/jmg.30.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fick GM, Johnson AM, Strain JD, et al. Characteristics of very early onset autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3:1863–1870. doi: 10.1681/ASN.V3121863. [DOI] [PubMed] [Google Scholar]
- 8.Torra R, Badenas C, Perez-Oller L, et al. Increased prevalence of polycystic kidney disease type 2 among elderly polycystic patients. Am J Kidney Dis. 2000;36:728–734. doi: 10.1053/ajkd.2000.17619. [DOI] [PubMed] [Google Scholar]
- 9.Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41:1311–1319. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- 10.Torres VE, Harris PC. Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J Intern Med. 2007;261:17–31. doi: 10.1111/j.1365-2796.2006.01743.x. [DOI] [PubMed] [Google Scholar]
- 11.European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 13.Rossetti S, Kubly VJ, Consugar MB, et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009;75:848–855. doi: 10.1038/ki.2008.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopp K, Ward CJ, Hommerding CJ, et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest. 2012;122:4257–4273. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson AD, Magistroni R, He N, et al. Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2005;16:755–762. doi: 10.1681/ASN.2004090758. [DOI] [PubMed] [Google Scholar]
- 16.Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6:197–206. doi: 10.1038/nrneph.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman AB, Rubinstein D, Hughes R, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Eng J Med. 1992;327:916–920. doi: 10.1056/NEJM199209243271303. [DOI] [PubMed] [Google Scholar]
- 18.Schievink W, Torres V, Wiebers D, Huston J., III Intracranial arterial dolichoectasia in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8:1298–1303. doi: 10.1681/ASN.V881298. [DOI] [PubMed] [Google Scholar]
- 19.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 20.Chauveau D, Pirson Y, Verellen-Dumoulin C, Macnicol A, Gonzalo A, Grünfeld J-P. Intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int. 1994;45:1140–1146. doi: 10.1038/ki.1994.151. [DOI] [PubMed] [Google Scholar]
- 21.Bobrie G, Brunet-Bourgin F, Alamowitch S, et al. Spontaneous artery dissection: is it part of the spectrum of autosomal dominant polycystic kidney disease? Nephrol Dial Transplant. 1998;13:2138–2141. doi: 10.1093/ndt/13.8.2138. [DOI] [PubMed] [Google Scholar]
- 22.Irazabal MV, Huston J, 3rd, Kubly V, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:1274–1285. doi: 10.2215/CJN.09731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu HW, Yu SQ, Mei CL, Li MH. Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke. 2011;42:204–206. doi: 10.1161/STROKEAHA.110.578740. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs FF, Huston J, III, Qian Q, et al. Follow-up of intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int. 2004;65:1621–1627. doi: 10.1111/j.1523-1755.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 25.Belz MM, Hughes RL, Kaehny WD, et al. Familial clustering of ruptured intracranial aneurysms in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2001;38:770–776. doi: 10.1053/ajkd.2001.27694. [DOI] [PubMed] [Google Scholar]
- 26.Huston J, Torres VE, Sullivan PP, Offord KP, Wiebers DO. Value of magnetic resonance angiography for detection of intracranial aneurysm in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3:1871–1877. doi: 10.1681/ASN.V3121871. [DOI] [PubMed] [Google Scholar]
- 27.Burns JD, Huston J, 3rd, Layton KF, Piepgras DG, Brown RD., Jr Intracranial aneurysm enlargement on serial magnetic resonance angiography: frequency and risk factors. Stroke. 2009;40:406–411. doi: 10.1161/STROKEAHA.108.519165. [DOI] [PubMed] [Google Scholar]
