Abstract
Background:
Farmers have applied Cynanchum mongolicum (Maxim) to control crop pests. The aim of this study was to analyze composition of essential oil from C. mongolicum, evaluate insecticidal activities against Aphis glycines, and lethal doses.
Materials and Methods:
Essential oil from C. mongolicum was efficiently extracted by steam distillation. The main components of the oil were analyzed with a gas chromatography/mass spectrometry (GC/MS) system, and the insecticidal activity of the essential oil on soybean aphids Aphis glycines was tested using a variety of methods.
Results:
The components of the essential oil from C. mongolicum mainly included terpenes and ester compounds, of which (Z)-3-Hexen-1-ol acetate, cis-3-hexenyl isovalerate, α-farnesene, and β-caryophyllene accounted for 15.8, 10.4, 8.4, and 5.5%, respectively. With 1- and 2-day exposure, the essential oil showed pronounced contact toxicity (median lethal concentration (LC50) =37.8 and 38.4 μL/mL, respectively), weak fumigant toxicity (LC50 = 139.7 and 139.9 μL/L, respectively). The essential oil showed strong deterrent activity on soybean aphids in 2 and 4 h.
Conclusion:
The essential oil of C. mongolicum contained insecticidal components and possessed contact toxicity and deterrent activity to A. glycines.
Keywords: Aphis glycines, Cynanchum mongolicum, essential oils, insecticidal activity
INTRODUCTION
Pests including weeds, pathogens, and insects are a substantial competitor of agricultural crops and often cause crop production losses of 25-50%.[1] For this reason, large amounts of broad-spectrum synthetic insecticides have been applied to protect crops, these insecticides have resulted in the development of resistant insect populations.[2] As a result, many people have been considering the use of natural pesticides. Natural substances are an alternative to conventional pesticides and plant essential oils have traditionally been used to kill or repel insects.[3] Natural plant products, including essential oils, hold good promise in pest and disease control.[1]
Essential oils are effective against insect pests with varying potencies, acting as toxins, growth inhibitors, development disruptors, deterrents, or repellents. Phytophagous insects commonly find their host plants by plant volatiles so that using nonhost essential oils to repel insect pests is a potential alternative for pest management. Behavioral effects and sensory detection of drimane deterrents in Myzus persicae and Aphis gossypii nymphs have been demonstrated.[4]
The soybean aphid A. glycines (Homoptera: Aphidiae) is an important agricultural pest that feeds on a range of leguminous plants, including many agricultural crops in large parts of China, and this aphid acts as a vector for more than 30 plant virus diseases.[5] The species is frequently used as a model organism for other piercing-sucking insects. Control of A. glycines is challenging because the long-term use of synthetic broad-spectrum insecticides has led to the widespread development of resistance.[6] Environment friendly insecticides must be found as alternatives of synthetic chemical insecticides for controlling the aphids, of which botanical insecticides are a promising alternative.[7]
Cynanchum mongolicum (Maxim) is an herb found in rangelands in several parts of the world, including Northern China. Chinese farmers have applied C. mongolicum to control crop pests and Tibetan doctors have used its seeds for treating fever, diarrhea, and cholecystitis.[8] However, the study on essential oil from rhizome of C. mongolicum by means of gas chromatography or mass spectrometry (GC/MS) technology has not been reported until now. Research on bioactivity and components of plant-derived pesticides has been an important step towards developing botanical insecticides and environment friendly control techniques for aphids. Hence, a study on composition of essential oil of C. mongolicum and insecticidal activities to A. glycines was considered worthwhile. These results will form a basis of further research on insecticidal mechanisms of essential oil of C. mongolicum.
MATERIALS AND METHODS
Plant materials
Cynanchum mongolicum were collected from the Inner Mongolia Erdos prairie in September 2009. The plant was identified by Prof. Qingru Wu (Department of Biology, Inner Mongolia Polytechnic University, Huhehaote) and a voucher specimen (NO: 09009) was lodged in the laboratory of the Inner Mongolia Polytechnic University.
Insects
Wingless soybean aphid adults A. glycines tested in the study came from a laboratory colony originating from stock obtained from the Key Laboratory for Biocontrol of the Chinese Ministry of Agriculture in Beijing that was initially established with A. glycines collected in Haidian, Beijing during 2003. The aphids were reared on cultivated soybeans in an incubator (RXZ-430B, Ningbo Equipment Factory) at 27 ± 1°C, under a light-dark cycle of 14:10 h, and 60-70% relative humidity (RH). The soybean aphids without wings were selected for experiments testing biological activity of the essential oil. The study protocol was approved by the Institutional Animal Ethical Committee (Approval No. UICT/PH/IAEC/0405/8).
