Abstract
Background:
Aconitum gymnandrum is a Chinese traditional herb used as carminative and analgesic. In this study, A. gymnandrum was used as an experimental matrix.
Materials and Methods:
Optimized ultrasonic extraction technology of total flavonoids from the A. gymnandrum Maxim was studied by using the methodology of single factor and orthogonal design to study the effects of operation conditions, such as ethanol content, ultrasonic wave power, temperature, ultrasonic wave radiation time, and the ratio of sample weight to solvent volume.
Result:
Through the orthogonal experiment, the optimal extraction conditions were determined as follows: Ultrasonic power 100 W, ultrasonic temperature 45°C, 60% ethyl alcohol, extraction time 30 min, and solid–liquid ratio 1:20.
Conclusion:
Under the optimum parameters, the extraction ratio of total flavonoids from the A. gymnandrum Maxim is about 1.278%.
Keywords: Aconitum gymnandrum, orthogonal design, total flavonoids, ultrasound-assisted extraction
INTRODUCTION
Chinese traditional medicinal herb, Aconitum gymnandrum Maxim, the only species in subgen. Gymnaconitum, Genus Aconitum L., has been documented to possess several therapeutic effects, such as activating meridians to stop pain, promoting Qi to activate blood, and carminative and analgesic effect. Modern pharmacological researches show that medicinal plants in Genus Aconitum have an extensive pharmacological action and clinical application such as anti-inflammation,[1] immunosuppression,[2] narcotic analgesic action,[3] anti-tumor, and so on.[4] Due to the medical significances of plants in Aconitum L., in-depth exploration and research around contend determination of alkaloids, chemical structure analysis and determination of bioactivity have been conducting for a long time.
Over 2000 kinds of flavonoids have been found, which have been proved to have a variety of pharmacological actions as anti-cancer, anti-aging, anti-inflammation, decreasing blood sugar and pressure, endocrine regulation, eliminating active oxygen radicals, and anti-inflammatory immunity and so on.[5,6] Thus, flavonoids gradually draw the emphasis of scientists and with the deepening research into the SAR (Structure-Activity Relationship), part of the pharmacological functioning mechanism of flavonoids was discovered, which provided a theoretical basis for their application in the fields of medicine and food and also accelerated the development and utilization of flavonoids at the same time[7,8] whereas, there have been comparatively only a few reports on flavonoids from Aconitum.[9,10,11,12]
Many researchers have been keeping on the research of extraction of flavonoids from various plants. Several extraction techniques and methods have been gradually set up, such as water-extracting method, organic solvent extraction, microwave method, ultrasonic-assisted extraction, enzyme decomposition method, macro-porous resin adsorption method, supercritical extraction[13] and semi-bionic extraction, and so on.[14]
In current research, optimized ultrasonic extraction technology of total flavonoids from the A. gymnandrum Maxim was reported firstly, which was established by using the methodology of single factor and orthogonal design experiments. The best extraction conditions were determined as follows: Ultrasonic power 100 W, ultrasonic temperature 45°C, 60% ethyl alcohol, 30 min of extraction time, and solid–liquid ratio 1:20. Under the optimized conditions, the extraction percent of total flavonoids from the A. gymnandrum Maxim is about 1.278%.
MATERIALS AND METHODS
Plant material
The whole herb of A. gymnandrum Maxim was collected from Kangding City of Sichuan, China in October 2011, and identified by Prof. Feng Gao of Sichuan Agricultural University. A voucher specimen (No. 2011-10-10) was prepared from the entire plants of A. gymnandrum Maxim and deposited in the herbarium of the Department of Chinese Traditional Herb, Agronomy College, Sichuan Agricultural University.
Making standard curve
Preparation of standard substance
The standard Rutin, which has been dried to consistent weight under 120°C, was precisely weighed to 10.30 mg and put into a 50 ml beaker. Then we added a few volumes by 70% ethyl alcohol into the beaker and transferred the solution into a 25 ml beaker after ultrasonic dissolution, and settled it to scale. After shacked up, we gained Rutin standard solution with a concentration of 0.412 mg/ml.
