Abstract
Aims:
The aim of this study is to monitor the changes in the chemical composition of Ziziphora clinopodioides Lam. throughout nine different growth stages.
Materials and Methods:
Volatile components such as essential oils were analyzed using the gas chromatography (GC) and GC-mass spectrometry, and the contents of non-volatile components were determined by a visible spectrophotometer.
Results:
Hydro-distilled essential oil content ranged from a minimum of 1.1% (in the post-flowering stage) to a maximum of 1.8% (in the flowering stage). The essential oils included pulegone, which was the most abundant component (77.48-87.3%), p-menthanone (2.79-12.39%), trans-isopulegone (1.04-2.06%), d-limonene (0.51-3.03%) and eucarvone (1.5-4.48%). The contents of non-volatile components, such as that of total phenolics (TPC), total flavonoids (TFC), total triterpenoids content (TTC) and total free amino acids content (TFAAC) were measured using visible spectrophotometry. In the growing stage, TPC, TFC, TTC and TFAAC were 9.91-12.80 mg/g, 29.84-50.63 mg/g, 0.57-1.41 mg/g and 13.33-28.56 mg/g, respectively.
Conclusion:
These data can be used as a basis to determine the optimal harvest time of Z. clinopodioide Lam.
Keywords: Chemical composition, dynamic changes, Ziziphora clinopodioide Lam.
INTRODUCTION
Ziziphora clinopodioide Lam. is a traditional Uygur medicinal plant widely distributed in China, Mongolia, Turkey, Kazakhstan and Kyrgyzstan Liu 1985.[1] In Iran, this plant is mostly used in traditional medicine as a sedative, carminative, anti-emetic, anti-inflammatory and antiseptic substance in food Maya 2011.[2] The Uygur use Z. clinopodioides Lam. as an edible medicinal plant and its leaves, flowers and stems as a wild vegetable or an additive in food for richer aroma and flavor. This plant is also used to treat different diseases as an antiseptic, cold, cough and wound-healing medicine. Studies on Z. clinopodioides Lam. have mainly focused on their chemical constituents and biological activity Senejoux et al. 2012; Ji et al. 2012.[3,4] Much of the biological activity of the plant has been reported, such as its antibacterial, antimicrobial and antioxidant properties, its relaxing effect on the vascular system and its immunity-boosting effect on laying hens Ali et al. 2012 and Soltani 2012.[5,6]
Our research team has previously studied the stability of the essential oils of the plant Shi et al. 2009,[7] described the differences in the essential oil content of four Z. clinopodioides Lam. types growing in northern Xinjiang, China Zhou et al. 2011,[8] investigated the total polyphenolic and flavonoid content and antioxidant activity of plant extracts of different polarities Tian et al. 2011,[9] determined the oleanolic and ursolic acid content in the plant by using the high-performance liquid chromatography Tian et al. 2010,[10] identified diosmin, linarin and pulegone content in the plant from different places in Xinjiang Halmuart et al. 2012[11] as well as its caffeic and rosmarinic acid content Zhou et al. 2011[12] established the fingerprint of the plant Yu et al. 2012.[13] and established the quality standard of Z. clinopodioide Lam. initially Yu et al. 2012,[14] However, the changes in the chemical composition of Z. clinopodioide Lam. throughout different growth stages have not been reported and the harvest date affects the chemical composition of the plant. Thus, this study was undertaken to determine the dynamic changes of chemical composition of Z. clinopodioide Lam. in different growth stages as well as its optimum harvest time.
EXPERIMENTAL
Standards and solvents
Standards of rutin, uralic acid were purchased from National Institute for The Control of Pharmaceutical and Biological Products (China), arginine and gallic acid were from Wuxi Jinghai Amino Acid Co. (Wuxi, China).
Solvents and reagents were all of analytical grade from Tianjin Fu-Yu Chemical Ltd., Co. (Tianjin, China).