Extraction and composition of essential oil
The plant's essential oils were extracted by steam distillation method of Bruce et al.[9] Plant rhizomes were cleaned and dried in an outdoor area under full shade. Dried plants were finely ground (HY-04B Beijing Xinhuanya Technol. Ltd.) and sieved through a 40-mesh sieve, and the resulting powder kept in a sealed container until use. Fifty grams of the crushed plant rhizome powder was put into 300 mL of distilled water (solid-liquid ratio = 1:6) in a 500 mL round bottom flasks and extracted by water vapor extraction distillation for 3 h using a Clevenger-type apparatus. The extracted solution was then further extracted three times by ethyl ether (l:l V/V) and the extracted solutions were dried by anhydrous sodium sulfate, then filtered, and concentrated with a rotary evaporator (RE52-98, Shanghai Yarong Biochemical Instrument Factory) at a temperature of 40°C. A total of 1.39 g of yellow essential oil was collected: The oil was easily recognized by its characteristic odor. The essential oil was stored at 4°C in a refrigerator until used in tests.
GC-MS analysis was performed using an Agilent 7890 GC equipped with 5,975 N mass selective detectors and an HP-5 (5% phenyl methylpolysiloxane) capillary column. The oven temperature was programmed to increase from 35 to 280°C at the rate of 4°C/min and kept at the highest temperature for 5 min. The inlet and interface temperatures were 250 and 280°C, respectively. The carrier gas was helium at a flow rate of 1.0 mL/min (constant flow). The sample (0.2 μL) was injected with a split of 20:1. Electron impact mass spectrometry was carried out at 70 eV, while ion source and quadrupole temperatures were maintained at 250 and 150°C, respectively. Scan area ranged from 30 to 350 Da. The volatile material was injected and analyzed through GC-MS. Each chromatographic peak was retrieved and its retention time compared to known compounds from previous literature as stored in a computer based NIST08 standard mass spectrometry atlas. The relative amounts of each essential oil compound were calculated using the peak area normalization method.
Contact toxic activity test
Test solutions diluted with acetone were prepared at the following concentrations: 3.125, 6.25, 12.5, 25.0, and 50.0 μL/mL; with acetone alone used as a negative control and pyrethrum extract as a positive control. The toxicity of the oils was studied by the leaf dipping method, the wax on the surface of soybean leaf was scraped off with knife, and then a 2 × 3 cm rectangle was punched out of the leaf.[4] Soybean leaves were then dipped in the selected solutions for 3 s and then air-dried. Twenty-five adult aphids were gently dislodged from the undersides of leaves of plants in the rearing colony and then put on the treated leaves in Petri dishes with a brush; sick or molting aphids were not transferred or were immediately removed from dishes upon discovery. Each test was replicated three times, with mortality recorded after 24 and 48 h. A toxic regression equation was calculated and a (median lethal concentration) LC50 obtained using probit analysis. Aphids were considered alive if their appendages moved or flinched, including tremors induced by the test solution. Otherwise, aphids were considered to have died, which was easy to confirm by gently prodding them with an insect pin and watching for any postmortem color change. The percentage of mortality was corrected for control mortality using Abbott's formula.
Fumigant activity test
Test concentrations were as follows: 6.25, 12.5, 25.0, 50.0, and 100.0 μL/L air. A pad (1 × 3 cm) of Whatman No. 1 filter paper was dipped into one of the solutions for 3 s, air dried, and then hung up within a 500 mL glass bottle. Twenty-five aphids were put into the glass bottle, which was then sealed with an airtight lid. Control insects were kept in bottle with pad of filter paper treated with acetone. Each treatment was replicated three times. The bottles were put in an incubator and the numbers of living and dead aphids were counted in each glass bottle after 24 and 48 h fumigation. The percentage mortality was corrected for mortality in controls using Abbott's formula.[10]
Deterrent bioassay
The filter paper (9 cm in diameter) was cut in half and one half was dipped into acetone as a control, while the other was dipped for 3 s into one the following solutions: 0.625, 1.25, 2.5, 5.0, and 10.0 μL/mL. Both control and treated papers were air-dried, and then put into a Petri dish, into which 25 aphids were placed using a brush. The Petri dish was wrapped with parafilm to prevent aphids escaping. Each treatment was performed with three replicates. The aphids were placed in an incubator and the number of aphids on control and treated papers were counted after 2, 4, and 24 h. A deterrence index was calculated for each dish as follows: (C - T)/(C + T), where C is the number of aphids on the control paper and T is the number of aphids on the treated paper.
