Abstract
Introduction:
Clover has attracted considerable interest not only as a valuable livestock forage plant, but also as an important source of isoflavones. The current study was aimed to assess the variation of concentration of three isoflavones in clover species grown under a cool temperate climate environment in Lithuania.
Materials and Methods:
Isoflavone contents were quantified in the plant parts of 21 accessions belonging to five perennial species of genus Trifolium (T. pratense, T. repens, T. medium, T. rubens, and T. pannonicum). Daidzein, formononetin, and genistein concentrations were quantified in separate plant parts by reversed-phase high-performance liquid chromatography. The analyte extraction was performed from dried and ground leaves, stems, flowers, and roots. The procedure included acid hydrolysis of isoflavone glycosides to aglycones.
Results:
According to the averaged sum of the three isoflavones quantified in leaves-stems-flowers, the five clover species ranked as follows: T. medium (7.54-3.62-2.31 mg/g) >T. pratense> T. rubens> T. pannonicum> T. repens (0.191-0.204-0.171 mg/g). The contribution of individual compound to the total isoflavone content depended on the species, accession, and plant part. The major part of the isoflavones is concentrated in leaves or stems; however, there is a great variation also.
Conclusion:
There exists a large variation in the total as well as in individual concentration of isoflavones among the clover species and plant parts and within species. With regard to isoflavone concentration and variability within species, some accessions of T. medium and T. pratense can be considered a highly promising source of phytoestrogens.
Keywords: Concentration variation, daidzein, formononetin, genistein, plant part, Trifolium species and accessions
INTRODUCTION
The Trifolium genus (Fabaceae) comprises about 300 species, found chiefly in north temperate regions, but also, like other north temperate genera, on the mountains in the tropics.[1,2] Traditionally, clover is used as a livestock forage and is highly valued due to its ability to fix atmospheric nitrogen in the soil. Moreover, it could be used to meet specific agricultural and environmental needs: For the conservation of eroded or agriculturally disadvantaged soils,[3] for planting on dry hill slopes,[1] and on acid, saline, dry soils, as well as by using it in apiculture because of the high nectar content etc.[4] Due to the highly ornamental flowers, the plants of T. fragiferum L., T. pannonicum Jacq. T. rubens L. can be used for the establishment of flowering meadows.[5]
Isoflavones are a subgroup of phytoestrogens, natural plant substances with a structure similar to 17-β-estradiol and capable of binding to estrogen receptors.[6] Thanks to the phytoestrogenic effect, isoflavones can prevent or slow up some forms of hormone-dependent cancers, osteoporosis, Alzheimer, cardiovascular pathologies, and relieve symptoms of menopause.[6,7,8,9] Different isoflavones are specific to fabaceous plants. Many isoflavone products derived from fabaceous are currently widely used as phytoestrogen food supplements (iseren, estro-cal, ateroklefit, mabelle, and meflovan, etc.). At present, soybean is the most important isoflavone source. However, its use is limited because of the allergenic reactions it can cause in some people.
Moreover, production area of genetically modified soy is steadily increasing and many people do not approve of the use of such plants.[10] Therefore, scientists are seeking for new isoflavone resources. Since olden times, red clover (T. pratense L.) has been used in folk medicine for treatment of some diseases (eczemas, asthma, and eye conditions, etc.).[11] About 40 different isoflavones are detected in red clover,[12,13] of which the most common are formononetin, biochanin A, daidzein, and genistein[14,15] and the last two compounds are the primary isoflavones in soy. Research related to isoflavone distribution in clover is predominantly limited to one species-red clover.[16,17,18] Little evidence is available on isoflavones in other Trifolium species and their variation within and between species. The current study focuses on the aspects of variation of isoflavone concentration in five clover species grown under a cool temperate climate environment in Lithuania.
MATERIALS AND METHODS
Experiments were conducted in the central lowland of Lithuania (55°23′49″N; 23°51′40″E), at the Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry during 2007–2008. The soil of the experimental site is Endocalcari-Epihypogleyic Cambisol (CMg-p-w-can). The isoflavone studies involved germplasm collection of the following perennial clover species: T. medium L., T. pannonicum Jacq., T. pratense L., T. repens L., and T. rubens L. The collection included commercial varieties and wild ecotypes, collected in natural or seminatural habitats in Lithuania and the Ukraine, and seeds of one wild population of T. pannonicum were provided by the French colleagues, the seed origin was not indicated [for details see Table 1].
Table 1.