Determination of the wavelength
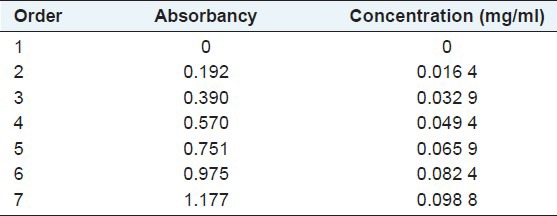
We precisely measured Rutin reference solution to 0, 1, 2, 3, 4, 5, and 6 ml, respectively, into seven 25 ml volumetric flasks. Then we added 1 ml 10% NaNO2 into the seven volumetric flasks respectively and shook them up. Next, the solution was remained for 6 min. Then added 1 ml 10% Al(NO3)3 respectively into the seven flasks and again we shook them up and remained for 6 min. Next we added 4% NaOH solution (4 ml) into the mixture and let them diluted by 70% ethanol respectively to scale, shook them up and put still for 15 min. Finally, determining absorbency under the wavelength range among 300-600 nm, and obtained the maximum absorption under the wavelength of 510 nm, which is correspondent to the data checked from references. Regression curve were made using concentration with absorbancy, and draw a conclusion that the regression equation Y = 0.0844X + 0.0005 (R2 = 0.9991) appeared linear relation among 0.0164-0.0988 mg/ml [Figure 1] and results got from standard curve are shown in Table 1.
Figure 1.
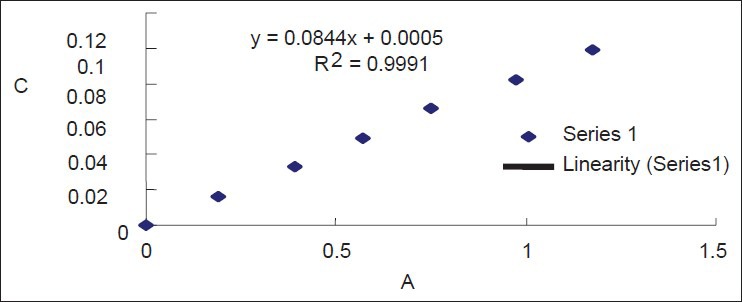
Standard curve protraction
Table 1.
The measured result of standard curve
Determination of total flavonoids and calculation of extraction ratio
Based on the regression equation of standard curve, we get the extraction concentration using ultraviolet spectrophotometer and then get the extraction ratio of total flavonoids using formula. Extraction ratio of total flavonoids (%) = (extract concentration × volume × diluted multiples)/raw materials quality × 100%.
Orthogonal design based on single factor test under ultrasonic extraction
Impact of ethanol concentration on extraction ratio of flavonoids
Precisely weighing five pieces of 1.000 g A. gymnandrum Maxim powder, and prepared into solution using volume by 50%, 60%, 70%, 80%, and 90% ethyl alcohol, respectively, under 1:20 solid–liquid ratio, then extracting under ultrasonic power 100 W and ultrasonic temperature 50°C for 30 min. Next we filtered the solution and cleaned the filter residue for 2 times using ethyl alcohol of relevant volume concentration. Settling all the five bottles of solution using 100 ml volumetric flask, and pipetting 10 ml solution respectively into five 25 ml volumetric flask sighed 1, 2, 3, 4, and 5. Volumetric flask signed 0 was set as reference substance without adding of sample. Then adding solution following procedures showed in wavelength selection determination, next we settled the solution with relevant ethyl alcohol. Taking appropriate amount of solution into curette and determining absorbency under 510 nm wavelength. Next, the extraction ratio of total flavonoids was calculated. According to the result, we chose the three optimal concentrations as the three levels of concentration factors in orthogonal test.
Impact of solid–liquid ratio on extraction ratio of flavonoids
Precisely weighing five pieces of 1.000 g A. gymnandrum Maxim powder into five 100 ml conical flasks signed 1, 2, 3, 4, and 5, respectively, and then prepared into solution using volume by 70% ethyl alcohol under 1:10, 1:20, 1:30, 1:40, and 1:50 solid–liquid ratio, respectively, following by ultrasonic extraction under ultrasonic power 100 W and ultrasonic temperature 40°C for 60 min. Then we determined absorbency following the steps showed above and calculating the extraction ratio of total flavonoids. According to the result, we chose the three optimal solid–liquid ratios as the three levels of solid–liquid ratio in orthogonal test.
Impact of extraction time on extraction ratio of flavonoids
Precisely weighing five pieces of 1.000 g A. gymnandrum Maxim powder into five 100 ml conical flasks signed 1, 2, 3, 4, and 5, respectively and then prepared into solution using volume by 70% ethyl alcohol under 1:20 solid–liquid ratio, then extracting under ultrasonic power 100 W and ultrasonic temperature 35°C for 20, 30, 40, 50, and 60 min, respectively. Then we determined absorbency following the steps as above and calculating the extraction ratio of total flavonoids. According to the result, we chose the three optimal extraction times as the three levels of time factors in orthogonal test.
