Abstract
Background:
Artemisinin, a sesquiterpene lactone endoperoxide isolated from the medicinal plant Artemisia annua L., is a choice and effective drug for malaria treatment. Due to the low yield of artemisinin in plants, there is a need to enhance the production of artemisinin from A. annua and biotechnological technique may be one of the methods that can be used for the purpose.
Aim:
To study the transformation efficiency of Agrobacterium tumefaciens in A. annua that could be applied to enhance the production of artemisinin by means of transgenic plants.
Setting and Designs:
The factors influencing Agrobacterium-mediated transformation of A. annua were explored to optimize the transformation system, which included A. tumefaciens strain and effect of organosilicone surfactants. Three strains of A. tumefaciens, that is, LBA4404, GV1301, and AGL1 harboring the binary vector pCAMBIA 1303 have been used for transformation. The evaluation was based on transient β-glucuronidase (GUS).
Materials and Methods:
Plant cell cultures were inniatiated from the seeds of A. annua using the germination Murashige and Skoog medium. A. tumefaciens harboring pCAMBIA were tranformed into the leaves of A.annua cultures from 2-week-old-seedling and 2-month-old-seedling for 15 min by vacuum infiltration. Transformation efficiency was determinated by measuring of blue area (GUS expression) on the whole leaves explant using ImageJ 1.43 software. Two organosilicon surfactants, that is, Silwet L-77 and Silwet S-408 were used to improve the transformation efficiency.
Results:
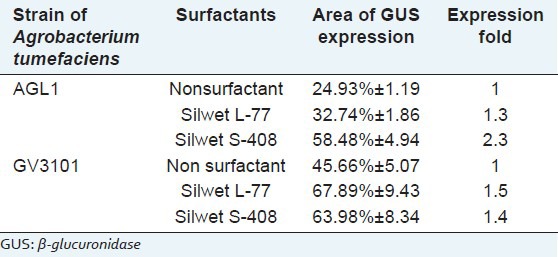
The transformation frequency with AGL1 strain was higher than GV3101 and LBA4404 which were 70.91, 49.25, and 45.45%, respectively. Effect of organosilicone surfactants, that is, Silwet L-77 and Silwet S-408 were tested on A. tumefaciens AGL1 and GV3101 for their level of transient expression, and on A. rhizogenes R1000 for its hairy root induction frequency. For AGL1, Silwet S-408 produced higher level of expression than Silwet L-77, were 2.3- and 1.3-fold, respectively. For GV3101, Silwet L-77 was still higher than Silwet S-408, were 1.5- and 1.4-fold, respectively. However, GV3101 produced higher levels of expression than AGL1. The area of GUS expression spots of AGL1, LBA4404, and GV3101 strains was 53.43%, 41.06%, and 30.51%, respectively.
Conclusion:
A. tumefaciens AGl1 strain was the most effective to be transformed in to A. annua than GV3101 and LBA4404 strain. Surfactant Silwet S-408 produced the highest efficiency of transformation.
Keywords: Artemisinin, Artemisia annua L., Agrobacterium transformation, malaria, pCAMBIA
INTRODUCTION
Artemisinin is a sesquiterpene lactone endoperoxide extracted from Artemisia annua L. (Asteraceae) and highly effective against multidrug-resistant Plasmodium.[1] However, the low level of artemisinin in the plant (0.01-1.4% of dry weight) has made artemisinin-based drugs relatively expensive because of its short supply in industry.[2] Currently, plant resources cannot meet the increasing worldwide demands, while chemical synthesis is difficult and expensive because of its endoperoxide bridge.[3] Therefore, biotechnology approach for increasing of artemisinin level via metabolic engineering in transgenic A. annua plants and in genetically modified microbes are sought as novel means for large-scale production and cost-effective commercialization of artemisinin.[4]
Numerous efforts focusing on enhancing the production of artemisinin have been made for a long time. However, conventional breeding of high artemisinin yielding plants;[5] tissue and cell cultures via manipulation of culture conditions, growth media, feeding of precursor, and elicitation;[6,7] and organ or culture transformation of A. annua via Agrobacterium (Paniego and Giulietti, 1995)[8,9,10] to increase the yield of artemisinin have not been successful yet.