Statistical analysis
One-way analysis of variance (ANOVA) and least significant difference (LSD) multiple range tests were performed on the data to determine significant (P < 0.05) differences among concentrations. Percent mortalities were transformed into arcsine square root values for analysis. The average larval mortality data were subjected to probit analysis for calculating LC50 and other statistics at 95% confidence limits of upper and lower confidence limit (SPSS 11.5).
RESULTS
Yield and components of essential oils
The yield expressed of essential oil from the hydrodistillation of C. mongolicum (Maxim) was 2.79% on a dry weight basis. A total of 15 compounds were identified in the oil from the rhizomes of C. mongolicum (Maxim) [Figure 1]. The identified compounds represent 97.6% of the essential oils collected. The main constituents included terpenes and ester compounds, of which (Z)-3-hexen-1-ol, acetate (15.8%), cis-3-hexenyl isovalerate (10.4%), α-farnesene (8.4%), and β-caryophyllene (5.5%) were in higher concentration.
Figure 1.
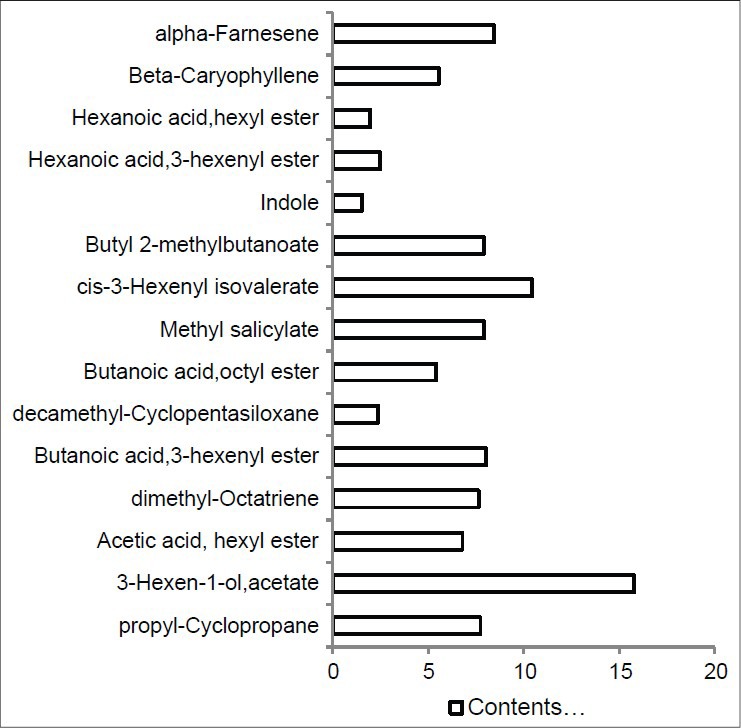
Composition of essential oils from Cynanchum mongolicum (Maxim)
Contact toxic activity
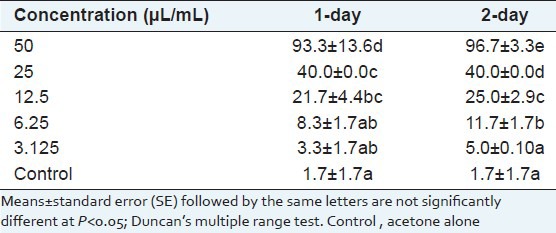
Essential oil of C. mongolicum (Maxim) and pyrethrum extract had a substantial contact toxic effect on A. glycines. At higher doses of essential oils, aphid mortality was high after 24 h, with little further mortality on the second day; 48 h after treatment with the essential oil, the aphid mortalities increased significantly with the test concentrations of the essential oil [Table 1]. The regression analysis between concentration and mortality demonstrated correlation coefficients >0.90. With 1- and 2-day exposure, the respective LC50 values were 37.8 and 38.4 μL/mL, respectively.
Table 1.