List of germplasm collection of the Trifolium spp. examined for isoflavones
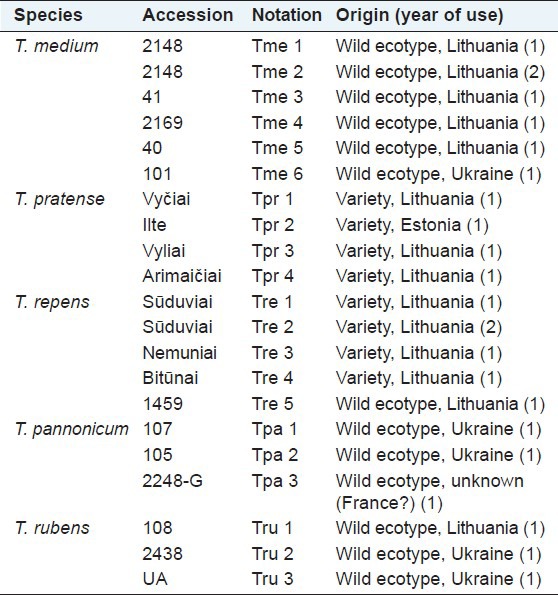
Two samples (one wild ecotype of T. medium no. 2148 with a high isoflavone content and one variety of T. repens “Sϋduviai” characterized by a negligible isoflavone concentration) were tested for these compounds in the first (1) and second (2) years of their cultivation. In 2007 and 2008, genetic collections of clover species were established in a trial field using seedlings grown in a greenhouse. Thirty plants per accession (2 replications, 15 plants per replication) were planted at 50 × 50 cm distances. The clover plants were cut at full flowering stage, aerial part of plants was separated into three fractions: Stems, leaves together with petioles, and flowers. Fresh samples of each fraction were immediately chopped into particles of 3-5 cm, fixed at 105°C for 20 min, dried at (65 ± 5) °C and ground in a cyclonic mill with a 1 mm sieve. Dried samples were assayed for the following isoflavones: Daidzein, formononetin, and genistein.
Chemicals
An HPLC grade acetonitrile and trifluoracetic acid were purchased from Sigma-Aldrich (Steinheim, Germany), ethanol from Stumbras AB (Kaunas, Lithuania). Ultrapure water (>18 MΩ cm) was used throughout the HPLC experiment. Standards of daidzein or 4’, 7- dihydroxyisoflavone (purity 99.9%) and genistein also known as 4’, 5, 7-trihydroxyisoflavone (purity 99.3%) were obtained from ChromaDex (Irvine, CA, USA) and formononetin (7-hydroxy-4′-methoxyisoflavone)(purity ≥ 99%) from Fluka-Sigma-Aldrich (Steinheim, Germany). The standard solutions were prepared by dissolving standard compounds in methanol (0.2-30 μg/mL).
HPLC equipment and isoflavone quantification
The sample preparation procedure included acid hydrolysis of glycoside forms and was performed under slightly modified conditions described in the USP monograph.[19] The isoflavones were extracted with 60 mL of 50% v/v ethanol from 1500 mg of accurately weighed ground plant material (orbital shaker, 12 h). After extraction, the solution was filtered. A volume of 50 mL of the filtrate was transferred to a round-bottom flask and evaporated to dryness under reduced pressure. The residue was rinsed with 10 mL of 96.3% v/v of ethanol and 10 mL of water and 5 mL of 10 M HCl was added into the flask and the mix was heated for 30 min on the water bath using the reflux condenser. The filtered hydrolyzate was mixed with 50% v/v of ethanol to bring the volume up to 30 mL and final solution was filtered through a nylon membrane having a 0.45 μm porosity (Millipore, MA, USA). The analyses were carried out with a Waters 2695 Alliance HPLC system (Milford, MA, USA), equipped with a Waters 2487 UV/Vis detector and a Waters XTerra RP18 150 × 3.9 mm column with a guard column. The dual λ absorbance detector allowed monitoring of two wavelengths, 258 nm (for genistein analyte) and 301 nm (for daidzein and formononetin analytes). Waters 996 photodiode array detector (PDA) detector was used for peak spectral identification. Chromatographic and PDA data were recorded and peak area responses were integrated by the software Waters Empower2 (Milford, MA, USA). The gradient chromatographic elution was carried out according to Raudonis et al.,[20] The linear through zero type calibration curves were generated using external standard solutions, prepared by dissolving standard compounds in methanol (0.2-30 μg/mL). The dependence between peak area and concentration was linear with the determination coeficient R2 ≥ 0.9998 for each of the three isoflavone. The regression equations for the standard curve of genistein, daidzein, and formononetin were respectively: y = 160329691·x, y = 55761134·x, and y = 55059861·x (y refers to the peak area, x - to the concentration of the respective reference). Limit of detection was determined with standard solution at a signal-to-noise ratio of three and was < 0.078 μg/mL(for daidzein), <0.032 μg/mL (for genistein), and < 0.072 μg/mL (for formononetin). Isoflavone concentrations were expressed as mg/g of sample dry matter (DM).