In recent years, artemisinin biosynthesis has been the subject of intensive research. Genes-encoding enzymes of the pathway, such as farnesyl diphosphate synthase (FDS), amorpha-4,11-diene synthase (ADS), and cytochrome P450 monooxygenase (CYP71AV1), and the genes of the enzymes relevant to the biosynthesis of artemisinin, such as squalene synthase (SQS), have been isolated and cloned from A. annua.[11] Thus, we can enhance of artemisinin production by genetic engineering in plant or microbe using these enzymes. Numerous efforts on genetic modification of A. annua have been made for enhance of artemisinin production. These efforts include overexpression of FDS[12,13] and isopentenyl transferase,[14] and inhibition of SQS expression.[15] Besides in plants, these genes have been transformed and expressed in microbes through genetic engineering. Artemisinic acid, a precursor of artemisinin, has been produced using Saccharomyces cerevisiae which engineered with FDS, ADS, and CYP71AV1 genes of A. annua. ADS gene which is a key enzyme in the cyclization of farnesyl pyrophosphate has also been expressed in Aspergilus nidulans[16] and Escherichia coli[17] to produce amorphadiene. However, these efforts have not shown a dramatic increase in the production of artemisinin and its precursor. Therefore, in this work, we focused on exploring the factors influencing Agrobacterium-mediated transformation of A. annua to optimize the transformation system. The optimum transformation system will be used in our next study to enhance the ADS gene expression in A. annua through plant genetic engineering.
MATERIALS AND METHODS
Plant materials and tissue culture conditions
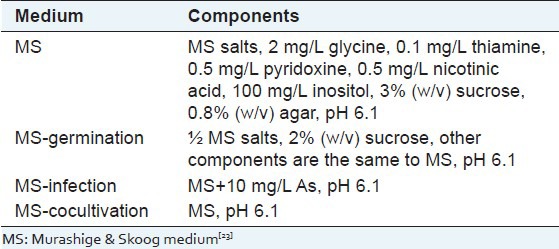
Seeds of A. annua L. were collected from medicinal plant cultivation Manoko, Lembang, Bandung, Indonesia. Seeds were surface sterilized by soaking in 5% NaOCl for 20 min after immersing in 70% ethanol for 2 min. The seeds were rinsed three times with sterile distilled water and then germinated under sterile conditions in 100 mL flask containing 20 mL germination Murashige and Skoog (MS) medium[23] [Table 1]. The pH was adjusted to 6.1 with 1 N NaOH before addition of agar. The medium was autoclaved at 121°C for 20 min. Germination started within 2 or 3 days using fluorescent lamps for 16 h and at temperature of 25 ± 2°C.
Table 1.
Media used in our case
Agrobacterium strains and plant expression vector
Several A. tumefaciens strains (LBA4404, GV3101, and AGl1) and binary vector pCAMBIA 1303 were tested for their competence to transform A. annua L. The binary vector contains the NPT II (npt II) gene conferring for bacterial kanamycin resistance and hpt II gene for plant hygromycin selection. This vector has a gusA-mgfp5-His6 fusion as reporter genes. These reporter genes were placed under the control of the CaMV 35S promoter.
Transformation of Agrobacterium tumefaciens
Vector pCAMBIA 1303 was transformed into A. tumefaciens using heat-shock method.[18] The transformant cells of Agrobacterium harboring the binary vector pCAMBIA were confirmed by crude polymerase chain reaction (PCR) and electrophoresis. The forward primer was 5’-AGTGGCAGTGAAGGGCGAACAGT-3’ and reverse 5’- AATAACGGTTCAGGCAC AGCACA-3’, designed according to the sequence of gus gene. The 25 mL of PCR reactions included 0.5 mM forward primer, 0.5 mM reverse primer, 2.5 μl 10 × Taq DNA Polymerase buffer, 0.2 mM dNTPs, 1 U Taq DNA Polymerase, and 2 mM MgCl2 (Promega). Amplification was performed in a thermal cycler (Applied Biosystem - 2720 Thermal cycler) as follows: 1 min at 94°C; 30 cycles of 30 s at 94°C; 30 s at 72°C; followed by 7 min at 72°C. The 989 bp amplification fragments were electrophoresis on a 0.8% agarose gel (Boehringer Mannheim) containing ethidium bromide (Promega) and then observed.
