Abstract
Objective:
The present study examined the pharmacokinetic profiles of two iridoid glycosides named morroniside and loganin in rat plasma after oral administration of crude and processed Cornus officinals.
Materials and Methods:
A rapid, selective and specific high-performance liquid chromatography/electrospray ionization tandem mass spectrometry with multiple reactions monitoring mode was developed to simultaneously investigate the pharmacokinetic profiles of morroniside and loganin in rat plasma after oral administration of crude C. officinals and its jiuzhipin.
Results:
The morroniside and loganin in crude and processed C. officinals could be simultaneously determined within 7.4 min. Linear calibration curves were obtained over the concentration ranges of 45.45-4800 ng/mL for all the analytes. The intra-and inter-day precisions relative standard deviation was lesser than 2.84% and 4.12%, respectively.
Conclusion:
The pharmacokinetic parameters of two iridoid glucosides were also compared systematically between crude and processed C. officinals. This paper provides the theoretical proofs for further explaining the processing mechanism of Traditional Chinese Medicines.
Keywords: High performance liquid chromatography-triple quadrupole mass spectrometry, morroniside and loganin, multiple reactions monitoring mode, pharmacokinetics, rat plasma
INTRODUCTION
Cornus officinals, a famous Chinese herbal medicine, is derived from the dry ripe sarcocarp of C. officinalis Sieb. et Zucc (Cornaceae) and recorded in Chinese Pharmacopoeia, which is a classic resource on Traditional Chinese Medicine (TCM).[1] Owing to its biological and pharmacological activities such as anti-inflammation, anti-virus and anti-oxidation, C. officinals has increasingly drawn much attentions as one of the most popular and cherished herbal medicine in the clinic in the world and can be used for medicine, hygienic food and cosmetic.[2,3]
The crude TCM and its processed product-jiuzhipin (JZP) are often used clinically. Appropriate pharmaceutical processing may reduce toxicity or side-effects, potentiate the beneficial effects, change the pharmacological properties, preserve active constituents, facilitate administration, improve flavor or eliminate unpleasant taste and increase purity of Chinese medicine.[4,5] In China, the processing methods for crude TCM have been practiced since the Tang dynasty and are documented in Chinese pharmacopoeia.[6] Previous studies showed that the crude C. officinals is better in astringing yin for arresting sweating, which has been used intensively for the treatment of spontaneous sweating, night sweating, spermatorrhea and enuresis.[7,8] The typical example is C. officinals powder for treating kidney deficiency and frequent micturition incontinence. C. officinals after stewed with yellow rice wine is stronger in nourishing kidney, astringing semen and reducing urination, which has been used diffusely for curing dizziness, coldness and pain in waist, frequent micturition, enuresis, impotence and prospermia. Simultaneously, C. officinals warmly dredges up by dint of wine and its acidity is reduced.[9,10]
A previous report revealed that the 39 compounds in crude and JZP and 10 compounds in rat plasma after oral administration of C. officinals were detected.[11] It provides a helpful chemical proof for further pharmacology and active mechanism research. However, biological studies with C. officinals have mainly been focused on the pharmacokinetics of one or a few bioactive components.[12] This approach offers limited information toward studying the pharmacology of C. officinals since the pharmacokinetic properties of each chemical constituent have not been taken into account. Until now, most investigations have been based on the pharmacodynamics or pharmacology of crude C. officinals and very little attention has been devoted to the pharmacokinetic study of multiple components of processed C. officinals in vivo.
Iridoid glycosides are the major active components widely distributed in crude C. officinals and its processed products. Exploring dynamic of iridoid glycosides in dialysate from C. officinals and its processed products may help to explain why crude and processed C. officinals have traditionally been used for treating different clinical symptoms and to expatiate upon the processing mechanism.[13] To the best of our knowledge, several high-performance liquid chromatography (HPLC)/ultraviolet methods have been applied to the quantification of single morroniside or loganin from crude C. officinals, but not from its processed products.[14] However, these methods cannot be used to study the pharmacokinetics of multiple absorbed components, due to the fact that the plasma sample used for testing at each time point cannot exceed 10% of the total plasma volume of an animal if multiple time points need to be measured. Thus, it is necessary to develop a more comprehensive and global assay to fully evaluate the pharmacokinetics of iridoid glycosides from crude and processed C. officinals.