Impact of ultrasonic temperature on extraction ratio of flavonoids
Precisely weighing five pieces of 1.000 g A. gymnandrum Maxim powder into five 100 ml conical flasks signed 1, 2, 3, 4, and 5, respectively. And then prepared into solution using volume by 70% ethyl alcohol under 1:20 solid–liquid ratio. Then extracting for 30 min under ultrasonic power 100 W and ultrasonic temperature 35°C, 45%, 55%, 65%, and 75%, respectively. According to the result, we chose the three optimal ultrasonic temperatures as the three levels of ultrasonic temperature in orthogonal test.
Orthogonal test
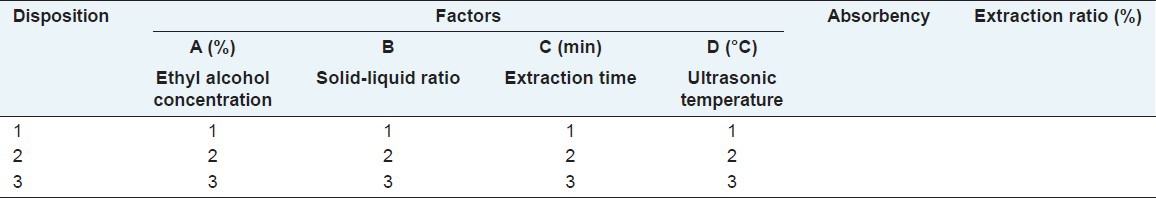
Results get from single factor test were used to conduct orthogonal test and the interaction effects between factors were not under consideration. Each factor levels are shown in Table 2 and then the L9(34) orthogonal design was conducted based on Table 2.
Table 2.
Factor levels of orthogonal test
RESULTS AND DISCUSSION
Single factor test results
Impact of ethyl alcohol concentration on extraction ratio of flavonoids
From Figure 2, we can see that the extraction ratio reached top using volume by 70% ethyl alcohol, after which the extraction ratio decreased with the increase of ethyl alcohol concentration. This is probably because that the decrease of water contend would lead to part of the water soluble not fully dissolved. What's more, because that the solubility of proteins, saccharides and pectin in water is greater than that in ethyl alcohol, lower ethyl alcohol concentration made an increase in dissolved quantity of these macromolecules as well as the viscosity of extracts, which led to slower filtration rate, longer waiting time and lower efficiency. After a comprehensive consideration, volume by 50%, 60%, and 70% ethyl alcohol was chosen as the levels of ethyl alcohol concentration in orthogonal test.
Figure 2.
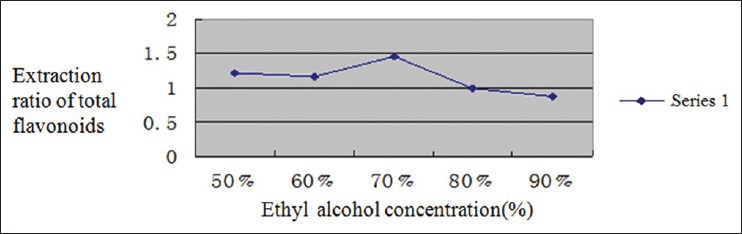
Impact of ethyl alcohol concentration on extraction ratio of flavonoids
Impact of solid–liquid ratio on extraction ratio of flavonoids
According to Figure 3, the extraction ratio of total flavonoids increased with the increasing of solid–liquid ratio, and reached top when solid–liquid ratio was 1:30, then the extraction ratio decreased for a bit and after that maintained constant. This is because that with the increment of solvent amounts, active constituent in solvent was low in concentration, which led to a big difference between active constituent concentration in material and solvent boundary layer and big active diffusion force. As a result, get a high extraction ratio. When active constituents were fully extracted, it led to a constant extraction ratio. We decided to choose 1:20, 1:30, and 1:40 as the three levels of solid–liquid ratios in orthogonal test.
Figure 3.