Transformation of Artemisia annua
The A. tumefaciens strains LBA4404, GV3101, and AGl1 harboring the binary vector pCAMBIA 1303 were used for transformation. These strains were grown in yeast extract peptone (YEP) medium supplemented with kanamycin (50 mg/L) and rifampycin (50 mg/L) for LBA4404 and GV3101, and carbenicilin/ampicilin (50 mg/L) for AGl1. The monoclones were picked out and incubated at 28°C for 36 h, then 1 mL bacterial suspension was diluted to 50 mL YEP medium followed by being incubated to reach OD600 ≈ 0.5. The bacterial suspension was centrifuged at 4°C, 4000 rpm for 10 min and then resuspended with 50 mL liquid MS-infection [Table 1]. They were continually incubated for 3 h and then used as infection bacterial suspension to infect the leaves explants from 2-week-old-seedling and 2-month-old-seedling for 15 min by vacuum infiltration. The infected leaves were blotted on sterile filter paper, then cocultivated on MS-cocultivation medium [Table 1] in the dark for 3 days. After cocultivation, the leaves were transferred to sterile distilled water with cefotaxime (500 mg/L) for LBA4404 and GV3101, and augmentin (400 mg/L) for AGl1, to wash and destroy the Agrobacterium cells.
Effect of surfactants on the tranformation efficiency of A. annua was performed using two organosilicon surfactants, that is, Silwet L-77 and Silwet S-408. Transformation procedure was the same as described above using leaves explants from 14-day-old seedling of A. annua. Surfactants with concentration 0.002% (v/v) was added into bacterial suspension for leaves infection. After 3 days cocultivation, leaves explants were washed with antibiotic to remove residual bacteria. Transformation efficiency was determinated by measuring of blue area (GUS expression) on the whole leaf explant using ImageJ 1.43 software.
B-glucuronidase assay
B-glucuronidase (GUS) assays were performed on transformant leaves with the pCAMBIA 1303 binary vector according to the method described by Jefferson (1987).[19] The amount of transient GUS expression was calculated based on the ratio of the area of spots or blue area on the whole leaves area using ImageJ 1.43 software.
RESULTS AND DISCUSSION
Cultivation of A. annua on MS medium from fresh seeds has been successfully and established in our laboratory [Figure 1] Previously, callus of A. annua has also been successfully induced and maintenanced in cell suspension cultures for artemisinin enhancement via feeding of precursor and elicitation, but the results showed there were no enhancement of artemisinin either in calli or cell suspension cultures (unpublished). Therefore, we have tried to enhance artemisinin content by plant genetic engineering via A. tumefaciens-mediated transformation of A. annua. The first step, optimization of transformation system, has been performed to find the most effective strains of A. tumefaciens. Several strains of A. tumefaciens have been reported successfully to infect the A. annua with various plant expression vectors.[20,21] However, the frequency of infection and regeneration of A. annua is still low.
Figure 1.

Artemisia annua L.: 3-months-old, (a) flower on 6-month-old, (b), seeds, (c) and 1-month-old-seedling on Murashige and Skoog medium (d and e)
In this work, we used three A. tumefaciens strains, that is, LBA4404, GV3101, and AGl1. There have been no reports on the ability of the last two strains to mediate transformation of A. annua. These two strains are known to be powerful to mediate transformation on plants. We used pCAMBIA 1303 as a binary vector which contain the NPT II (npt II) gene conferring for bacterial kanamycin resistance and hpt II gene for plant hygromycin selection. This vector has a gusA-mgfp5-His6 fusion as reporter genes. All reporter genes were placed under the control of the CaMV 35S promoter.
The binary vector pCAMBIA 1303 was successfully transformed into competent cells of A. tumefaciens using heat-shock method. The transformant cells of Agrobacterium were analyzed by PCR [Figure 2]. The A. tumefaciens strains LBA4404, GV3101, and AGl1 harboring the binary vector pCAMBIA 1303 were used for transformation on leaves explants of A. annua. We have adapted Han et al., (2005)[21] method for high efficiency of genetic transformation of A. annua. We have used transient gus gene expression to monitor the transformed leaves.
Figure 2.
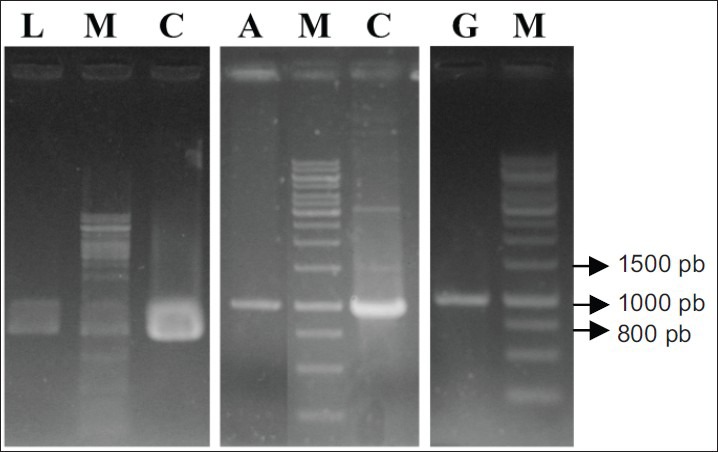
Polymerase chain reaction analysis of pCAMBIA 1303 from transformant of Agrobacterium tumefaciens strains: LBA4404 (L), AGl1 (A), GV3101 (G), Marker 1 kb (M) and pCAMBIA 1303 as a gus-positive control (C)
The result in Figure 3 showed the bacterial strains have important role in plant transformation. Transformation frequency of AGL1 was 70.91% from the total leaves explants of A. annua, higher than GV3101 and LBA4404 strains, which were 49.25% and 45.45%, respectively. These transformation frequencies was calculated based on the presence of GUS transient expression on leaves explants. The ability of Agrobacterium to infect the plants is very dependent on their chromosome and their virulence. The AGL1, GV3101, and LBA4404 strains are not only different in their chromosome, but also the level of genes activation in virulence region. Perhaps this cause why AGL1, a succinamopine strain was known highly virulent. It has a higher infection frequency than nopaline strain (GV3101) and octopine strain (LBA4404).