RESULTS AND DISCUSSION
It is commonly known that red clover is a rich source of isoflavones, which have been extracted and commercialized as phytoestrogen products or nutraceuticals Rimostil, Promensil from Novogen, Australia,[21,22] Menoflavon; from Melbrosin, Austria.[23] Our experimental evidence suggests that this species is quite abundant in isoflavones (formononetin + daidzein + genistein): Averaged total concentration depending on an aerial plant part amounted to 2.22 (flowers)-3.32 (stems) mg/g [Table 2]. The total concentration of these three isoflavones of similar order in T. pratense (3.25 mg/g) was observed also by Zgórka.[24] Such concentration of the three phytoestrogens tested (excluded biochanin A) in red clover is equal or even higher than the total isoflavone concentration in soy: In soybean it ranges from 0.360 to 2.24 mg/g.[25]
Table 2.
Variation of the total isoflavone concentration among aerial plant parts and clover species
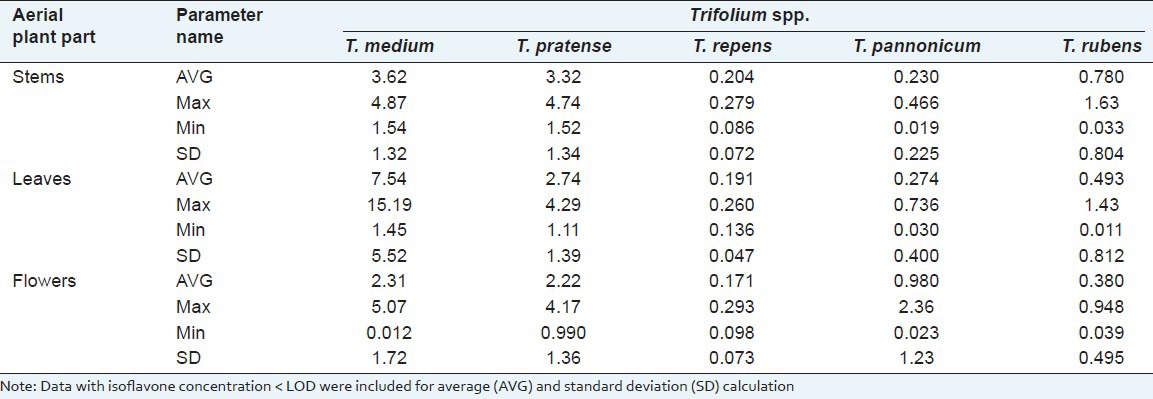
Although T. pratense has received a lot of researchers’ interest, the highest isoflavone contents are accumulated by the aerial plant parts of perennial T. medium. During flowering stage, the leaves of the T. medium contained, on average, the highest total concentration (7.54 mg/g) of the three isoflavones. However, depending on an accession, this concentration varied within a very wide range from 1.45 to 15.2 mg/g with the standard deviation equal to 5.52. The average total isoflavone concentration in stems of this species was 3.62 mg/g and it varied in a narrower range than in leaves: From 1.54 to 4.87 mg/g. The lowest average isoflavone concentration (2.307 mg/g) was found in flowers of T. medium and ranged from 0 to 5.07 mg/g. Only negligible concentrations of the compounds assayed were determined in all aerial plant parts of T. repens, T. pannonicum, and T. rubens. Screening of 57 Trifolium species for total isoflavone concentration, done by Oleszek et al.,[26] showed also that there are a number of species with particularly high amounts of these compounds. However, the regularity of isoflavone content variation between species significantly differed from that established in our study as well as Zgórka[24] research: According to Oleszek et al.,[26] isoflavone concentration in T. pratense ranged from 10.39 to 39.51, and in T. medium it was as low as 1.58 mg/g DM. Considering the average isoflavone content, our findings in general are consistent with the regularity recently established by Zgórka[24]: Lower isoflavone contents have been determined for white and white-pink flowering clover species (T. repens and T. pannonicum) and in clover species, whose flowers are characterized by red color, isoflavone content was higher, except for T. rubens. Dark red color is specific to T. rubens flowers and the isoflavone contents detected varied within 0.011-1.63 mg/g range [Table 2]. There is considerable variation in total and individual isoflavone concentrations between the plant parts. Research sources provide contradictory findings on which above-ground plant parts accumulate maximal isoflavone contents.[17,27] According to Vetter,[16] isoflavone accumulation in different plant organs is species-specific. In our study, for T. pratense, T. rubens, and T. repens higher isoflavone concentrations were detected in stems, while for T. pannonicum in flowers. Leaves of T. medium were richer in isoflavones than any other above-ground plant part.