Therefore, we hypothesized that two iridoid glucosides named morroniside and loganin in JZP may exhibit pharmacokinetic properties presenting with increased Cmax and delayed T1/2 following oral administration, which appear to be beneficial for the pharmacological activities. In the present study, a rapid, selective and specific HPLC- electrospray ionization tandem mass spectrometry (ESI-MS/MS) with multiple reactions monitoring (MRM) mode was firstly developed to simultaneously investigate the pharmacokinetic profiles of morroniside and loganin in vivo and to screen potentially bioactive components in rat plasma after oral administration of crude C. officinals and its JZP. The pharmacokinetic behaviors of two iridoid glucosides were also compared systematically between the crude and processed C. officinals. This paper provides the theoretical proofs for further explaining the processing mechanism of TCMs.
EXPERIMENTAL
Materials and reagents
The crude and processed forms of C. officinals and its JZP were collected from Henan suppliers. Loganin was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Morroniside was obtained from Shanghai Shangyi Biotechnology Co. Ltd., (Shanghai, China). The purity of each standard compound was greater than 98% by HPLC analysis. The structures of these two compounds are shown in Figure 1. HPLC-grade acetonitrile and methanol was obtained from Merck (Darmstadt, Germany) and Fisher Scientific Corporation (Loughborough, UK), respectively. Deionized water was purified using the Milli-Q system (Millipore, Bedford, MA, USA) and HPLC grade formic acid was purchased from Honeywell Company (Morristown, New Jersey, USA). All other chemicals were of analytical grade and commercially available.
Figure 1.
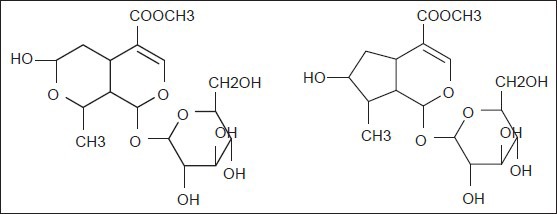
The chemical structures of two iridoid glucosides in Fructus corni
Instrument and analytical conditions
Chromatographic experiments were performed on an Agilent 1200 series HPLC system (Santa Clara, USA) with the mobile phase consisting of (A) methanol acetic acid (0.1%, v/v) and (B) aqueous acetic acid (0.1%, v/v) using a gradient elution of 10-90% B at 0-10 min. An Agilent Zorbax Extend C18 (100 mm × 3.0 mm, 3.5 μm) was employed with a flow rate of 0.6 mL/min. The column temperature was set at 40°C and the injection volume was 5 μL.
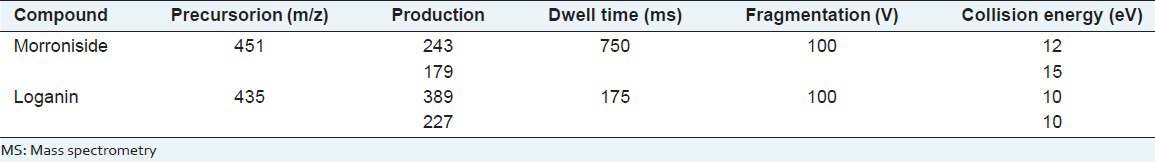
Determination was performed using an Agilent Technologies 6410 Triple Quad liquid chromatography/MS equipped with electrospray ionization (ESI). The compounds were ionized in the positive and negative ion polarity mode. The ionization source conditions were as follows: Spray voltage of 4000 V (+), 3600 V (−), source temperature of 100°C and desolvation temperature of 350ΊC. Nitrogen was used as nebulizer gas and pressure was set at 40 p.s.i at a flow rate of 10 L/min. The pressure of high purity nitrogen was 0.15 MPa for collision-induced dissociation (CID). Quantification was operated at MRM modes. The delta potential of the electron multiplier (EMV) was set to 400 V for data acquisition. The summary of MS/MS detection parameters is shown in Table 1. Data acquisition and processing were performed by Agilent Mass Hunter Workstation.