- 28.Matsubara S, Hadeishi H, Suzuki A, Yasui N, Nishimura H. Incidence and risk factors for the growth of unruptured cerebral aneurysms: observation using serial computerized tomography angiography. J Neurosurg. 2004;101:908–914. doi: 10.3171/jns.2004.101.6.0908. [DOI] [PubMed] [Google Scholar]
- 29.Miyazawa N, Akiyama I, Yamagata Z. Risk factors for growth of unruptured intracranial aneurysms: follow-up study by serial 0.5-T magnetic resonance angiography. Neurosurgery. 2006;58:1047–1053. doi: 10.1227/01.NEU.0000217366.02567.D2. [DOI] [PubMed] [Google Scholar]
- 30.Wermer MJ, van der Schaaf IC, Velthuis BK, Majoie CB, Albrecht KW, Rinkel GJ. Yield of short-term follow-up CT/MR angiography for small aneurysms detected at screening. Stroke. 2006;37:414–418. doi: 10.1161/01.STR.0000199077.06390.35. [DOI] [PubMed] [Google Scholar]
- 31.Wiebers DO, Whisnant JP, Sundt TM, Jr, O'Fallon WM. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987;66:23–29. doi: 10.3171/jns.1987.66.1.0023. [DOI] [PubMed] [Google Scholar]
- 32.Griffin MD, Torres VE, Grande JP, Kumar R. Vascular expression of polycystin. J Am Soc Nephrol. 1997;8:616–626. doi: 10.1681/ASN.V84616. [DOI] [PubMed] [Google Scholar]
- 33.Torres VE, Cai Y, Chen X, et al. Vascular expression of polycystin 2. J Am Soc Nephrol. 2001;12:1–9. doi: 10.1681/ASN.V1211. [DOI] [PubMed] [Google Scholar]
- 34.Ibraghimov-Beskrovnaya O, Dackowski WR, Foggensteiner L, et al. Polycystin: In vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc Natl Acad Sci USA. 1997;94:6397–6402. doi: 10.1073/pnas.94.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharif-Naeini R, Folgering JH, Bichet D, et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 36.Qian Q, Li M, Cai Y, et al. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol. 2003;14:2280–2287. doi: 10.1097/01.asn.0000080185.38113.a3. [DOI] [PubMed] [Google Scholar]
- 37.Qian Q, Hunter LW, Li M, et al. PKD2 haploinsufficiency alters intracellular calcium in vascular smooth muscle cells. Hum Mol Genet. 2003;12:1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]
- 38.Hassane S, Claij N, Lantinga-van Leeuwen IS, et al. Pathogenic sequence for dissecting aneurysm formation in a hypomorphic polycystic kidney disease 1 mouse model. Arterioscler Thromb Vasc Biol. 2007;27:2177–2183. doi: 10.1161/ATVBAHA.107.149252. [DOI] [PubMed] [Google Scholar]
- 39.Schievink WI, Torres VE, Piepgras DG, Wiebers DO. Saccular intracranial aneurysms in autosomal dominant polycystic disease. J Am Soc Nephrol. 1992;3:88–95. doi: 10.1681/ASN.V3188. [DOI] [PubMed] [Google Scholar]
- 40.van Dijk MA, Chang PC, Peters DJM, Breuning MH. Intracranial aneurysms in polycystic kidney disease linked to chromosome 4. J Am Soc Nephrol. 1995;6:1670–1673. doi: 10.1681/ASN.V661670. [DOI] [PubMed] [Google Scholar]
- 41.Rossetti S, Strmecki L, Gamble V, et al. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet. 2001;68:46–63. doi: 10.1086/316939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossetti S, Chauveau D, Walker D, et al. A complete mutation screen of the ADPKD genes by DHPLC. Kidney Int. 2002;61:1588–1599. doi: 10.1046/j.1523-1755.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 43.Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 44.Veldhuisen B, Saris JJ, de Haij S, et al. A spectrum of mutations in the second gene for autosomal dominant polycystic kidney disease (PKD2) Am J Hum Genet. 1997;61:547–555. doi: 10.1086/515497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watnick T, Phakdeekitcharoen B, Johnson A, et al. Mutation detection of PKD1 identifies a novel mutation common to three families with aneurysms and/or very-early-onset disease. Am J Hum Genet. 1999;65:1561–1571. doi: 10.1086/302657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ADPKD. PKD Foundation; 2010. Autosomal Dominant Polycystic Kidney Disease: Mutation Database. http://pkdb.mayo.edu. [Google Scholar]
