Abstract
Background:
Myoporum bontioides A. Gray, an evergreen shrub from the Myoporaceae family, is a commonly used medicinal plant. Many studies have been conducted on the biologically active constituents of whole parts of M. bontioides. However, the endophytes of M. bontioides have not been intensively investigated. A new chlorine-containing isocoumarin, named dichlorodiaportinol A (1) was isolated from the endophytic fungus Trichoderma sp. 09 isolated from the root of M. bontioides. Its cytotoxic activity against human breast cancer (MCF-7) and human liver cancer (HepG2) cell lines was evaluated.
Materials and Methods:
Different open silica gel column chromatographic techniques with different solvent systems were used for the separation of the constituents of the ethyl acetate extract of the culture broth of the endophytic fungus Trichoderma sp. 09. The structure of compound one was identified by analysis of spectroscopic data [one-dimensional (1D), two-dimensional (2D)-nuclear magnetic resonance (NMR), ultraviolet (UV), infrared (IR) and Mass spectrometry (MS)]. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay method was used for the evaluation of cytotoxic activity of compound one against MCF-7 and HepG2 cell lines.
Results:
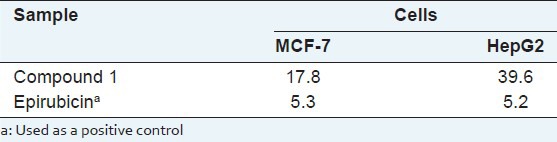
Compound one was identified as 3-(3,3-dichloro-2,3-dihydroxy-propyl)-8-hydroxy-6- methoxy-isochromen-1-one. It inhibited MCF-7 and HepG2 cell lines, with half maximal inhibitory concentration (IC50) values of 17.8 and 39.6 μg/mL, respectively.
Conclusions:
Compound one is a new chlorine-containing isocoumarin with moderate cytotoxic activity against MCF-7 and HepG2 cell lines. Thus, endophytes of M. bontioides are worthy of consideration for the development and research of antitumor agents.
Keywords: Cytotoxic activity, dichlorodiaportinol A, isocoumarin, Trichoderma sp.
INTRODUCTION
Endophytes are microorganisms that reside in the tissues of living plants without causing any damage to the host.[1] They have proven to be a rich source of structurally unique and biologically active secondary metabolites which are of interest for specific medicinal or agrochemical value.[2] Myoporum bontioides A. Gray belonging to genus Myoporum, family Myoporaceae, is a small evergreen shrub distributed along the coastal regions of southern Kyushu islands through Okinawa, Taiwan, southern China, and Indochina. In China, the decoction of Myoporum bontioides A. Gray is usually used as a folk medicine for antidermatosis and as antipyretic and antipsychotic agent.[3,4] The phytochemistry and pharmacological studies conducted on this medicinal plant have successfully isolated a serial of natural products including sesquiterpenoids, iridoids, monoterpenes, phenylethanoids, and flavonoids etc.[5,6,7,8] However, the chemical and medicinal values of its endophytes seldom have been investigated.
During our continuing search for structurally new bioactive secondary metabolites from endophytes from M. bontioides,[9] we examined the chemical constituents of Trichoderma sp. 09, an endophytic fungus isolated from the root of the semi-mangrove plant. In the study, we describe the isolation and characterization of a new chlorine-containing isocoumarin, 3-(3,3-dichloro-2,3-dihydroxy-propyl)- 8-hydroxy-6-methoxy-isochromen-1-one, Dichlorodiaportinol A (1) obtained from the culture broth of this fungus. Its in vitro cytotoxic activity against human breast cancer (MCF-7) and human liver cancer (HepG2) cell lines were evaluated.
MATERIALS AND METHODS
General
Melting points were detected on X-4 micromelting point apparatus (Cany Precision Instruments Co., Ltd., Shanghai, China), uncorrected. Ultraviolet (UV) absorptions were measured in MeOH on a Shimadzu UV-2450 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Infrared (IR) spectra were obtained on a Nicolet 5DX-Fourier transform infrared (FTIR) spectrophotometer (Thermo Electron Corporation, Madison, USA). Nuclear Magnetic Resonance (NMR) data were recorded on a Bruker AVIII 600MHz NMR spectrometer (Bruker BioSpin GmbH company, Rheinstetten, Germany), using deuterated acetone (CD3COCD3) as solvent and tetramethylsilane (TMS) as internal standard,and coupling constants (J) are in Hz. Electrospray ionization mass (ESIMS) and high resolution electrospray ionization mass (HRESIMS) were operated on LCQ-DECA-XP (Thermo,USA), and LCMS-IT-TOF (Shimadzu, Janpan) mass spectrometers, respectively. Chromatography was carried out on silica gel column (200-300 mesh; Qingdao haiyang chemicals Co., Ltd., Qingdao, China). All other reagents used were analytical grade.