Table 1.
MS/MS detection parameters for morroniside and loganin in Fructus corni
Preparation of sample solutions
The 100 g powder of C. officinals and its JZP samples were transferred to the round-bottom flasks with ten-fold volumes of distilled water for about 2 h, respectively. A few grains of pumice were added and then boiled on a water-bath under a reflux condenser for about 2 h. After cooling to the room temperature, the sample solutions were filtered and the filtrates were collected. The solid residues were treated again as described above (for 1 h). The collected filtrates were mixed and evaporated to a final volume of 50 mL under reduced pressure at a temperature not exceeding 60°C. The extracted solutions were filtered through 5 layer gauzes and concentrated to approximately 2 g crude drug per milliliter (this concentration of the solution was used for oral administration) and finally the solutions were lyophilized.
Animals and blood sampling
Sprague-Dawley rats (8 weeks old, 200 ± 20 g) were obtained from the Laboratory Animal Center of Zhejiang Chinese Medical University (Zhejiang, China). The experimental protocol was approved by the University Ethics Committee for the use of experimental animals and conformed to the Guide for Care and Use of Laboratory Animals. Rats were housed in groups of two or three with 12 h light/12 h dark cycle at a temperature of 22°C ± 1°C, relative humidity of 60% ±10%, for 1 week. Immediately before the day of administration, the rats were fasted for 12 h, but were allowed water ad libitum. Then, aqueous solutions of crude and processed C. officinals extracts were administered to the rats at a dose of 1.0 mL/100 g body weight, respectively. The rats were anesthetized by inhalation of diethyl ether after administration. At 5, 10, 15, 30, 45, 60, 90, 120, 180, 240 and 360 min following oral administration, the blood samples were collected from the caudal vein into heparinized tubes and centrifuged at 10,000 rpm for 15 min to separate plasma and stored at −80°C until analysis.
A simple liquid-liquid extraction method was applied to extract two iridoid glucosides from rat plasma. Briefly, to 200 μL of the plasma sample, 15 μL of methanol was added (volume of the corresponding working solution for calibration curve and quality control [QC] samples). Then, the mixture was vortexed for 1 min and extracted with 2 mL ethyl acetate by vortex-mixing for 5 min. The upper layer was transferred to a clean tube after centrifugation at 12,000 rpm for 10 min. The organic phase was evaporated to dryness under a gentle stream of nitrogen gas. The obtained residue was reconstituted in 50 μL methanol and centrifuged at 12,000 rpm for another 5 min. The above operations were carried out at room temperature. And then, an aliquot of 5 μL supernatant was injected into HPLC-MS system for analysis.
Pharmacokinetic analysis
The data of plasma concentration versus time was subjected to a non-compartmental pharmacokinetic analysis using Kinetica 5.0 (Thermo Electron Corp., Philadelphia, PA) to obtain an estimate of various pharmacokinetic parameters. Maximum plasma concentration (Cmax) and time of maximum concentration (Tmax) were obtained directly from the plasma concentration-time plots. The elimination rate constants (k) were determined by linear regression on the logarithmic transformation of the last four data points of the curve. The elimination half-life (T1/2) was calculated by the following equation: T1/2 = 0.693/k. The area under the plasma concentration versus time curve up to the last time (t) (AUC0−t) was determined using the trapezoidal rule. The AUC0−∞ values were calculated by adding the value of Ct × k−1 to AUC0−t.