Differences between specific isoflavones also varied greatly depending on species and plant part [Figure 1]. From some literature sources, it follows that formononetin like biochanin A (not analyzed in our work) concentration in whole plant and/or individual plant parts is from twice to much more times higher than that of daidzein and genistein.[18,24,28,29,30] In our study, in most cases formononetin accounted for more than half of the total concentration of the three isoflavones [Figure 1]. In average for T. pratense accessions, formononetin content ranged from 54.9% (in leaves) to 74.95% (in flowers). More even distribution of isoflavones was determined in aerial parts of T. medium plants, with prevalence of genistein in leaves (46.6%) and formononetin in flowers and stems (40.9% and 49.3%, respectively). The average composition of the three isoflavones in leaves and stems of T. pannonicum species was exceptional: Genistein was a predominant isoflavone in leaves (65.0%) and stems (80.9%), while the concentration of formononetin in these aerial plant parts was only negligible. However, its share in flowers accounted for 51.3% of the total content of the tested compounds.
Figure 1.
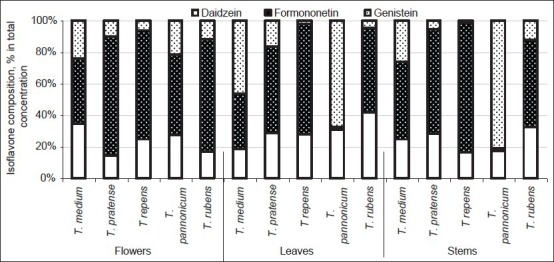
Variation of isoflavone composition among clover species and aerial plant parts
The concentration as well as composition of isoflavones in different accessions within a clover species may differ significantly [Figure 2]. We will pay more attention to intraspecific variation of this attribute in aerial plant parts of the two species, containing high average isoflavone concentration, that is, T. medium and T. pratense. The concentrations of the isoflavones in six samples of zigzag clover T. medium [Tme1-Tme6 in Figure 2] largely varied depending on an accession, plant part, and year of use. Nearly, all populations tested were characterized by a higher total isoflavone concentration in leaves than in other aerial plant parts. The local wild population of T. medium no. 2148 was distinguished by the abundance of these compounds in leaves in both years of use (12.9 and 15.2 mg/g in leaves of Tme1 and Tme2, respectively). The flowers of this population accumulated lower concentrations of these bioactive compounds: 2.64 mg/g in the flowers of plants in the first year of use and only trace levels in the second year of use. The leaves, like other aerial plant parts, of other populations also contained high concentrations of isoflavones: The total concentration of the three isoflavones was equal to or markedly exceeded that in soy, which so far has been the most popular source for the production of isoflavone supplements. The plant parts of the Lithuanian wild population Tme4 contained markedly lower concentrations of these compounds; however, their distribution in leaves-stems-flowers was the most even (1.45-1.54-1.15 mg/g).
Figure 2.
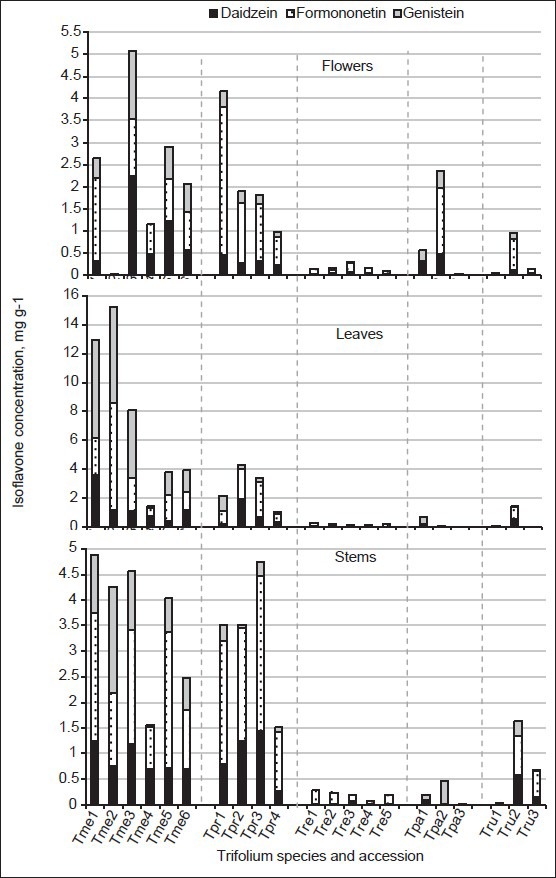
Variation of isoflavones among aerial plant parts, between and within two clover species. Note: Data with isoflavone concentration < LOD were included
Among the four T. pratense accessions, it is possible to distinguish only one Lithuanian variety “Arimaičiai” (Tpr4), in whose aerial plant parts the total isoflavone concentration was < 2.00 mg/g: From 0.990 mg/g in flowers to 1.52 mg/g in stems. The distribution of isoflavone contents in the plant parts of other accessions was uneven. Among aerial plant parts, the highest concentrations were established in the flowers of Tpr1 (4.17 mg/g), leaves of Tpr2 (4.29 mg/g), and stems of Tpr3 (4.74 mg/g).