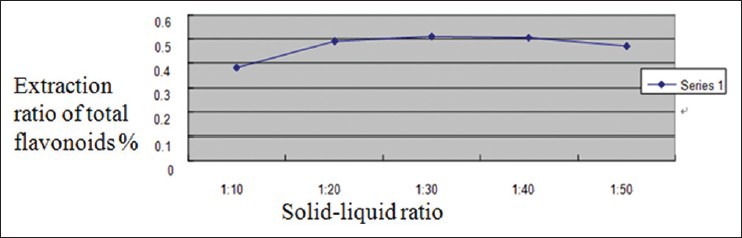
Impact of solid–liquid ratio on extraction ratio of flavonoids
Impact of extraction time on extraction ratio of flavonoids
According to Figure 4, the extraction ratio of total flavonoids increased with the increasing of extraction time, and reached top when extraction time was 50 min, after which, the extraction ratio decreased with the increasing of time. This is probably due to long time ultrasonic cavitations effect which destroyed the structure of flavonoids and led to the decrease of extraction ratio of flavonoids. Considering the energy consumption of long time extraction, we decided to choose 30, 40, and 50 min as the three levels of extraction time in orthogonal test.
Figure 4.
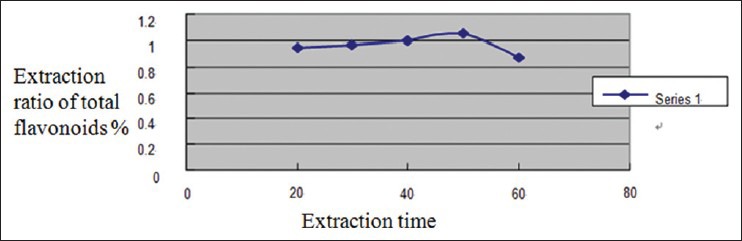
Impact of extraction time on extraction ratio of flavonoids
Impact of ultrasonic temperature on extraction ratio of flavonoids
According to Figure 5, the extraction ratio of total flavonoids reached top when ultrasonic temperature was 55°C. Then the extraction ratio remained relatively stable under ultrasonic temperature among 55-65°C. After 65°C, the extraction ratio had a trend of decreasing. This is probably because that excessively high temperature of solution would destroy the structure of flavonoids, which led to great quantities of volatilization of ethyl alcohol as well as a reduction in extraction ratio of flavonoids. After a comprehensive consideration, 45°C, 55°C, and 65°C were chosen as the three levels of ultrasonic temperature in orthogonal test.
Figure 5.
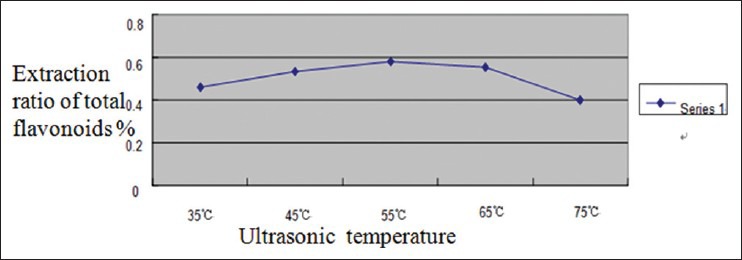
Impact of ultrasonic temperature on extraction ratio of flavonoids
Orthogonal test results and analysis
We can see from the single factor research above that the extraction ratio of flavonoids could be influenced by four factors: Ethyl alcohol concentration, solid–liquid ratio, extraction time, and ultrasonic temperature. In order to do an overall study on the impact of the four factors, orthogonal test with three levels of four factors was conducted. The following experiments were all carried on under ultrasonic power 100 W [Table 3]. Precisely weighing nine samples of 1 g (accurate to 0.001 g) were added into three conical flasks respectively. Then we extracted following L9 (34) orthogonal design shown in Table 1. The extracts of total flavonoids were used for the determination of extraction ratio.
Table 3.