Fungus and cell material
The Trichoderma sp. 09 strain was isolated from the root of M. bontioides A. Gray collected in Leizhou Peninsula, Guangdong Province, China. Stock cultures were maintained on slant solid cornmeal seawater agar. Plugs of agar supporting mycelia growth were cut and transferred aseptically to a 250 mL Erlenmeyer flask containing 100 mL of liquid medium (glucose 10g/L, Peptone 2 g/L, yeast extract 1 g/L, NaCl 30 g/L). The flask was incubated at 30°C on a rotary shaker for 5–7 days. The mycelium was aseptically transferred to 500 mL Erlenmeyer flasks containing culture liquid (200 mL). The flasks were then incubated at 30°C for 25 days.
Breast MCF-7 and Liver HepG2 cell lines were purchased from the American Type Culture Collection (ATCC). They were cultured in Dulbecco's modification Eagle's medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 2 mM L-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin (Invitrogen). The cells were incubated at 37°C in a humidified atmosphere with 5% CO2. Microplate reader (TECAN, Inc.). Flat-bottom microtiter plates, 96 well were from Falcon, NJ, USA. 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) for viability assay was purchased from Sigma (St. Louis, Mo., USA).
Extraction and isolation
The cultures (30 L) were filtered through cheesecloth. The filtrate was concentrated to 5 L in vacuo below 50°C and extracted five times by shaking with an equal volume of ethyl acetate. The combined extract (18.6 g) was subjected to silica gel column chromatography and eluted with petroleum ether–ethyl acetate (90:10, 80:20, 70:30, 50:50, and 0:100) to afford fractions 1-5 (0.9, 1.6, 2.2, 3.7, and 5.8 g, respectively). Fraction four (3.7 g) was subjected to silica gel column chromatography and eluted with petroleum ether-ethyl acetate in a gradient of ethyl acetate (petroleum ether-ethyl acetate, 100:0-50:50). Fifty-two fractions of 30 ml each were collected, combined on the basis of their thin layer chromatography profiles, and concentrated to dryness to give nine major fractions A–I. Repeated slow recrystallization of Fraction H (85 mg, petroleum ether-ethyl acetate 60:40, v/v) at room temperature from petroleum ether-ethyl acetate (65:35, v/v) yielded 3.2 mg white crystalline needle shape solid of Dichlorodiaportinol A (1).
Assessment of Antitumor Activity by 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) Assay
Cells were harvested during logarithmic growth phase and seeded in 96-well plates at a density of 1 × 104 cells/mL, and cultured at 37°C in a humidified incubator (5% CO2) for 24 h, followed by exposure to various concentrations of compound one tested for 48 h. Negative control cells were incubated without test samples. Cells treated with epirubicin served as a positive control. Subsequently, 20 μL of MTT (Genview, Houston, TX, USA) solution (5 mg/mL) was added to each well and mixed, the cells were then incubated for an additional 4 h. Culture supernatant was removed and 150 μL of Dimethyl sulfoxide (DMSO) (Sangon Biotech, Shanghai, China) was added to each well to fully dissolve the MTT-formazan crystals. Cell growth inhibition was determined by measuring the absorbance (Abs) at λ = 570 nm using a microplate reader and calculated according to the following equation: Growth inhibition = (1 − OD of treated cells/OD of control cells) ×100%. The half maximal inhibitory concentrations (IC50) were obtained from liner regression analysis of the concentration-response curves plotted for each tested compound.