- 47.Harris P, Rossetti S. Molecular diagnostics of autosomal dominant polycystic kidney disease (ADPKD) Nature Rev Nephrol. 2010;6:197–206. doi: 10.1038/nrneph.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet. 2003;361:2196–2201. doi: 10.1016/S0140-6736(03)13773-7. [DOI] [PubMed] [Google Scholar]
- 49.Ponting CP, Hofmann K, Bork P. A latrophilin/CL-1-like GPS domain in polycystin-1. Curr Biol. 1999;9:R585–R588. doi: 10.1016/s0960-9822(99)80379-0. [DOI] [PubMed] [Google Scholar]
- 50.Qian F, Boletta A, Bhunia AK, et al. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1- associated mutations. Proc Natl Acad Sci USA. 2002;99:16981–16986. doi: 10.1073/pnas.252484899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atkinson JL, Sundt TM, Jr, Houser OW, Whisnant JP. Angiographic frequency of anterior circulation intracranial aneurysms. J Neurosurg. 1989;70:551–555. doi: 10.3171/jns.1989.70.4.0551. [DOI] [PubMed] [Google Scholar]
- 52.Winn HR, Taylor J, Kaiser D. Prevalence of asymptomatic incidental aneurysms: Review of 4,568 arteriograms. Stroke. 1983;4:121. doi: 10.3171/jns.2002.96.1.0043. [DOI] [PubMed] [Google Scholar]
- 53.McCormick WF, Nofzinger JD. Saccular Intracranial Aneurysms: An Autopsy Study. J Neurosurg. 1965;22:155–159. doi: 10.3171/jns.1965.22.2.0155. [DOI] [PubMed] [Google Scholar]
- 54.Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol. 1990;34:361–365. doi: 10.1016/0090-3019(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 55.Inagawa T, Hirano A. Ruptured intracranial aneurysms: an autopsy study of 133 patients. Surg Neurol. 1990;33:117–123. doi: 10.1016/0090-3019(90)90020-p. [DOI] [PubMed] [Google Scholar]
- 56.Schievink WI, Wijdicks EF, Parisi JE, Piepgras DG, Whisnant JP. Sudden death from aneurysmal subarachnoid hemorrhage. Neurology. 1995;45:871–874. doi: 10.1212/wnl.45.5.871. [DOI] [PubMed] [Google Scholar]
- 57.Phillips LH, 2nd, Whisnant JP, O'Fallon WM, Sundt TM., Jr The unchanging pattern of subarachnoid hemorrhage in a community. Neurology. 1980;30:1034–1040. doi: 10.1212/wnl.30.10.1034. [DOI] [PubMed] [Google Scholar]
- 58.Sacco RL, Wolf PA, Bharucha NE, et al. Subarachnoid and intracerebral hemorrhage: natural history, prognosis, and precursive factors in the Framingham Study. Neurology. 1984;34:847–854. doi: 10.1212/wnl.34.7.847. [DOI] [PubMed] [Google Scholar]
- 59.Longstreth WT, Jr, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology. 1993;43:712–718. doi: 10.1212/wnl.43.4.712. [DOI] [PubMed] [Google Scholar]
- 60.Fogelholm R, Hernesniemi J, Vapalahti M. Impact of early surgery on outcome after aneurysmal subarachnoid hemorrhage. A population-based study. Stroke. 1993;24:1649–1654. doi: 10.1161/01.str.24.11.1649. [DOI] [PubMed] [Google Scholar]
- 61.Inagawa T, Tokuda Y, Ohbayashi N, Takaya M, Moritake K. Study of aneurysmal subarachnoid hemorrhage in Izumo City, Japan. Stroke. 1995;26:761–766. doi: 10.1161/01.str.26.5.761. [DOI] [PubMed] [Google Scholar]
- 62.Schievink WI, Schaid DJ, Rogers HM, Piepgras DG, Michels VV. On the inheritance of intracranial aneurysms. Stroke. 1994;25:2028–2037. doi: 10.1161/01.str.25.10.2028. [DOI] [PubMed] [Google Scholar]
- 63.Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders: A review. Stroke. 1994;25:889–903. doi: 10.1161/01.str.25.4.889. [DOI] [PubMed] [Google Scholar]
- 64.Chambers WR, Harper BF, Jr, Simpson JR. Familial incidence of congenital aneurysms of cerebral arteries: report of cases of ruptured aneurysms in father and son. J Am Med Assoc. 1954;155:358–359. doi: 10.1001/jama.1954.73690220001007. [DOI] [PubMed] [Google Scholar]
- 65.Norrgard O, Angquist KA, Fodstad H, Forsell A, Lindberg M. Intracranial aneurysms and heredity. Neurosurgery. 1987;20:236–239. doi: 10.1227/00006123-198702000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Ronkainen A, Hernesniemi J, Puranen M, et al. Familial intracranial aneurysms. Lancet. 1997;349:380–384. doi: 10.1016/S0140-6736(97)80009-8. [DOI] [PubMed] [Google Scholar]