Orthogonal test and results
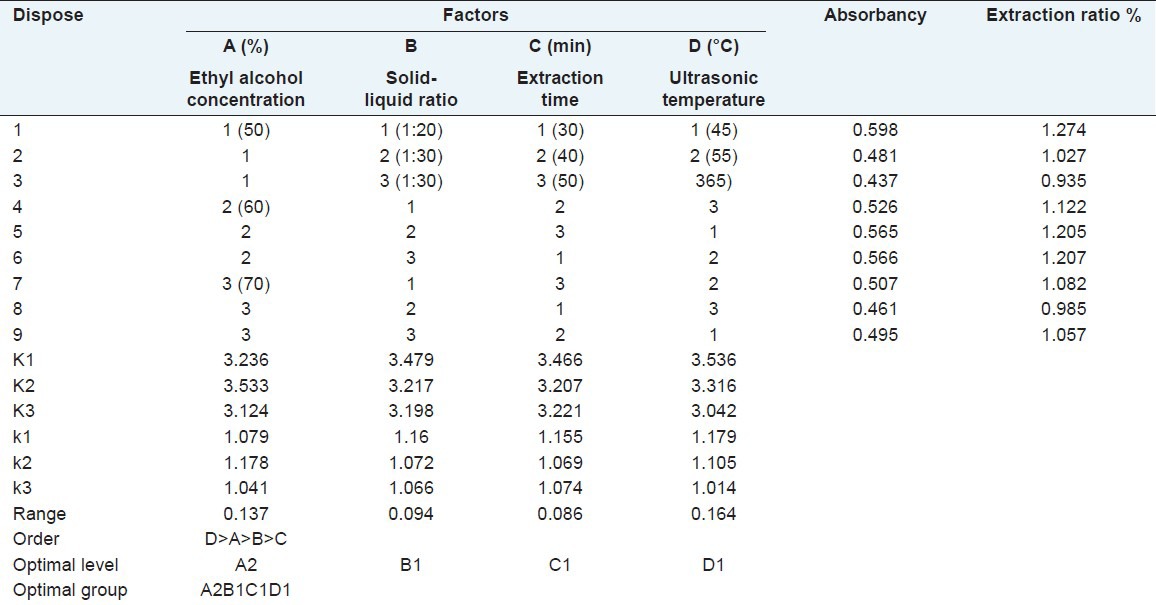
According to the result of L9 (34) orthogonal test and Range analysis, the order of affecting extraction ratio of flavonoids was ranked as D > A > B > C, which is ultrasonic temperature > ethyl alcohol concentration > solid–liquid ratio > extraction time. This means that extraction ratio of flavonoids was affected most by the ultrasonic temperature and affected least by extraction time. Then we get the theoretically optimal combination A2B1C1D1 from range analysis, which means that the technologically optimal condition of total flavonoids extraction from A. gymnandrum Maxim is A2B1C1D1. That is Ultrasonic power 100 W, ultrasonic temperature 45°C, volume by 60% ethyl alcohol, extraction time 30 min, solid–liquid ratio 1:20. The results of variance analysis by DPS (Data Processing System) are shown in Table 3. According to the results, the impacts of the four factors on extraction ratio of total flavonoids are all non-significant. Thus, referring to the results of range analysis and realistic cases to reduce consumption, we chose the optimal extracting condition of A2B1C1D1 get from range analysis.
Validation test
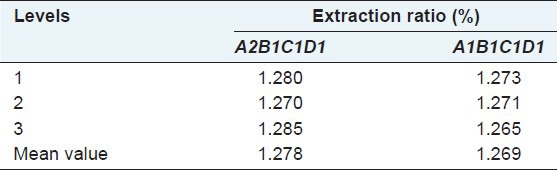
Repeating the experiments for 3 times respectively under the theoretically optimal extracting condition of A2B1C1D1 and the experimentally optimal extracting condition of A1B1C1D1, and the results are shown in Tables 4 and 5. The results showed that the extraction ratio was greater under theoretically optimal extracting condition of A2B1C1D1 than that under experimentally optimal extracting condition of A1B1C1D1, so that we should choose theoretically optimal extracting condition A2B1C1D1 when extracting total flavonoids from A. gymnandrum Maxim.
Table 4.
Analysis of variance
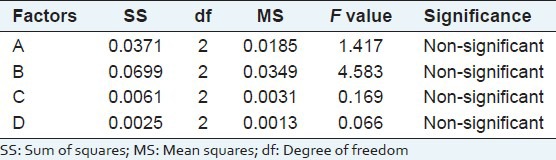
Table 5.
Validation test
CONCLUSIONS
This is the first report on total flavonoids contend from the Genus Aconitum and the optimization of its extraction technique. From the above experiments, we can draw a conclusion that when extracting total flavonoids from A. gymnandrum Maxim under ultrasonic conditions, we should choose Ultrasonic power 100 W, ultrasonic temperature 45°C, volume by 60% ethyl alcohol, extraction time 30 min, solid–liquid ratio 1:20 and the extraction ratio under this condition is 1.278%.
Ultrasonic extraction has several advantages like simple and efficient operation, requiring no heating, high extraction ratio, fast speed, without destruction of structure of extracts, fine effects and energy saving and what's more, it offers theoretical basis for industrial extraction of total flavonoids as well as laboratory.