In terms of isoflavone composition, all red clover accessions had formononetin as the predominating isoflavone in stems, flowers, and leaves; formononetin share in the total concentration of the three isoflavones ranged from 42.7% to 80.6%, except for Tpr1 leaves, where genistein accounted for a slightly larger share than formononetin-48.9% or 1.07 mg/g of the total amount 2.18 mg/g [Figure 2]. Similar concentrations of formononetin (2.10 mg/g) and daidzein (1.91 mg/g) were found in the leaves of the red clover variety “Ilte” (Tpr2) of Estonian origin. The major compound of the three isoflavones of some zigzag clovers may not always be formononetin as was the case with red clovers. The composition of the tested isoflavones in T. medium varied inconsistently. In leaf samples of Tme1, Tme3, and stem samples of Tme2, the contribution of genistein to the sum of isoflavones was ultimate among the compound tested: 52.3%, 57.6%, and 48.7%, respectively. Wild ecotype Tme4 was distinguished not only for lower total isoflavone concentration among zigzag clovers, but also for their composition with negligible content of genistein and quite high share of daidzein and formononetin, accounting for 40.2%-53.4% and from 36.2% to 59.4% of the sum of isoflavones in the aerial plant parts [Figure 2]. Isoflavone concentrations in clover, like those of many secondary metabolites, is primarily associated with the genetic peculiarities of species/accession.[28] Such differences were obvious not only among the T. medium and T. pratense accessions. In a flower sample of one of the three T. pannonicum populations (Tpa2 from Ukraine), a moderate isoflavone concentration, that is, 2.36 mg/g was determined. Similarly, in T. rubens species, the wild ecotype Tru2 of Ukrainian origin too, accumulated in the aerial plant parts from 0.948 (flowers) to 1.63 (stems) mg/g isoflavones, which is two to three times more than in average for the species. Some differences in isoflavone concentration as well as composition may be explained also by different growing conditions (temperature, light, nutrition, etc.),[30] but this factor in our study was not relevant. The cause of the fluctuations in isoflavone concentration and composition between years of use could not be defined correctly because only two populations were tested for isoflavones in the first and second years of their cultivation, and exact developmental stages were not recorded. As Sivesind and Seguin[31] observed, changes in isoflavone concentrations with increasing maturity varied depending on the plant part: The highest isoflavone concentrations were found in leaves and stems during the vegetative stages, with a decline until plants initiated flowering (principally at the expense of formononetin), and then the concentrations stabilized in both parts. The concentration of isoflavones in flowers decreased more sharply.
In order to further determine the role of plant part in the isoflavones concentration, the Lithuanian varieties of red clover “Vyčiai” and “Vyliai” were additionally analyzed for daidzein, genistein, and formononetin contents in roots [Figure 3]. The data are presented by comparing the distribution of these compounds in aerial plant parts. The roots are characterized by quite high total levels of isoflavones tested; moreover, the below-ground plant part was the most abundant in isoflavones (4.73 mg/g) among all plant parts of the variety “Vyčiai”. Other plant parts of this variety were ranked in the following order: Flowers (4.17 mg/g), stems (3.51 mg/g), and leaves (2.18 mg/g). Completely different isoflavone distribution per plant was characteristic of the variety “Vyliai”. With regard to the total concentration of phytoestrogens tested, plant parts were arranged in the following sequence: Stems (4.74 mg/g), roots (4.11 mg/g), leaves (3.40 mg/g), and flowers (1.82 mg/g). In terms of formononetin concentration differences in plant parts of “Vyčiai”, roots contained the largest amount of this isoflavone. Daidzein together with genistein represented only a small proportion, that is, 10.5% of the total isoflavone, whereas leaves accumulated considerably more genistein, which accounted for about half of the isoflavone sum. Fairly large amount of daidzein was found in stems of “Vyčiai” (0.813 mg/g in 3.51 mg/g of total), as well as in roots, stems, and leaves of “Vyliai” (1.06, 1.46, and 0.724 mg/g or 25.9%, 30.8%, and 21.3% of the total concentration of three isoflavones, respectively).