Figure 3.
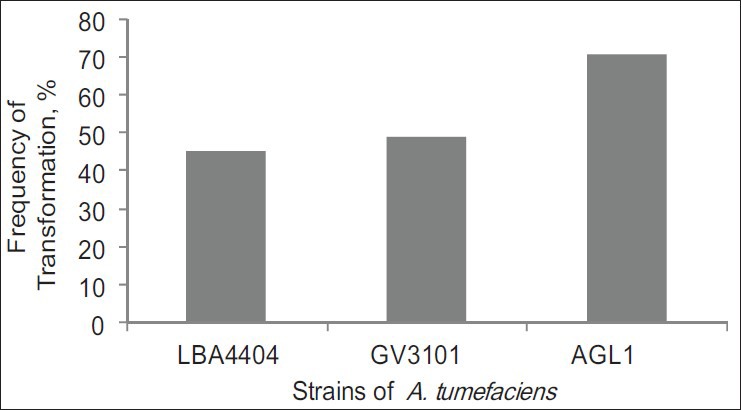
Transformation frequency of Artemisia annua with three Agrobacterium tumefaciens strain
The surfactants are known to increase the efficiency of plant transformation using Agrobacterium. The use of several types of surfactants by Kim et al., (2009)[22] enhanced the transient expression in Arabidopsis leaves. In our study, we used surfactant from organosilicon type, that is, Silwet L-77 and S-408. In addition for its function to decrease the surface tension between two phases, the surfactants are also able to enhance penetration into the cuticle, thereby increasing the movement of material into a plant cell, in this case the genetic material which is transferred by Agrobacterium.[22] Organosilicon surfactants which are a nonionic surfactants are less toxic to plant growth. Surfactant is added in infiltration medium at low concentrations 0.002% (v/v) in order to be able to facilitate the infection of plant tissues by Agrobacterium cells without damaging the growth of explants.
The leaves showed several blue spots caused by the transient GUS expression after 3 days cocultivation [Figure 4]. By contrast, untransformed did not show any blue staining [Figure 4d–f]. The results in Figure 3G–J showed the use of surfactants which enhanced the transient expression compared to transformation without of surfactants (A-C). On the AGL1 strain, level of transient expression with the Silwet S-408 was 2.3-fold higher than Silwet L-77 which was only 1.3-fold. While on the GV3101 strain, both of surfactants exhibited transient expression of GUS in the same level. The expression level of GUS on the Silwet L-77 was 1.5-fold higher than Silwet S-408. However, GV3101 strain showed the level of transient expression higher than AGL1, which can be seen from the area of GUS expression [Table 2].
Figure 4.

Histochemical β-glucuronidase assays of the transformed leaf of Artemisia annua: Without surfactants, (a-c), untransformed, (d-f) and with surfactant (g-j)
Table 2.