RESULTS
Dichlorodiaportinol A (1)
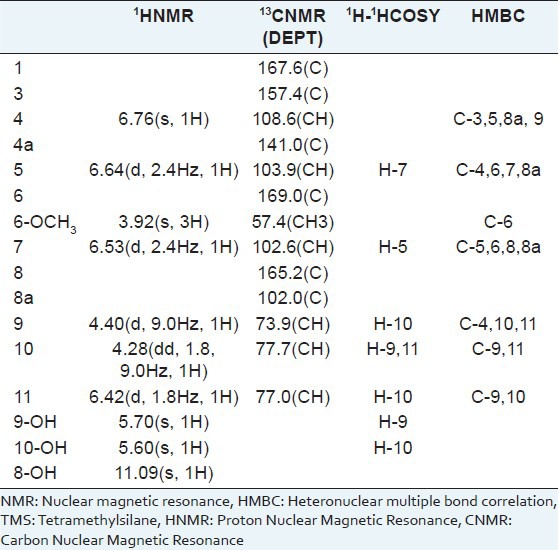
White needles, m.p. (CHCl3) 137-139°C [α]25D + 7.5° (c 0.04, CH3 COCH3). UV (MeOH) λmax (nm): 243, 280, 326. IR (KBr) umax (cm-1): 3,421, 2,925, 1,685, 1,637, 1,570, 1,508, 1,241, 1,207, 1,166, 1,047 cm-1. 1H-nuclear magnetic resonance (NMR) (600 MHz, CD3COCD3), 13C-NMR (150MHz, CD3COCD3), Heteronuclear Multiple Bond Correlation (HMBC) and 1H-1H COSY spectral data are listed in Table 1. ESIMS m/z 335[M+H]+, 356 [M+Na]+. High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) m/z 335.0082 ([M+H]+, calcd. 335.0084), 356.9902 ([M+Na]+, calcd. 356.9903).
Table 1.
NMR data for compound 1 (CD3COCD3, TMS)
DISSCUSSION
Dichlorodiaportinol A (1) m.p. 137–139°C, has a molecular formula of C13H12Cl2O6 as determined by HR-ESI-MS m/z 335.0082 ([M+H]+, calcd. 335.0084) and m/z 356.9902 ([M+Na]+, calcd. 356.9903). Its UV spectrum with absorption maxima at 243, 280 and 326 nm was indicative of a conjugated chromophore. Its IR spectrum with absorption bands at 3,421, 1,685, 1,637, 1,570, and 1,508 cm-1 suggested the presence of hydroxyl, α,β-unsaturated δ-lactone carbonyl, and olefinic functionalities, respectively. These spectra revealed the presence of typical isocoumarin moiety in comparison with known isocoumarins.[10,11] In the 1H-NMR spectrum, a lowfield, one-proton singlet signal at δ 10.47 (s, 1H, exchangeable) indicated the presence of a hydrogen-bonded phenolic proton at C-8.[12] Therefore, a pair of meta-coupled doublets (J = 2.4 Hz) at δ 6.53 and 6.64, were assigned to H-5 and H- 7, respectively. Another aromatic singlet at δ 6.76 (s, 1H), connected to the carbon at δ 108.6 (C-4) in the HSQC spectrum, was ascribed to H-4 of the isocoumarin moiety. A sharp three-proton singlet at δ 3.92 belonging to a methoxy group was connected to C-6 that was determined by the HMBC correlations from 6-OCH3, H-5, and H-7 to the carbon at δ 169.0 (C-6). The remaining substituent of the isocoumarin was revealed to contain a -CH-CH-CH- fragment by the 1H-1HCOSY correlations from H-9 to H-10 and H-10 to H-9 and H-11. Two hydroxyls at δ 5.60 (s, 1H, exchangeable) and 5.70 (s, 1H, exchangeable) were, respectively, assigned to be at the positions of C-9 and C-10 by the 1H-1HCOSY correlations from 9-OH to H-9 and 10-OH to H-10. Therefore, the two remaining chlorine atoms were both connected to C-11. The HMBC correlations from H-9 to C-4 and H-4 to C-9 further demonstrated the connectivity of C-3 and C-9. Hence, the structure of compound 1 was established as 3-(3,3-dichloro-2,3-dihydroxy-propyl)-8-hydroxy- 6-methoxy-isochromen- 1-one [Figure 1]. We named it Dichlorodiaportinol A, according to its similar structure with the known compound, Dichlorodiaportin.[13]
Figure 1.