Figure 3.
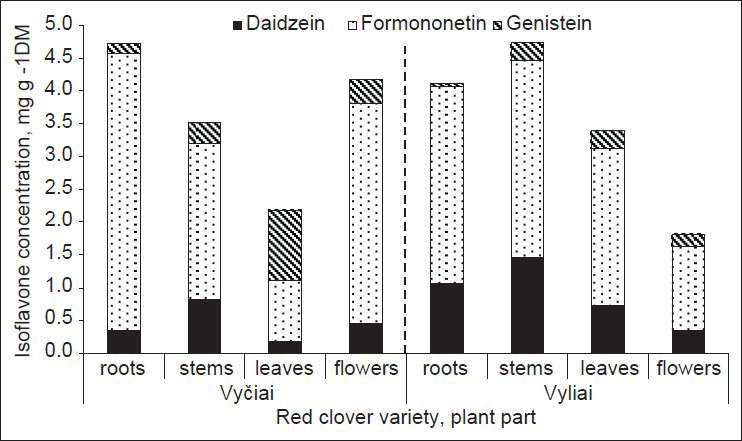
Pattern of isoflavones variation among plant parts of two red clover varieties
With regard to species practical application, a very important aspect is high DM yield, DM distribution among individual plant parts, as good reproductive plant characteristics. Previous research of Vilčinskas and Dabkevičienė[32,33] showed that DM yield greatly varied among clover species, and according to the productivity per plant averaged for species, five clovers were ranked in the following order: T. pannonicum, T. pratense, T. rubens, T. medium, and T. repens. High seed yield (4.43-2.42 g per plant) was noted for T. pratense, T. pannonicum, T. repens, and T. rubens. Although only moderately yielding T. medium plants exhibited low seed set, due to its specific root system (vegetative propagation by rhizomes), persistence and longevity as well as the highest isoflavone content, this species could be a potential medicinal plant in the countries of temperate climate. Furthermore, T. medium plants were characterized by quite high percentage of leaves (39.4%), and as is seen from Table 2, the largest isoflavone concentration was found in this aerial plant part of zigzag clover. Among Trifolium spp., the significantly highest stem weight (53.5%) was determined in T. pratense.[33] Since the most considerable isoflavone concentrations in average for red clover were accumulated in stems, this species trait, as well as high DM and seed yield make T. pratense one of the most plausible isoflavone sources under Lithuania's conditions and under those of cool temperate climate countries. Although T. pannonicum plants displayed high seed set and the highest DM yield per plant, there was a wide intraspecific variability in the content of isoflavones, and additional research for accession choice is needed, the same is true for T. rubens. All five T. repens accessions tested showed very low isoflavone concentrations in all aerial plant parts, thus, in our opinion, this species has no prospects as a source of isoflavones. The intraspecific variability in the total and individual isoflavone concentrations, in addition to the variability in agrobiological traits described previously,[32,33] indicates that it would be feasible to select populations for the desirable combination of chemical and agrobiological properties through a variety development program. Metabolic pathways of aromatic secondary metabolites, including isoflavones, are particularly complex.[34] Genetic peculiarities, environmental factors, agronomic practices, and plant maturity have a noticeable impact on the dynamics of qualitative and quantitative accumulation of these compounds. Therefore, it would be profitable to conduct a more comprehensive evaluation of the germplasm at different stages of maturity for the range of isoflavone, including biochanin A.
CONCLUSIONS
The present study revealed that total and individual concentrations of formononetin, daidzein, and genistein showed great variation among clover species and identified that the isoflavone content in T. medium and T. pratense species is much higher than that found in the other clover species; therefore, these two species may be valuable multifunctional plants for different use.
This study also confirmed the large variation in the isoflavone concentration and profile among aerial plant parts. Generally, leaves of the zigzag clover and stems of the red clover plants had the highest total isoflavone concentration among all plant parts. But a marked intraspecific difference was also established both in total isoflavone levels and distribution of individual compounds among plant parts.