ACKNOWLEDGMENTS
We are grateful to the NSFC (No. 81001383), the Doctoral Foundation of Ministry of Education of China (No. 20105103120009), Applied Basic Research Programs of Science and Technology Department of Sichuan Province (No. 2012JY0035) and the research fund of Chengdu Medical College (No. CYZ11-021).
Footnotes
Source of Support: Grant from the NSFC (No. 81001383), the Doctoral Foundation of Ministry of Education of China (No. 20105103120009), Applied Basic Research Programs of Science and Technology Department of Sichuan Province (No. 2012JY0035) and the research fund of Chengdu Medical College (No. CYZ11-021)
Conflict of Interest: None declared.
REFERENCES
- 1.Borcsa B, Csupor D, Forgo P, Widowitz U, Bauer R, Hohmann J. Aconitum lipo-alkaloids – Semisynthetic products of the traditional medicine. Nat Prod Commun. 2011;6:527–36. [PubMed] [Google Scholar]
- 2.Wang FP, Chen QH. The C19-diterpenoid alkaloids. Alkaloids Chem Biol. 2010;69:1–577. doi: 10.1016/s1099-4831(10)69001-3. [DOI] [PubMed] [Google Scholar]
- 3.Jian XX, Tang P, Liu XX, Chao RB, Chen QH, She XK, et al. Structure-cardiac activity relationship of C19-diterpenoid alkaloids. Nat Prod Commun. 2012;7:713–20. [PubMed] [Google Scholar]
- 4.Liu XX, Jian XX, Cai XF, Chao RB, Chen QH, Chen DL, et al. Cardioactive C 19-diterpenoid alkaloids from the lateral roots of Aconitum carmichaeli “Fu Zi”. Chem Pharm Bull (Tokyo) 2012;60:144–9. doi: 10.1248/cpb.60.144. [DOI] [PubMed] [Google Scholar]
- 5.Costa SS, Couceiro JN, Silva IC, Malvar Ddo C, Coutinho MA, Camargo LM, et al. Flavonoids in the therapy and prophylaxis of flu: A patent review. Expert Opin Ther Pat. 2012;22:1111–21. doi: 10.1517/13543776.2012.724062. [DOI] [PubMed] [Google Scholar]
- 6.Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: A review of the evidence. J Nutr Gerontol Geriatr. 2012;31:206–38. doi: 10.1080/21551197.2012.702534. [DOI] [PubMed] [Google Scholar]
- 7.Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–46. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 8.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41:1727–46. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Vitalini S, Braca A, Passarella D, Fico G. New flavonol glycosides from Aconitum burnatii Gáyer and Aconitum variegatum L. Fitoterapia. 2010;81:940–7. doi: 10.1016/j.fitote.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Mariani C, Braca A, Vitalini S, De Tommasi N, Visioli F, Fico G. Flavonoid characterization and in vitro antioxidant activity of Aconitum anthora L. (Ranunculaceae) Phytochemistry. 2008;69:1220–6. doi: 10.1016/j.phytochem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Shrestha BB, Dall’Acqua S, Gewali MB, Jha PK, Innocenti G. New flavonoid glycosides from Aconitum naviculare (Brühl) Stapf, a medicinal herb from the trans-Himalayan region of Nepal. Carbohydr Res. 2006;341:2161–5. doi: 10.1016/j.carres.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Luis JC, Valdés F, Martín R, Carmona AJ, Díaz JG. DPPH radical scavenging activity of two flavonol glycosides from Aconitum napellus sp. lusitanicum. Fitoterapia. 2006;77:469–71. doi: 10.1016/j.fitote.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Zhao YM, Guo CY, Zhang SM, Liu CL, Zhang DS, et al. Ultrasound-assisted extraction of total flavonoids from Inula helenium. Pharmacogn Mag. 2012;8:166–70. doi: 10.4103/0973-1296.96581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Wu BH, Fang JB, Liu YL, Zhang HH, Fang LC, et al. Analysis of flavonoids from lotus (Nelumbo nucifera) leaves using high performance liquid chromatography/photodiode array detector tandem electrospray ionization mass spectrometry and an extraction method optimized by orthogonal design. J Chromatogr A. 2012;1227:145–53. doi: 10.1016/j.chroma.2011.12.098. [DOI] [PubMed] [Google Scholar]