Percent mortality of Aphis glycines; 1- or 2-day after topical application of essential oils from Cynanchum mongolicum
Fumigant activity
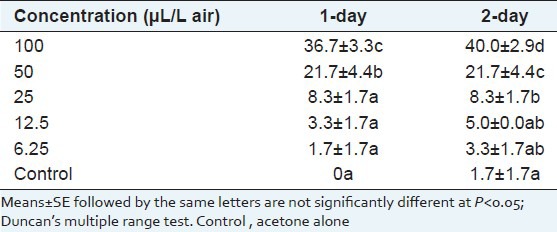
Cynanchum mongolicum oils showed a weak fumigant activity against A. glycines at the test concentrations. At the higher dose 100 μL/L, the oils caused less than 50% mortality after 24 and 48 h of exposure. But the activities of the oil at the above 50 μL/L doses were significantly higher than that of oil at low doses, the mortalities showed positive relationship with the concentration of essential oil within 48 h after treatment, the regression analysis between concentration and mortality showed those correlation coefficients above 0.98. With 1- and 2-day exposure, the respective LC50 values were 139.7 and 139.9 μL/L, respectively [Table 2].
Table 2.
Percent mortality of Aphis glycines after 1- or 2-day exposure to various aerial concentrations of essential oils from Cynanchum mongolicum
Deterrent activity
Aphis glycine was deterred significantly by the essential oil from C. mongolicum (Maxim) under choice conditions. The deterrence index was high at even low concentrations after exposure for 2 or 4 h and was highest at a concentration of 10.0 μL/L after exposure for 4 h [Figure 2].
Figure 2.
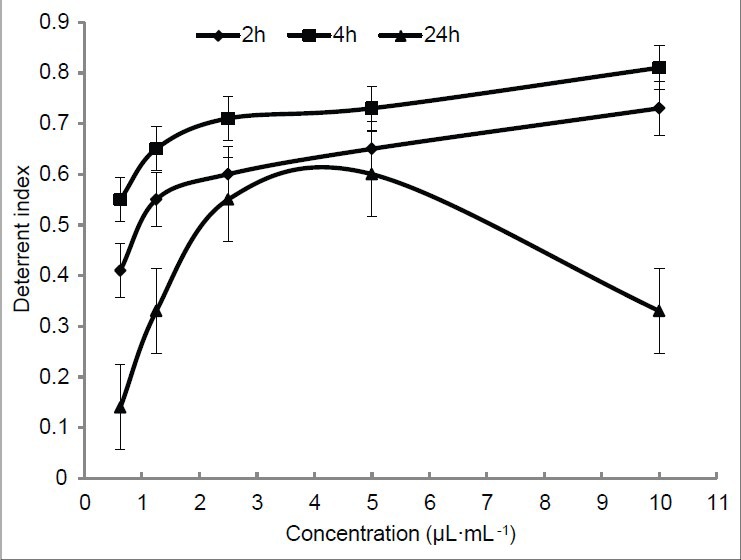
Deterrent activity of essential oils from Cynanchum mongolicum (Maxim) to Aphis glycine under choice conditions
DISCUSSION
The value of potential insecticidal components of plants depends on a combination of high yield in extraction or production of the components and their high virulence against target pests. The different components showed different modes of action. (S)-germacrene D from Nepeta ucrainica has been shown to be a potent arthropod repellent, effective against aphids.[9] Essential oils of cumin (Cuminum cyminum), anise (Pimpinella ansium), oregano (Origanum syriacum var. bevanii), and eucalyptus (Eucalyptus camaldulensis) were effective as fumigants against the cotton aphid (A. gossypii).[11] Similar activity of some essential oil-based insecticides (a proprietary mixture of eugenol, thymol, and phenethylpropionate as active ingredients) against aphids has been reported.[3] Several secondary metabolites of plants, especially volatile substances had also been explored to develop repellency against aphids.[12] However, most of these studies have been restricted to laboratory experiments. Volatile semiochemicals are used as olfactory cues for insects to recognize not only their hosts, but also shelter and oviposition sites.[13]
The essential oils from C. mongolicum (Maxim) were analyzed through GC-MS. These compounds were identified in essential oils of this plant and these compounds accounted for 97.6% of the volatile components. β-caryophyllene had significant effects on A. gossypii Glover including contact toxicity and deterrence activity, the compound also significantly reduced the fertility of this species and decreased the frequency and weight of honey dew excreted.[14] (E, E)-α-farnesene had attraction effects on the larvae of Cydia pomonella (L.), but its effects on the beetles depended on the concentration of the substance.[15] The insecticidal ingredients β-caryophyllene and α-farnesene contained in the essential oil from C. mongolicum accounted for 13.98% of the volatile components. The main substances from the extract of Cynanchum komarovii included C-21 steroidal glycoside, alkaloids, terpenes, flavonoids, polysaccharide, fatty acid, phospholipids, amino acids, vitamins, and showed insecticidal activities;[16] the essential oil from the herb possibly contains similar composition and activities to that of C. mongolicum.