Plant material
Fresh plants (i.e., the entire plant) were collected from Tuoli, a township in Urumq (N 43°26’ 51.2”, E 87°39’ 18.9”) from May 1 to August 31, 2012, once every 2 weeks of harvest, authenticated by Yonghe Li, the chief apothecary of Chinese medicine at the Hospital of Xinjiang and dried naturally in the shade. Voucher specimens were deposited in the Department of Traditional Chinese Medicine Ethnical Herbs Specimen Museum of Xinjiang Medical University.
Analysis of essential oil composition
The whole plants from nine different growth stages (50 g) were extracted through hydro-distillation by using a Clevenger-type apparatus for 6 h. The essential oils were dried over anhydrous Na2 SO4 and stored in sealed vials under refrigeration prior to analysis. Gas chromatography (GC) analyses were carried out on essential oil samples by using a Shimadzu QP-2010 GC-MS system equipped with DB-5 ms (30 m, 0.25 mm, film thickness of 0.25 μm) capillary column and connected to a flame ionization detector. The temperatures of the injector and detector were both 250°C. Helium was used as the carrier and the flow rate was 1.0 mL/min. The temperature program was 40°C to 250°C at a rate of 5°C/min. The split ratio was 1:100. GC-MS analyses were performed in the electron-impact ionization mode with energy of 70 eV. The inlet temperature was set at 200°C and the transfer line temperature was 250°C. The temperature program used was similar to that adopted for GC analysis. The injected volume was 0.2 μL. Finally, the components were identified by comparing the retention time of each component with the n-alkane series (C6-C22) internal standards in identical experimental conditions. The mass spectra and relative retention index of the components were compared with those of commercial samples National Institute of Standards and Technology (NIST 05 and NIST 05 s). The relative amounts of individual components were calculated based on GC integrator peak areas without the use of correction factors.
Ethanol extract
Samples from nine different growth stages of Z. clinopodioide Lam. were pulverized into a fine powder by using a stainless steel blender. The dried and powdered plant materials (1 g) were extracted with 25 mL of 70% ethanol with 100 W ultrasonic bath (KQ2200E, Kunshan Ultrasonic Instrument Co., Jiangsu, China) for 30 min and were finally filtered.
Water extract
The powders from the nine stages of Z. clinopodioide Lam. were accurately weighed (2 g) and placed in a 250 mL round bottom flask. Boiling water was added three times. The volume of the first addition of water was 100 mL. The solution underwent reflux extraction for 1 h and was filtered while hot. The volume of the second addition of water was 50 mL. The solution was boiled for 0.5 h and filtered while hot. The volume of the third addition of water was 50 mL. The solution was boiled for 0.5 h and filtered while hot. The filtrate that was merged three times was concentrated to 100 mL as the test solution.
Total phenolic content
TPC was determined using the Folin-Ciocalteu method. First, 1 mL of ethanol was extracted and diluted to 10 mL with 70% ethanol. About 0.5 mL of the diluents was then mixed with 1 mL of the Folin-Ciocalteu reagent and was left to stand for 1 min at room temperature. About 2 mL of sodium carbonate (20% Na2 CO3) solution was then added and the volume of distilled water was increased to 10 mL. The solution was left to stand at room temperature for 10 min. Supernatant absorbance was measured at 760 nm with a visible spectrophotometer (Vis)-722S, Shanghai Jinghua, China and the quantification was done on the basis of the standard curve (Y = 14.31X + 0.1173) of gallic acid concentration ranging between 0.01 and 0.05 mg/mL (r2 =0.9949). The results were different from the standards prepared similarly with known gallic acid concentrations. All samples were analyzed three times.
Total flavonoid content
TFC was determined by the method of Lv Lin and Ningping Tao Lv et al. 2009[15] at 510 nm with a Vis-722S spectrophotometer. The quantification was performed on the basis of the standard curve (Y = 10.7007X + 0.0136) of rutin concentration ranging between 0.02 and 0.06 mg/mL (r2 =0.9949).