RESULTS AND DISCUSSION
Method validation
Selectivity
The specificity of the method was presented by comparing MRM chromatograms of morroniside and loganin from crude and processed C. officinals for a blank rat plasma sample, a spiked plasma sample and a plasma sample from a rat 3 h after oral administration of crude and processed C. officinals extracts. The morroniside and loganin were eluted at 6.0 min and 7 min approximately. As shown in Figure 2, no interfering peaks were found at the retention time of morroniside and loganin. Two bioactive compounds were confirmed by the mass spectral analyses [Figure 3].
Figure 2.
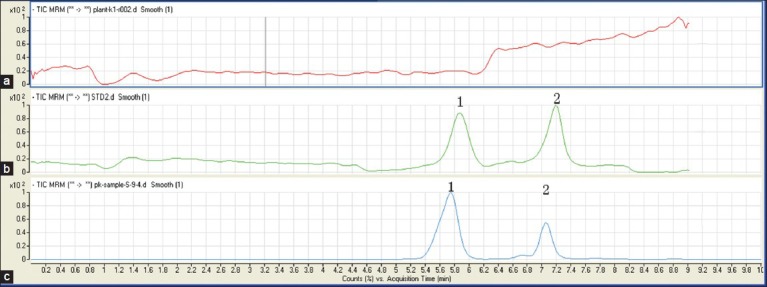
Liquid chromatography/mass spectrometry chromatographic profiles of Fructus corni samples. (a) Blank plasma; (b) blank plasma spiked with morroniside and logain; (c) plasma samples after oral administration Fructus Corni extracts; (1) morroniside; (2) logain
Figure 3.
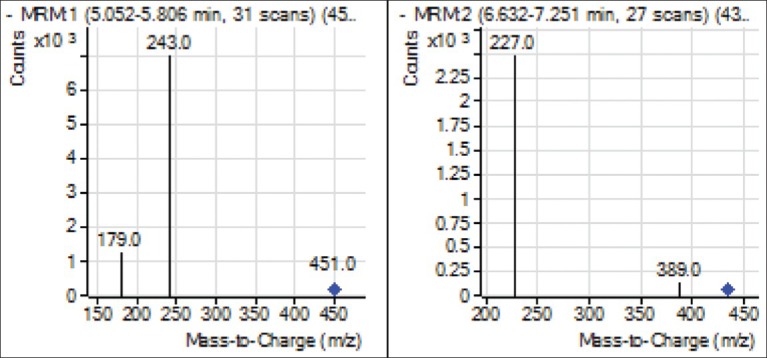
Mass spectra of two bioactive compounds. Each compound was collected from high-performance liquid chromatography and analyzed by mass spectra
Linearity of the calibration curve and lower limit of quantification (LLOQ)
The standard curves of the peak area (Y) to the concentration (C) were constructed using 1/x2 weighted linear least-squares regression model. The standard curves, correlation coefficients and linear ranges of morroniside and loganin in plasma were y = 0.6433 × -24.602 (45.45-2272.72 ng/mL) and y = 1.3161 × -51.830 (96.00-4800 ng/mL). All the marker substances showed good linearity (r2 ≥ 0.9994). LLOQ of the two analytes were 45.45-96.00 ng/mL.