The concentration of total and individual isoflavones (formononetin, daidzein, and genistein) in the aerial part of clover species was different for the studied accessions. Isoflavone concentration in leaves of the local wild population T. medium no. 2148 both of in the first and second years of use was much higher (12.9 and 15.2 mg/g) than that found in stems and flowers, as well as in the other accessions of Trifolium spp. There was established a large variation in the total as well as in individual concentration of isoflavones within other clover species too. The roots of red clover are characterized by high total levels of isoflavones tested; moreover, roots were the most abundant in isoflavones (4.73 mg/g) among all plant parts of the variety “Vyčiai”.
Screening of wild ecotypes and varieties could be useful to identify germplasm with a high potential of bioactive substances, for both wider cultivation of clovers as medicinal plants and for further breeding programs aimed to develop varieties with the desirable combination of chemical and agrobiological properties. To get a better insight into the factors responsible for optimal isoflavone concentrations in clover plants, a more extensive evaluation of germplasm at different stages of maturity for the range of isoflavones is needed.
ACKNOWLEDGMENTS
This work was partly supported by the long-term research programs “Genetics and purposeful change of genotypes of agricultural and forest plants” and “Biopotential and quality of plants for multifunctional use” implemented by Lithuanian Research Centre for Agriculture and Forestry.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gillett JM, Taylor NL, Collins M. The world of clovers. In: Collins M, editor. Iowa State, USA: University Press; 2001. p. 457. [Google Scholar]
- 2.Clover The 1911 Classic Encyclopedia. Based on the 11th Edition of the Encyclopaedia Britannica (Pub. 1911) [Last accessed on 2012 Sep 5]. Available from: http://www.1911encyclopedia.org/Clover .
- 3.Smith RR. Kura clover (Trifolium ambiguum M.B.): A forage legume with great potential for pasture and conservation. In: Boller B, Stadelman FJ, editors. Breeding for a Multifunction Agriculture. Zurich-Reckenholz: Swiss Federal Research Station for Agroecology and Agriculture; 1998. pp. 87–90. [Google Scholar]
- 4.Noor MJ, Khan MA, Camphor ES. Palynological analysis of pollen loads from pollen sources of honeybees in Islamabad, Pakistan. Pak J Bot. 2007;41:495–501. [Google Scholar]
- 5.Rumball W, Claydon RB. ’Gao 153’ turf type strawberry clover (Trifolium fragiferum L. N Z J Agric Res. 2005;48:421–2. [Google Scholar]
- 6.Pilšáková L, Riečanský I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010;59:651–64. doi: 10.33549/physiolres.931902. [DOI] [PubMed] [Google Scholar]
- 7.Setchell KD. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68(Suppl):1333S–46. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 8.Ososki AL, Kennelly EJ. Phytoestrogens: A review of the present state of research. Phytother Res. 2003;17:845–69. doi: 10.1002/ptr.1364. [DOI] [PubMed] [Google Scholar]
- 9.Albulescu M, Popovici M. Isoflavones – biochemistry, pharmacology and therapeutic use. Rev Roum Chim. 2007;52:537–50. [Google Scholar]
- 10.Aparicio-Fernandez X, Yousef GG, Loarca-Pina G, de Mejia E, Lila MA. Characterization of polyphenolics in the seed coat of black jamapa bean (Phaseolus vulgaris L.) J Agric Food Chem. 2005;53:4615–22. doi: 10.1021/jf047802o. [DOI] [PubMed] [Google Scholar]
- 11.Wren RC. Saffron Walden: C.W. Daniel Company Ltd; 1988. Potter's New Cyclopaedia of Botanical Drugs and Preparations; p. 112. [Google Scholar]
- 12.Kjeldus B, Vitamvasova D, Kuban V. Identification of isoflavone conjugates in red clover (Trifolium pratense) by liquid chromatography-mass spectrometry after two-dimensional solid-phase extraction. Anal Chim Acta. 2001;450:81–97. [Google Scholar]
- 13.Rijke E, Zappey H, Ariese F, Gooijer C, Brinkiman UA. Flavonoids in Leguminosae: Analysis of extracts of T. pratense L., T. dubium L., T. repens L., and L. corniculatus L. leaves using liquid chromatography with UV, mass spectrometric and fluorescence detection. Anal Bioanal Chem. 2004;378:995–1006. doi: 10.1007/s00216-003-2310-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–9. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 15.Booth NL, Overk CR, Yao P, Totura S, Deng Y, Hedayat AS. Seasonal variation of red clover (Trifolium pratense L., Fabaceae) isoflavones and estrogenic activity. J Agric Food Chem. 2006;54:1277–82. doi: 10.1021/jf052927u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vetter J. Isoflavones in different parts of common Trifolium species. J Agric Food Chem. 1995;43:106–8. [Google Scholar]