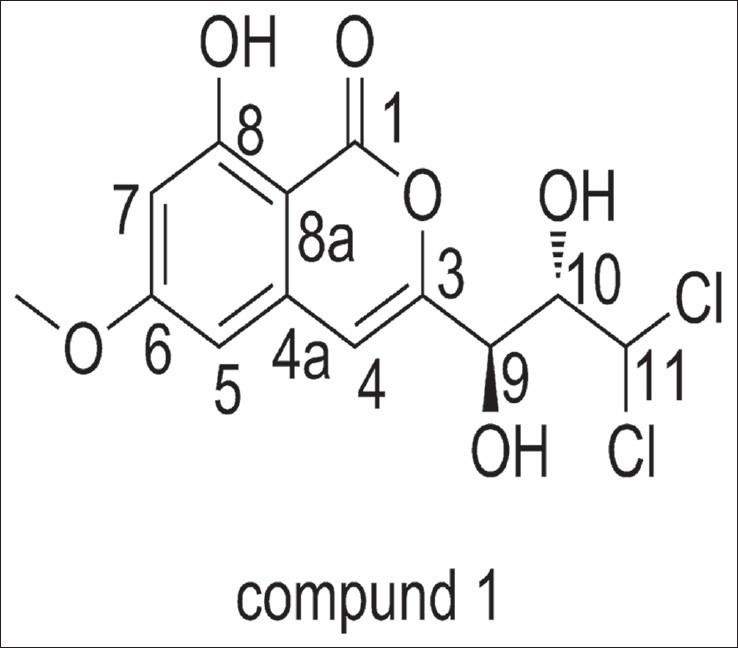
The structure of compound 1
The relative configuration of compound one was assigned based on the method of J-based configurational analysis and Nuclear Overhauser effect (NOE) data.[14,15] For compound one, H-9 and H-10 were anti due to their large vicinal coupling constant (9.0 Hz). In this case, C-11 and C-3 might be gauche in the threo diastereomer or anti in the erythro diastereomer. Since the NOE interactions between 9-OH and H-11 were also observed, it suggested that 9-OH and H-11 were gauche, and C-11 and C-3 were anti. Accordingly, compound one was confirmed as the erythro diastereomer. The key Nuclear Overhauser effect spectroscopy (NOESY) correlations were shown in Figure 2.
Figure 2.
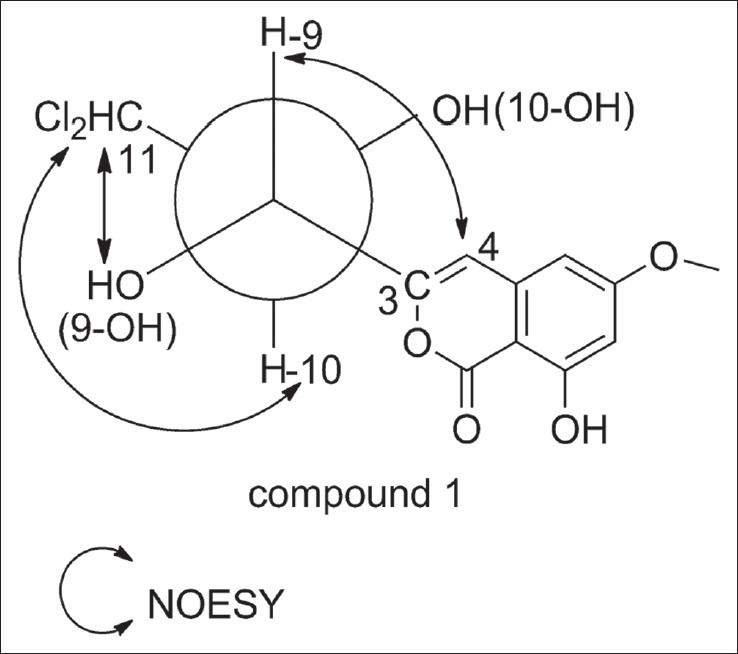
The key NOESY correlations of compound 1
Compound one was evaluated for in vitro cytotoxic activity against two human cancer cell lines including human breast MCF-7 and human Liver HepG2 by MTT assay[16,17] using epirubicin (an anticancer drug used widely in the clinic[18,19]) as positive control. The results are summarized in Table 2. Compound one was moderately active against MCF-7 and HepG2 cells as compared with the standard. Thus, this study suggests that endophytes of M. bontioides are worthy of consideration for the development and research of antitumor agents.
Table 2.
Cytotoxicity of compound 1 against MCF-7 and HepG2 cells (IC50, μg/mL)
Footnotes
Source of Support: The National Natural Science Foundation of China (21102049), the Natural Science Foundation of Guangdong Province (9451064201003751), the Science and Technology Project of Guangzhou City (11C12100771), the Key Academic Program of the 3rd Phase “211 Project” of South China Agricultural University (2009B010100001), the national scholarship fund for studying abroad of China(file No.201208440268)
Conflict of Interest: None declared.