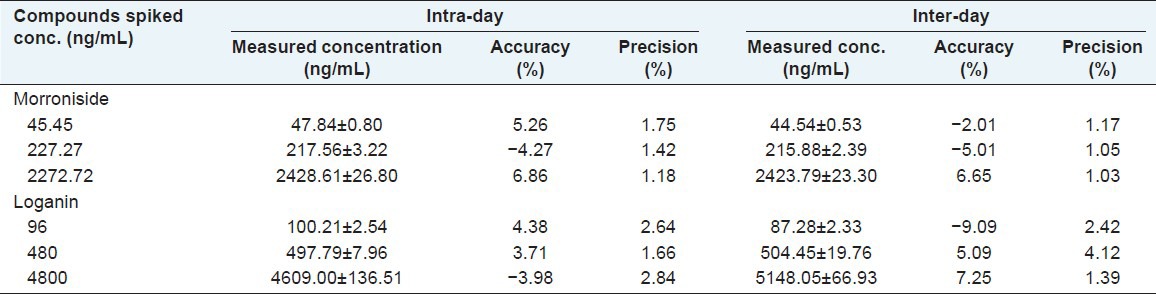
Accuracy and precision
Accuracy, intra- and inter-day precisions were evaluated from the results of QC samples. Six replicates of QC samples at three concentration levels were determined on three different days. The mean values and relative standard deviation (RSD) for QC samples were calculated over three validation days. The intra- and inter-day precisions were expressed by RSD. Table 2 summarizes the intra- and inter-day precisions and accuracies of morroniside and loganin at three concentration levels (low, middle and high). The intra- and inter-day precisions (RSD) of these analytes were all lesser than 2.84% and 4.12%. These results were within the acceptance criteria and indicated that the method was accurate, reliable and reproducible.
Table 2.
The intra- and inter-day accuracy and precisions of morroniside and loganin in rat plasma at low, medium, and high concentration levels
Recovery
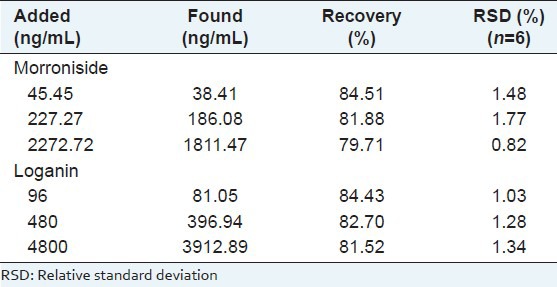
The extraction recovery of analytes at three QC levels was conducted by calculating at each standard concentration as the ratio of the peak area for extracted blank plasma spiked before extraction relative to peak area of the equivalent blank plasma samples spiked after the extraction.
The recoveries of morroniside and loganin from rat plasma are shown in Table 3. The mean recoveries of morroniside and loganin were more than 79% at three concentration levels (low, medium and high). This result indicated that the efficiency was acceptable and the method was accurate.
Table 3.
Recoveries of morroniside and loganin at three different spiked level in rat plasma samples
Stability
QC samples of morroniside and loganin at three concentrations were used for stability experiments. The stabilities of morroniside and loganin were tested under different conditions. It was indicated that this new method for the simultaneous determination of morroniside and loganin in rat plasma offered satisfactory stability, with accuracy in the range between 7.48% and 14.82%.
Application to pharmacokinetic study
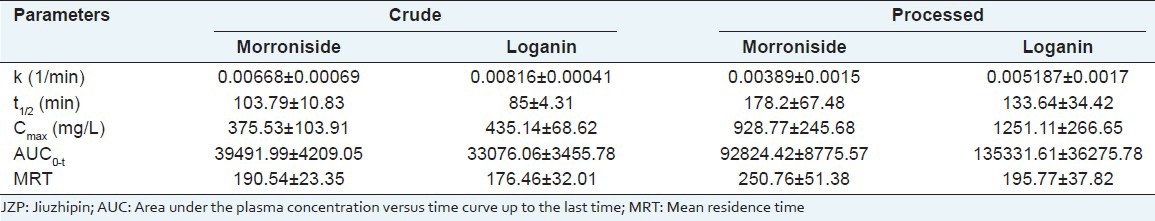
This HPLC-MS/MS method was developed and used successfully in the pharmacokinetic studies in rats. The mean plasma concentration-time curves of morroniside and loganin in rats after oral administration of crude C. officinals and its JZP extracts are shown in Figures 4 and 5. Their pharmacokinetic parameters are listed in Table 4. In general, morroniside and loganin could be absorbed and eliminated rapidly in rats, for it was detected in plasma at 5 min. As shown in Figures 4 and 5, the plasma levels of morroniside and loganin after administration of JZP extracts were much higher than those after oral administration of crude extracts. The T1/2 of morroniside and loganin after used with JZP extracts were longer than that after used with crude extracts. The AUC0−t and Cmax of morroniside and loganin after administration of JZP were significantly higher than those after administration of crude product. The information described above might be helpful for further studies on the pharmacokinetics of C. officinals and beneficial for application of this TCM in clinical therapy.