Total triterpenoid content
In this assay, the ethanol extracts needed to be purified because the other impurities in the extract would affect the measurement results. The ethanol extracts were concentrated and filtrated with a rotary evaporator, dissolved with 30 mL of chloroform and then moved to a separatory funnel. The saturated sodium bicarbonate extraction of chloroform was performed four times (15 mL × 4) and was combined with a sodium bicarbonate solution. The chloroform extraction was performed four times (15 mL × 4) with the pH regulated between 2 and 3 by using 6 mol/mL hydrochloric acid. The chloroform liquid was merged and the chloroform solution was washed with water. The water was then left to stand. The chloroform solution with anhydrous sodium sulfate was dried and filtered. The anhydrous sodium sulfate was washed with chloroform three times (15 mL × 3). The washing liquid and filtrate were merged. The solution was decompressed and evaporated until dry. The residue with the anhydrous ethanol solution was set at a constant volume of 5 mL. The solution was shaken and the product solution was obtained. TTC was determined through colorimetry. An accurate quantity of the purification extract was taken (0.4 mL in a test tube). Anhydrous ethanol was placed in a boiling water bath. The solution was then cooled. About 5 mL of 5% vanillin-glacial acetic acid solution and 1 mL of concentrated sulfuric acid solution were prepared. The test tube was heated to 60°C in the water bath for 10 min and the solution was cooled to room temperature. A constant volume of glacial acetic acid was added to the solution, which was then agitated. Each sample solution's absorbance was measured at 553 nm by using a Vis-722S spectrophotometer. Uralic acid (1.2 mg/mL to 7.2 mg/mL) was used as a standard. All samples were analyzed three times.
Total free amino acids content
The TFAAC of the extracts was determined based on the method of Rosen (1957). First, 0.5 mL of water extractions was added to 1 mL of sodium carbonate buffer salts and 2 mL of 2% ninhydrin solution in a tube. The tubes were heated in a boiling water bath for 15 min and allowed to cool at room temperature for 15 min. A constant volume of water was added to the solution, which was then agitated. Each sample solution's absorbance was measured at 566 nm by using a Vis-722S spectrophotometer. Arginine (4 μg/mL to 16 μg/mL) was used as a standard. All samples were analyzed three times.
RESULTS AND DISCUSSIONS
Volatile components
The growth stages were as follows: May 3 and May 17 were the seedling stage, June 2 and June 17 were the pre-flowering stage, July 2 was the initial flowering stage, July 2 to August 1 was the flowering stage, August 16 was the blossom stage and August 31 was the post-flowering stage. The essential oil compositions in different growth stages are presented in Table 1 and the dynamic changes are shown in Figure 1. The nine essential oils from different growth stages were mainly composed of oxygenated monoterpenes and had no significant differences in contents. The main component was pulegone in each stage, especially in the pre-flowering stage (June 17), where it was as high as 86.05%. p-Menthanone was as high as 12.39% on August 16, the blossoming stage. However, the content of d-limonene was lower during the flowering stage, but was the highest in the seeding stage, May 3 (3.5%). However, the trans-isopulegone content was higher in both the seeding and flowering stages at 2.06% and 2.01%, respectively. Overall, both the content of essential oils and the main chemical compositions in Z. clinopodioides Lam. were higher in the flowering stage than in other stages. Thus, Z. clinopodioides Lam. should be harvested during the flowering stage if its essential oils need to be studied.
Table 1.
Chemical compositions of the essential oil of Ziziphora clinopodioide Lam in different growth stages
Figure 1.
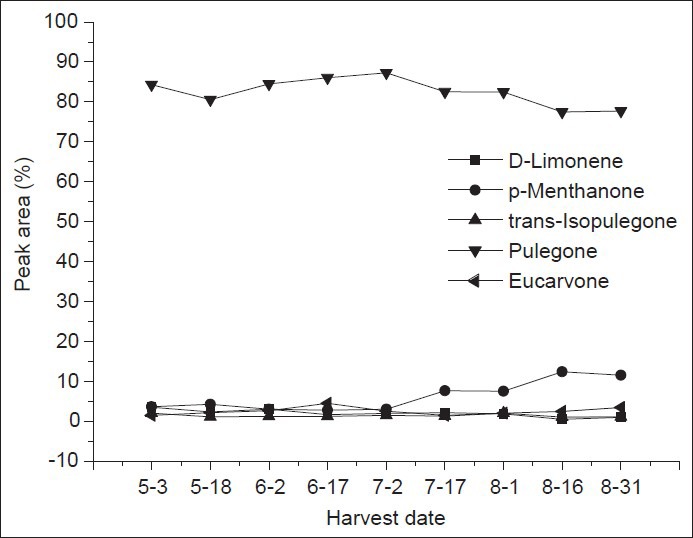
The dynamic changes of main chemical compositions of essential oils in the Ziziphora clinopodioide Lam.