The results here showed that essential oils from C. mongolicum are very promising aphidicides because of their high deterrent and insecticidal activities. This combination of activities are likely a result of essential oils being complex mixtures of various volatile metabolites, some of which are deterrents while others cause direct mortality. Future research should be focused on both, laboratory studies on the mechanism of botanical essential oils controlling the pests as well as field trials to see what effects are evident in the field as part of determining what role such compounds might have in integrated pest management.
ACKNOWLEDGMENTS
This research was supported by a grant from “the National Li Chanye Jishu Tixi” (CARS-29-05B).
Footnotes
Source of Support: This research was supported by a grant from “the National Li Chanye Jishu Tixi” (CARS-29-05B)
Conflict of Interest: None declared.
REFERENCES
- 1.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils – a review. Food Chem Toxicol. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 2.Bughio FM, Wilkins RM. Influence of Malathion resistance status on survival and growth of Tribolium castaneum (Coleoptera: Tenebrionidae), when fed on flour from insect-resistant and susceptible grain rice cultivars. J Stored Prod Res. 2004;40:65–75. [Google Scholar]
- 3.Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000;19:603–8. [Google Scholar]
- 4.Messchendorp L, Gols GJ, van Loon JJ. Behavioral effects and sensory detection of drimane deterrents in Myzus persicae and Aphis gossypii nymphs. J Chem Ecol. 1998;24:1433–46. [Google Scholar]
- 5.Blackman RL, Eastop VF. 2nd ed. England: John Wiley & Sons; 2000. Aphids of the World's Crops: An Identification and Information Guide; p. 466. [Google Scholar]
- 6.Sharma HC, Sharma KK, Crouch JH. Genetic transformation of crops for insect resistance: Potentials and limitations. Crit Rev Plant Sci. 2004;23:47–72. [Google Scholar]
- 7.Edwards OR, Franzmann B, Thackray D, Micic S. Insecticide resistance and implications for future aphid management in Australian grains and pastures: A review. Aust J Exp Agric. 2008;48:1523–30. [Google Scholar]
- 8.Lou HX, Li X, Zhu TY. Chemical constituents of Cynanchum mongolicum. J Shenyang Pharm Univ. 1990;7:286–91. [Google Scholar]
- 9.Bruce TJ, Birkett MA, Blande J, Hooper AM, Martin JL, Khambay B, et al. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manag Sci. 2005;61:1115–21. doi: 10.1002/ps.1102. [DOI] [PubMed] [Google Scholar]
- 10.Ma YH, Zhao Z, Jiang ZL. Fumigant activity of essential oil from bitter apricot kernel to insects. Northwest Plant Acta. 2007;27:1879–83. [Google Scholar]
- 11.Tunç I, Şahinkaya Ş. Sensitivity of two greenhouse pests to vapours of essential oils. Entomol Exp Appl. 1998;86:183–7. [Google Scholar]
- 12.Beale MH, Birkett MA, Bruce TJ, Chamberlain K, Field LM, Huttly AK, et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc Natl Acad Sci U S A. 2006;103:10509–13. doi: 10.1073/pnas.0603998103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleeker PM, Diergaarde PJ, Ament K, Guerra J, Weidner M, Schütz S, et al. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009;151:925–35. doi: 10.1104/pp.109.142661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YQ, Xue M, Zhang QC. Toxicity and control mechanism of β-caryophyllene to Aphis gossypi. Entomol Knowl. 2010;53:396–404. [Google Scholar]
- 15.Landolt PJ, Brumley JA, Smithhisler CL, Biddick LL, Hofstetter RW. Apple fruit infested with codling moth are more attractive to neonate codling moth larvae and possess increased amounts of (E, E)-α-farnesene. J Chem Ecol. 2000;26:1685–99. [Google Scholar]
- 16.Sun H, Blanford S, Guo Y, Fu X, Shi W. Toxicity and influences of the alkaloids from Cynanchum komarovii AL. Iljinski (Asclepiadaceae) on growth and cuticle components of Spodoptera litura Fabricius (Noctuidae) larvae. Nat Prod Res. 2012;26:903–12. doi: 10.1080/14786419.2010.533665. [DOI] [PubMed] [Google Scholar]