Figure 4.
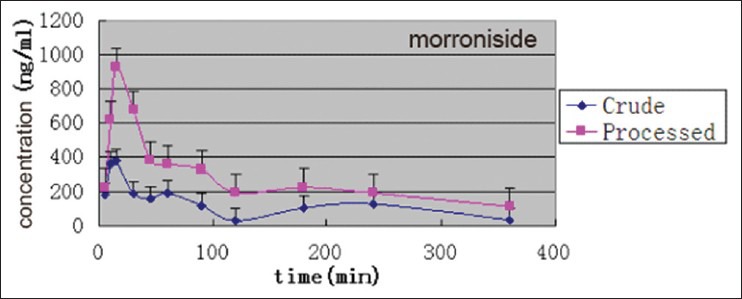
Mean plasma concentration-time curves of morroniside after oral administration of crude Fructus corni extracts to rats (n = 6)
Figure 5.
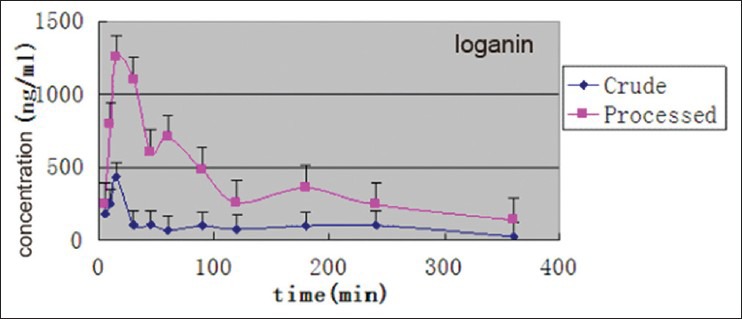
Mean plasma concentration-time curves of logain after oral administration of processed Fructus corni extracts to rats (n = 6)
Table 4.
Mean pharmacokinetic parameters of morroniside and loganin in rat plasma after oral administration of crude processed Cornus officinals and JZP extracts
CONCLUSION
A novel HPLC-ESI-MS/MS with MRM mode was firstly developed and validated for comparison of pharmacokinetics of two iridoid glucosides in rat plasma. The analytical procedure was then successfully applied to a pharmacokinetic study of the analytes after oral administration of crude C. officinals and its jiuzhipin and it could help to investigate the processing mechanism of C. officinals. To the best of our knowledge, this paper is the first study reporting the comparison of pharmacokinetics of bioactive compounds after oral administration of Chinese herbal medicine extracts to rats. The described novel method has high sensitivity and specificity and would offer a good alternative for simultaneous analysis of the process of absorbing multi-components into the body after administration of Chinese herbal medicine.