Non-volatile components
Z. clinopodioides Lam. is rich in total flavonoids, total polyphenols and total free amino acids [Table 2]. The harvest date had a significant effect on its content [Figure 2]. However, TTC in the plant was much lower and had a little change during the different growth stages. Overall, TPC was higher on May 3 and in August and reached values as high as 12.80 and 13.18 mg/g, respectively. TFC increased from May 18 to August 16 from 29.84 mg/g to 50.63 mg/g and then decreased to 48.01 mg/g on August 31. TFAAC was rich in the seeding stage, followed by a rapid decrease on June 2 and became relatively stable in the subsequent growth stage. TTC was always maintained less than the others we had determined, a trend similar to that of TFAAC.
Table 2.
The dynamic changes of chemical components of Ziziphora clinopodioide Lam
Figure 2.
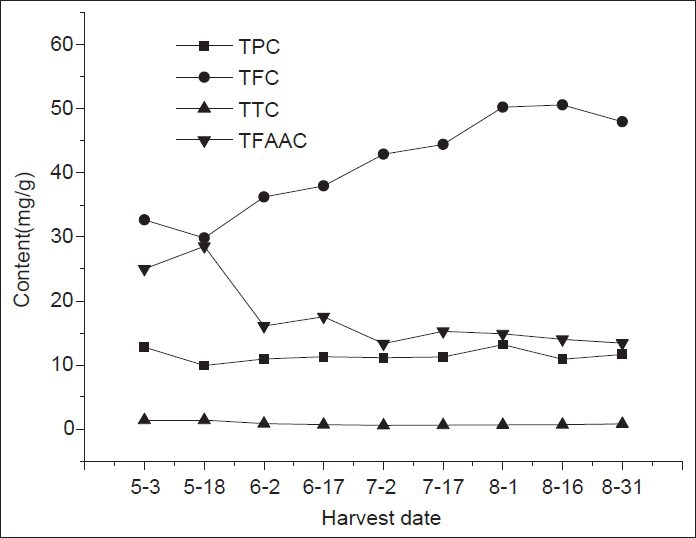
Content of chemical composition in Ziziphora clinopodioide Lam. at different growth stage
CONCLUSIONS
Based on the investigation of the essential oils in Z. clinopodioides Lam., the contents during the growth stage is different. However, in all stages, the content accounts for 1% or above. The common dates were very rich in pulegone, especially at the flowering stage. Thus, the best harvesting time for the essential oils of Z. clinopodioides is during the flowering stage.
Z. clinopodioides Lam. is rich in total phenolics, total flavoniods and total free amino acids. Both TFC and TFAAC significantly varied in the growth process, whereas TPC was relatively stable. TTC was low in these varieties and similar in all stages.
The differences in the content of chemical components may be attributed to the different harvest dates. Growth time also affects the change in chemical constituents in the plants. The influence of growth phase on the essential oil composition of Z. clinopodioides Lam. in Iran has been previously investigated Amiri 2009.[16] However, the present study is the first to determine simultaneously the volatile and non-volatile components of Z. clinopodioides Lam. in Xingjiang, China at different growth stages. This method can also be used to monitor and rapidly predict the quality of Z. clinopodioides Lam. to decide the optimal harvest date.