- 67.Ronkainen A, Hernesniemi J, Ryynanen M. Familial subarachnoid hemorrhage in east Finland, 1977–1990. Neurosurgery. 1993;33:787–796. doi: 10.1227/00006123-199311000-00001. discussion 96–97. [DOI] [PubMed] [Google Scholar]
- 68.Schievink WI, Schaid DJ, Michels VV, Piepgras DG. Familial aneurysmal subarachnoid hemorrhage: a community-based study. J Neurosurg. 1995;83:426–429. doi: 10.3171/jns.1995.83.3.0426. [DOI] [PubMed] [Google Scholar]
- 69.De Braekeleer M, Perusse L, Cantin L, Bouchard JM, Mathieu J. A study of inbreeding and kinship in intracranial aneurysms in the Saguenay Lac-Saint-Jean region (Quebec, Canada) Ann Hum Genet. 1996;60:99–104. doi: 10.1111/j.1469-1809.1996.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 70.Bromberg JE, Rinkel GJ, Algra A, et al. Subarachnoid haemorrhage in first and second degree relatives of patients with subarachnoid haemorrhage. BMJ. 1995;311:288–289. doi: 10.1136/bmj.311.7000.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nahed BV, Bydon M, Ozturk AK, Bilguvar K, Bayrakli F, Gunel M. Genetics of intracranial aneurysms. Neurosurgery. 2007;60:213–225. doi: 10.1227/01.NEU.0000249270.18698.BB. discussion 25–6. [DOI] [PubMed] [Google Scholar]
- 72.Ruigrok YM, Rinkel GJ. Genetics of intracranial aneurysms. Stroke. 2008;39:1049–1055. doi: 10.1161/STROKEAHA.107.497305. [DOI] [PubMed] [Google Scholar]
- 73.Ruigrok YM, Rinkel GJ, Wijmenga C. Genetics of intracranial aneurysms. Lancet Neurol. 2005;4:179–189. doi: 10.1016/S1474-4422(05)01015-X. [DOI] [PubMed] [Google Scholar]
- 74.Ruigrok YM, Elias R, Wijmenga C, Rinkel GJ. A comparison of genetic chromosomal loci for intracranial, thoracic aortic, and abdominal aortic aneurysms in search of common genetic risk factors. Cardiovasc Pathol. 2008;17:40–47. doi: 10.1016/j.carpath.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Bilguvar K, Yasuno K, Niemela M, et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet. 2008;40:1472–1477. doi: 10.1038/ng.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yasuno K, Bilguvar K, Bijlenga P, et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat Genet. 2010;42:420–425. doi: 10.1038/ng.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Visel A, Zhu Y, May D, et al. Targeted deletion of the 9p21 noncoding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsui T, Kanai-Azuma M, Hara K, et al. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- 79.Sakamoto Y, Hara K, Kanai-Azuma M, et al. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem Biophys Res Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- 80.Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 82.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leung TH, Ching YP, Yam JW, et al. Deleted in liver cancer 2 (DLC2) suppresses cell transformation by means of inhibition of RhoA activity. Proc Natl Acad Sci USA. 2005;102:15207–15212. doi: 10.1073/pnas.0504501102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 85.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 86.Thompson AR, Golledge J, Cooper JA, Hafez H, Norman PE, Humphries SE. Sequence variant on 9p21 is associated with the presence of abdominal aortic aneurysm disease but does not have an impact on aneurysmal expansion. Eur J Hum Genet. 2009;17:391–394. doi: 10.1038/ejhg.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 88.Pasmant E, Sabbagh A, Vidaud M, Bieche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2011;25:444–448. doi: 10.1096/fj.10-172452. [DOI] [PubMed] [Google Scholar]
- 89.Gretarsdottir S, Baas AF, Thorleifsson G, et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42:692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoneyama T, Kasuya H, Onda H, et al. Association of positional and functional candidate genes FGF1, FBN2, and LOX on 5q31 with intracranial aneurysm. J Hum Genet. 2003;48:309–314. doi: 10.1007/s10038-003-0030-6. [DOI] [PubMed] [Google Scholar]