Effect of surfactant on the levels of β-glucuronidase transient expression on Artemisia annua
CONCLUSION
The transfer of pCAMBIA 1303 via Agrobacterium tumefaciens and expression of gus reporter gene on leaf of Artemisia annua were successfully performed. Strain of A. tumefaciens AGl1 was the most effective to be transformed to this plant than GV3101 and LBA4404 strain. Surfactant Silwet S-408 produced the highest efficiency of transformation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–3. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 2.Dewick PM. 3rd ed. UK: John Wiley and Sons Ltd; 2009. Medicinal natural products: A biosynthetic approach. [Google Scholar]
- 3.Hong GJ, Hu WL, Li JX, Chen XY, Wang LJ. Increased accumulation of artemisinin and anthocyanins in Artemisia annua expressing the Arabidopsis blue light receptor CRY1. Plant Mol Biol Rep. 2009;27:334–41. [Google Scholar]
- 4.Zeng QP, Zeng XM, Yin LL, Yang RY, Feng LL, Yang XQ. Quantification of three key enzymes inolved in artemisinin biogenesis in Artemisia annua by policlonal antisera-based elisa. Plant Mol Biol Rep. 2009;27:50–7. [Google Scholar]
- 5.Qian Z, Gong K, Zhang L, Jianbing L, Jing F, Wang Y, et al. A simple and efficient procedure to enhance artemisinin content in Artemisia annua L. by seeding to salinity stress. AJB. 2007;6:1410–3. [Google Scholar]
- 6.Putalun W, Luealon W, De-Eknamkul W, Tanaka H, Shoyama Y. Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L. Biotechnol Lett. 2007;29:1143–6. doi: 10.1007/s10529-007-9368-8. [DOI] [PubMed] [Google Scholar]
- 7.Baldi A, Dixit VK. Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresour Technol. 2008;99:4609–14. doi: 10.1016/j.biortech.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 8.Paniego NB, Giulietti AM. Artemisinin production by Artemisia annua L.-transformed organ cultures. Enzyme Microb Technol. 1996;18:526–30. [Google Scholar]
- 9.Ghosh B, Mukherjee S, Jha S. Genetic transformation of Artemisia annua by Agrobacterium tumefaciens and artemisinin synthesis in transformed cultures. Plant Sci. 1997;122:193–9. [Google Scholar]
- 10.Wang JW, Tan RX. Artemisinin Production in Artemisia annua hairy root cultures with improved growth by altering the nitrogen source in the medium. Biotechnol Lett. 2002;24:1153–6. [Google Scholar]
- 11.Covello PS. Making artemisinin. Phytochemistry. 2008;69:2881–5. doi: 10.1016/j.phytochem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Liu C, Ye H, Li G, Liu BY, Meng YL, et al. Ri-mediated transformation of Artemisia annua with a recombinant farnesyl diphosphate synthase gene for artemisinin production. Plant Cell Tissue Organ Cult. 1999;57:157–62. [Google Scholar]
- 13.Chen D, Ye H, Li G. Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plant via Agrobacterium tumefaciens-mediated transformation. Plant Sci. 2000;155:179–85. doi: 10.1016/s0168-9452(00)00217-x. [DOI] [PubMed] [Google Scholar]
- 14.Sa G, Mi M, He-chun Y, Ben-ye L, Guo-feng L, Kang C. Effects of ipt gene expression on the physiological and chemical characteristics of Artemisia annua L. Plant Sci. 2001;160:691–8. doi: 10.1016/s0168-9452(00)00453-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Jing F, Li F, Li M, Wang Y, Wang G, et al. Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA-mediated gene silencing. Biotechnol Appl Biochem. 2009;52:199–207. doi: 10.1042/BA20080068. [DOI] [PubMed] [Google Scholar]
- 16.Lubertozzi D, Keasling JD. Expression of a synthetic Artemisia annua amorphadiene synthase in Aspergillus nidulans yields altered product distribution. J Ind Mirobiol Biotechnol. 2008;35:1191–8. doi: 10.1007/s10295-008-0400-3. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruta H, Paddon CJ, Eng D, Lenihan JR, Horning T, Anthony LC, et al. High-level production of amorpha-4.11-diene. A precursor of the antimalarial agent artemisinin. in Escherichia coli. PLoS One. 2009;4:e4489. doi: 10.1371/journal.pone.0004489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K. 2nd ed. Vol. 1. New Jersey: Humana Press; 2006. Methods in molecular biology: Agrobacterium protocols. [Google Scholar]
- 19.Jefferson RA. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–05. [Google Scholar]
- 20.Vergauwe A, Van Geldre E, Inzé D, Van Montagu M, Van den Eeckhout E. Factors influencing Agrobacterium tumefaciens-mediated transformation of Artemisia annua L. Plant Cell Rep. 1998;18:105–10. doi: 10.1007/BF00231590. [DOI] [PubMed] [Google Scholar]
- 21.Han JL, Wang H, Ye HC, Liu Y, Li ZQ, Zhang L, et al. High efficiency of genetic tranformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci. 2005;168:73–80. [Google Scholar]
- 22.Kim MJ, Baek K, Park CM. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009;28:1159–67. doi: 10.1007/s00299-009-0717-z. [DOI] [PubMed] [Google Scholar]
- 23.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;51:473–97. [Google Scholar]