- 17.Lin LZ, He XG, Lindenmaier M, Yang J. LC-ESI-MS study of the flavonoid glycoside malonates of red clover (Trifolium pratense) J Agric Food Chem. 2000;48:354–65. doi: 10.1021/jf991002+. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, Wang M, Simon JE. Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J Chromatogr A. 2003;1016:195–209. doi: 10.1016/j.chroma.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Sharaf MH. Red clover. In: Williams RL, editor. US Pharmacopeia 32-NF27. Rockville: United States Pharmacopeia; 2009. pp. 983–6. [Google Scholar]
- 20.Raudonis R, Jakstas V, Burdulis D, Benetis R, Janulis V. Investigation of contribution of individual constituents to antioxidant activity in herbal drugs using post column HPLC method. Medicina. 2009;45:382–94. [PubMed] [Google Scholar]
- 21.Clifton-Bligh PB, Baber RJ, Fulcher GR, Nery ML, Moreton T. The effect of isoflavones extracted from red clover (Rimostil) on lipid and bone metabolism. Menopause. 2001;8:259–65. doi: 10.1097/00042192-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Van de Weijer PH, Barentsen R. Isoflavones from red clover (Promensil®) significantly reduce menopausal hot flush symptoms compared with placebo. Maturitas. 2002;42:187–93. doi: 10.1016/s0378-5122(02)00080-4. [DOI] [PubMed] [Google Scholar]
- 23.Dornstauder E, Jisa E, Unterrieder I, Krenn L, Kubelka W, Jungbauer A. Estrogenic activity of two standardized red clover extracts (Menoflavon®) intended for large scale use in hormone replacement therapy. J Steroid Biochem Mol Biol. 2001;78:67–75. doi: 10.1016/s0960-0760(01)00075-9. [DOI] [PubMed] [Google Scholar]
- 24.Zgórka G. Ultrasound-assisted solid-phase extraction coupled with photodiode-array and fluorescence detection for chemotaxonomy of isoflavone phytoestrogens in Trifolium L. (Clover) species. J Sep Sci. 2009;32:965–72. doi: 10.1002/jssc.200800456. [DOI] [PubMed] [Google Scholar]
- 25.Seguin P, Zheng W, Smith DL, Deng W. Isoflavone content of soybean cultivars grown in eastern Canada. J Sci Food Agric. 2004;84:1327–32. [Google Scholar]
- 26.Oleszek W, Stochmal A, Janda B. Concentration of isoflavones and other phenolics in the aerial parts of Trifolium species. J Agric Food Chem. 2007;55:8095–100. doi: 10.1021/jf072024w. [DOI] [PubMed] [Google Scholar]
- 27.Seguin P, Zheng W, Souleimanov A. Alfalfa phytoestrogen content: Impact of plant maturity and herbage components. J Agron Crop Sci. 2004;190:211–7. [Google Scholar]
- 28.Tsao R, Papadopoulos Y, Yang R, Young JC, McRae K. Isoflavone profiles of red clovers and their distribution in different parts harvested at different growing stages. J Agric Food Chem. 2006;54:5797–805. doi: 10.1021/jf0614589. [DOI] [PubMed] [Google Scholar]
- 29.Ramos P, Dias PM, Morais CB, Froehlich PE, Dall’Agnol M, Zuanazzi JA. LC Determination of four isoflavone aglycones in red clover (Trifolium pratense L.) Chromatographia. 2008;67:125–9. [Google Scholar]
- 30.Saviranta NM, Anttonen MJ, von Wright A, Karjalainen RO. Red clover (Trifolium pratense L.) isoflavones: Determination of concentrations by plant stage, flower colour, plant part and cultivar. J Sci Food Agric. 2008;88:125–32. [Google Scholar]
- 31.Sivesind E, Seguin P. Effects of the environment, cultivar, maturity, and preservation method on red clover isoflavone concentration. J Agric Food Chem. 2005;53:6397–402. doi: 10.1021/jf0507487. [DOI] [PubMed] [Google Scholar]
- 32.Vilčinskas E, Dabkevičienė G. Yield structure and dry matter qualitative characteristics of clover (Trifolium spp.) species. Žemės ūkio mokslai. 2010;17:18–24. [Google Scholar]
- 33.Vilčinskas E, Dabkevičienė G. Qualitative and quantitative characteristics of clover (Trifolium spp.) species in the first year of growing. Zemdirbyste-Agriculture. 2009;96:170–80. [Google Scholar]
- 34.Graham TL. A rapid, high resolution high performance liquid chromatography profiling procedure for plant and microbial aromatic secondary metabolites. Plant Physiol. 1991;95:584–93. doi: 10.1104/pp.95.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]