ACKNOWLEDGMENTS
The authors are grateful to the financial support of National Natural Science Foundation of China (No. 81202918), the Project of Science and Technology for Chinese Medicine of Zhejiang province, China (No. 2013KYB183), International Science and Technology Cooperation Project of Jiangsu Province (No. BZ2011053), Science and Technology of Chinese Medicine of Zhejiang Province (No. 2009CB008), Zhejiang Province Chinese Medicine Research Program (2011ZB101), the International Science and Technology Cooperation Project of Zhejiang Province, China (No. 2012D60SA1C0065 and No. 2012D60SA1C0066), the Chinese Medicine Research Program of Zhejiang Province, China (No. 2008ZA002), the Science Foundation of Zhejiang Chinese Medical University (No. 2011ZY25, 7211093) and the Fund of Zhejiang Modernization of Traditional Chinese Medicine Item (No.[2008] 436 and[2012] 680).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cao G, Zhang C, Zhang Y, Cong X, Cai H, Cai B, et al. Global detection and identification of components from crude and processed traditional Chinese medicine by liquid chromatography connected with hybrid ion trap and time-of-flight-mass spectrometry. J Sep Sci. 2011;34:1845–52. doi: 10.1002/jssc.201100211. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Sun A, Wu S, Liu R. Preparative purification of morroniside and loganin from Fructus corni by combination of macroporous absorption resin and HSCCC. J Chromatogr Sci. 2009;47:333–6. [PubMed] [Google Scholar]
- 3.Yokozawa T, Park CH, Noh JS, Tanaka T, Cho EJ. Novel action of 7-O-galloyl-D-sedoheptulose isolated from Corni fructus as a hypertriglyceridaemic agent. J Pharm Pharmacol. 2009;61:653–61. doi: 10.1211/jpp/61.05.0015. [DOI] [PubMed] [Google Scholar]
- 4.Cao G, Cai H, Zhang Y, Cong X, Zhang C, Cai B. Identification of metabolites of crude and processed Fructus corni in rats by microdialysis sampling coupled with electrospray ionization linear quadrupole ion trap mass spectrometry. J Pharm Biomed Anal. 2011;56:118–25. doi: 10.1016/j.jpba.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhou LL, Wu GG, Liu ZQ, Liu SY. Studies on the components of crude and processed Fructus corni by ESI-MSn. Chem Res Chin Univ. 2008;24:270–4. [Google Scholar]
- 6.Li FM, Xiong ZL, Lu XM, Qin F, Li XQ. Strategy of quality control for traditional Chinese medicines and chromatographic technology. Chin J Chromatogr. 2006;6:537–44. [PubMed] [Google Scholar]
- 7.Fu ZQ, Wang MY, Cai BC. Discussion of 5-hydroxymethyl furfural in Chinese native medicine research present situation. Chin Arch Tradit Chin Med. 2008;26:508–10. [Google Scholar]
- 8.Gao XM. Beijing. People Sanitation Press; 2000. Handbook of Traditional Chinese Medicine; p. 1914. [Google Scholar]
- 9.Ding X, Wang MY, Yu ZL, Hu W, Cai BC. Studies on separation, appraisal and the biological activity of 5-HMF in Cornus officinalis. Chin. J. Chin Mater Med. 2008;33:392–6. 484. [PubMed] [Google Scholar]
- 10.Cao G, Zhang Y, Cai H, Cong XD, Cai BC. Research progress on the chemical constituents and pharmacological activities of Fructus corni. J Chin Pharm Sci. 2009;18:208–13. [Google Scholar]
- 11.Xu HQ, Hao HP, Zhang X, Pan Y. Morroniside protects cultured human umbilical vein endothelial cells from damage by high ambient glucose. Acta Pharmacol Sin. 2004;25:412–5. [PubMed] [Google Scholar]
- 12.Cao G, Shan Q, Zhang C, Zhang Y, Cai H, Cong X, et al. Pharmacokinetic parameters of morroniside in iridoid glycosides of Fructus corni processing based on back-propagation neural network. Pharm Biol. 2011;49:989–93. doi: 10.3109/13880209.2010.551780. [DOI] [PubMed] [Google Scholar]
- 13.Du W, Cai H, Wang M, Ding X, Yang H, Cai B. Simultaneous determination of six active components in crude and processed Fructus corni by high performance liquid chromatography. J Pharm Biomed Anal. 2008;48:194–7. doi: 10.1016/j.jpba.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Wang Q, Zhang X, Sheng X, Zhou Y, Li M, et al. HPLC study of pharmacokinetics and tissue distribution of morroniside in rats. J Pharm Biomed Anal. 2007;45:349–55. doi: 10.1016/j.jpba.2007.05.013. [DOI] [PubMed] [Google Scholar]