REFERENCES
- 1.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan RX, Zou WX. Endophytes: A rich source of functional metabolites. Nat Prod Rep. 2001;18:448–59. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 3.Singh UP, Prithiviraj B, Wagner KG, Schumacher KP. Effect of ajoene, a constituent of garlic (Allium sativum) on Powdery mildew (Erysiphe pisi) of pea (Pisum sativum) J Plant Dis Protect. 1995;102:399–406. [Google Scholar]
- 4.Huang LL, Li JW, Ni CL, Gu WX, Li CY. Isolation, crystal structure and inhibitory activity against Magnaporthe Grisea of (2R,3R)-3,5,7-trihydroxyflavanone 3-acetate from Myoporum Bontioides A. Gray. Chin J Struct Chem. 2011;30:1298–304. [Google Scholar]
- 5.Yecheng D, Zhen Y, Yanzhen Y, Xiulian B. Inhibitory activity against plant pathogenic fungi of extracts from Myoporum bontioides A. Gray and indentification of active ingredients. Pest Manag Sci. 2008;64:203–7. doi: 10.1002/ps.1491. [DOI] [PubMed] [Google Scholar]
- 6.Wang QG, Ma CL, Zhai JJ. Furanoeudesmane-B, a new eudesmane sesquiterpenoid from Myoporum bontioides. Acta Cryst C. 2000;56:e569. [Google Scholar]
- 7.Kanemoto M, Matsunami K, Otsuka H, Shinzato T, Ishigaki C, Takeda Y. Chlorine-containing iridoid and iridoid glucoside, and other glucosides from leaves of Myoporum bontioides. Phytochemistry. 2008;69:2517–22. doi: 10.1016/j.phytochem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Li XZ, Li CY, Wu LX, Yang FB, Gu WX. Chemical constituents from leaves of Myoporum bontioides. Chin Tradit Herb Drugs. 2011;42:2204–7. [Google Scholar]
- 9.Ding WJ, Zhang SQ, Wang JH, Lin YX, Liang QX, Zhao WJ, et al. A new di-O-prenylated flavone from an actinomycete Streptomyces sp. MA-12. J Asian Nat Prod Res. 2013;15:209–14. doi: 10.1080/10286020.2012.751979. [DOI] [PubMed] [Google Scholar]
- 10.Ichihara A, Hashimoto M, Hirai T, Takeda I, Sasamura Y, Sakamura S, et al. Structure, synthesis, and stereochemistry of (+)-orthosporin, a phytotoxic metabolite of Rhynchosporium orthosporium. Chem Lett. 1989;18:1495–8. [Google Scholar]
- 11.Yang SX, Gao JM, Zhang Q, Laatsch H. Toxic polyketides produced by Fusarium sp., an endophytic fungus isolated from Melia azedarach. Bioorg Med Chem Lett. 2011;21:1887–9. doi: 10.1016/j.bmcl.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 12.Sheng L, Yoshikazu S, Shosuke Y, Kazuaki K, Hideyuki F. Three new phenolic metabolites from Penicillium species. Heterocycles. 1991;32:297–305. [Google Scholar]
- 13.Larsen TO, Breinholt J. Dichlorodiaportin, diaportinol, and diaportinic acid: Three novel isocoumarins from penicillium nalgiovense. J Nat Prod. 1999;62:1182–4. doi: 10.1021/np990066b. [DOI] [PubMed] [Google Scholar]
- 14.Bifulco G, Dambruoso P, Gomez-Paloma L, Riccio R. Determination of relative configuration in organic compounds by NMR spectroscopy and computational methods. Chem Rev. 2007;107:3744–79. doi: 10.1021/cr030733c. [DOI] [PubMed] [Google Scholar]
- 15.Kirk RG. NMR methods for stereochemical assignments. In: Ernesto F, William HG, Orazio TS, editors. Handbook of Marine Natural Products. Heidelberg, New York, London: Springer Dordrecht; 2012. pp. 547–71. [Google Scholar]
- 16.Huang CH, Pan JH, Chen B, Yu M, Huang HB, Zhu X, et al. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9-6B from the South China Sea. Mar Drugs. 2011;9:832–43. doi: 10.3390/md9050832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanavi M, Gheidarloo R, Sadati N, Ardekani MR, Nabavi SM, Tavajohi S, et al. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacogn Mag. 2012;8:60–4. doi: 10.4103/0973-1296.93327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonfante V, Bonadonna G, Villani F, Di Fronzo G, Martini A, Casazza AM. Preliminary phase I study of 4′-epi-adriamycin. Cancer Treat Rep. 1979;63:915–8. [PubMed] [Google Scholar]
- 19.Schauer PK, Wittes RE, Gralla RJ, Casper ES, Young CW. A phase I trial of 4′-epi-adriamycin. Cancer Clin Trials. 1981;4:433–7. [PubMed] [Google Scholar]