ACKNOWLEDGMENT
This work was supported by the Program for Xinjiang science and technology support plan(201233134).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Liu YM. Xinjiang: Science and Technology Health Pyblisher; 1985. Pharmacography of Uighur; pp. 353–7. [Google Scholar]
- 2.Maya B. The Evaluation of Medicinal Properties of Ziziphora clinopodioides. World Appl Sci J. 2011;9:1635–8. [Google Scholar]
- 3.Senejoux F, Demougeot C, Kerram P, Aisa HA, Berthelot A, Bévalot F, et al. Bioassay-guided isolation of vasorelaxant compounds from Ziziphora clinopodioides Lam. (Lamiaceae) Fitoterapia. 2012;83:377–82. doi: 10.1016/j.fitote.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Ji ZH, Yu Q, Zhou XY, Upur H. Effects of a aqueous extract of Ziziphora clinopodioides Lam on the growth of streptococcus mutans. J XinJiang Med Univ. 2012;35:1031–4. [Google Scholar]
- 5.Ali N, Navid HM, Mohanmmad AM. Effect of Melissa officinalis L., Tanacetum balsamita L. and Ziziphora clinopodioides L. on Performance, Blood Biochemical and Immunity parameters of Laying Hens. Asian J Anim Vet Adv. 2012;7:74–9. [Google Scholar]
- 6.Soltani NS. Chemical composition and in vitro antibacterial activity of Ziziphora clinopodioides Lam. essential oil against some pathogenic bacteria. Afr J Microbiol Res. 2012;7:1504–8. [Google Scholar]
- 7.Shi Y, Bi K, Xu TH, Tian SG. The stability of the essential oil from Uygur medicine Ziziphora clinopodioides Lam. Chin J Ethnomed Ethnopharm. 2009;18:1–3. [Google Scholar]
- 8.Zhou XY, Yu Q, Gong HY, Tian SG. GC-MS Analysis of Essential Oil Composition of Ziziphora clinopodioides Lam. in North Xinjiang, China. Nat Prod Commun. 2011;1:81–2. [PubMed] [Google Scholar]
- 9.Tian S, Shi Y, Zhou X, Ge L, Upur H. Total polyphenolic (flavonoids) content and antioxidant capacity of different Ziziphora clinopodioides Lam. extracts. Pharmacogn Mag. 2011;7:65–8. doi: 10.4103/0973-1296.75904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian SG, Shi Y, Yu Q, Upur H. Determination of oleanolic acid and ursolic acid contents in Ziziphora clinopodioides Lam. by HPLC method. Pharmacogn Mag. 2010;6:116–9. doi: 10.4103/0973-1296.62898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halmuart U, Tian SG, Yu Q. Development of a rapid resolution liquid chromatography-diode array detector method for the determination of three compounds in Ziziphora clinopodioides Lam from different origins of Xinjiang. Pharmacogn Mag. 2012;8:280–4. doi: 10.4103/0973-1296.103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou XY, Yu Q, Tian SG. Simultaneous Determination of Caffeic Acid and Rosmarinic Acid in Ziziphora clinopodioides Lam. from Different Sources in Xinjiang by a Novel Rapid Resolution Liquid Chromatography Method. Lat Am J Pharm. 2011;8:1651–5. [Google Scholar]
- 13.Yu Q, Halmuart U, Xin LD. Chemical Fingerprinting by RP-RRLC-DAD and Principal component analysis of Ziziphora clinopodioides lam from different locations. Nat Prod Commun. 2012;9:1181–4. [PubMed] [Google Scholar]
- 14.Yu Q, Shi Y, Yuan SN, Tian SG. Preliminary study of quality standards of Ziziphora clinopodioides Lam. J XinJiang Med Univ. 2012;35:301–5. [Google Scholar]
- 15.Lv L, Tao NP. Determination of total flavonoids content in citrus peels by ultraviolet spectrophotometry. Mod Food Sci Technol. 2009;2:217–20. [Google Scholar]
- 16.Amiri H. Influence of growth phase on the essential oil composition of Ziziphora clinopodioides Lam. Nat Prod Res. 2009;23:601–6. doi: 10.1080/14786410802113995. [DOI] [PubMed] [Google Scholar]