- 91.Ruigrok YM, Rinkel GJ, van't Slot R, Wolfs M, Tang S, Wijmenga C. Evidence in favor of the contribution of genes involved in the maintenance of the extracellular matrix of the arterial wall to the development of intracranial aneurysms. Hum Mol Genet. 2006;15:3361–3368. doi: 10.1093/hmg/ddl412. [DOI] [PubMed] [Google Scholar]
- 92.Krischek B, Inoue I. The genetics of intracranial aneurysms. J Hum Genet. 2006;51:587–594. doi: 10.1007/s10038-006-0407-4. [DOI] [PubMed] [Google Scholar]
- 93.Woo D, Broderick J. Genetics of intracranial aneurysm. J Stroke Cerebrovasc Dis. 2002;11:230–240. doi: 10.1053/jscd.2002.129598. [DOI] [PubMed] [Google Scholar]
- 94.Ruigrok YM, Rinkel GJ, Wijmenga C. The versican gene and the risk of intracranial aneurysms. Stroke. 2006;37:2372–2374. doi: 10.1161/01.STR.0000236499.55301.09. [DOI] [PubMed] [Google Scholar]
- 95.Shi C, Awad IA, Jafari N, et al. Genomics of human intracranial aneurysm wall. Stroke. 2009;40:1252–1261. doi: 10.1161/STROKEAHA.108.532036. [DOI] [PubMed] [Google Scholar]
- 96.Zhang B, Fugleholm K, Day LB, Ye S, Weller RO, Day IN. Molecular pathogenesis of subarachnoid haemorrhage. Int J Biochem Cell Biol. 2003;35:1341–1360. doi: 10.1016/s1357-2725(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 97.Santiago-Sim T, Mathew-Joseph S, Pannu H, et al. Sequencing of TGF-beta pathway genes in familial cases of intracranial aneurysm. Stroke. 2009;40:1604–1611. doi: 10.1161/STROKEAHA.108.540245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 99.Pezzini A, Drera B, Del Zotto E, et al. Mutations in TGFBR2 gene cause spontaneous cervical artery dissection. J Neurol Neurosurg Psychiatry. 2011;82:1372–1374. doi: 10.1136/jnnp.2010.231902. [DOI] [PubMed] [Google Scholar]
- 100.Ruigrok YM, Tan S, Medic J, Rinkel GJ, Wijmenga C. Genes involved in the transforming growth factor beta signalling pathway and the risk of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2008;79:722–724. doi: 10.1136/jnnp.2007.128041. [DOI] [PubMed] [Google Scholar]
- 101.van de Laar IM, Oldenburg RA, Pals G, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 102.Tromp G, Wu Y, Prockop DJ, et al. Sequencing of cDNA from 50 unrelated patients reveals that mutations in the triple-helical domain of type III procollagen are an infrequent cause of aortic aneurysms. J Clin Invest. 1993;91:2539–2545. doi: 10.1172/JCI116490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jeanne M, Labelle-Dumais C, Jorgensen J, et al. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am J Hum Genet. 2012;90:91–101. doi: 10.1016/j.ajhg.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Plaisier E, Chen Z, Gekeler F, et al. Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3(IV) domain. Am J Med Genet A. 2010;152A:2550–2555. doi: 10.1002/ajmg.a.33659. [DOI] [PubMed] [Google Scholar]
- 105.Plaisier E, Gribouval O, Alamowitch S, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 106.Lanfranconi S, Markus HS. COL4A1 mutations as a monogenic cause of cerebral small vessel disease: a systematic review. Stroke. 2010;41:e513–e518. doi: 10.1161/STROKEAHA.110.581918. [DOI] [PubMed] [Google Scholar]
- 107.Borck G, Beighton P, Wilhelm C, Kohlhase J, Kubisch C. Arterial rupture in classic Ehlers-Danlos syndrome with COL5A1 mutation. Am J Med Genet A. 2010;152A:2090–2093. doi: 10.1002/ajmg.a.33541. [DOI] [PubMed] [Google Scholar]
- 108.Peck G, Smeeth L, Whittaker J, Casas JP, Hingorani A, Sharma P. The genetics of primary haemorrhagic stroke, subarachnoid haemorrhage and ruptured intracranial aneurysms in adults. PLoS One. 2008;3:e3691. doi: 10.1371/journal.pone.0003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rossetti S, Irazabal MV, Hopp K, et al. Analysis of the genomic profile associated with the development of a vascular phenotype in autosomal dominant polycystic kidney disease. J Am Soc Neph. 2012;23:699A. [Google Scholar]