Abstract
Background:
A new method has been developed for the simultaneous determination of ferulic acid, senkyunolide A, and Z-ligustilide in Angelicae Sinensis Radix before and after sulfur-fumigation using quantitative analysis of multi-components by a single marker (QAMS).
Materials and Methods:
The feasibility and accuracy of QAMS were checked by the external standard method, and various high-performance liquid chromatographic instruments and chromatographic conditions were investigated to verify its applicability. Using ferulic acid as the internal reference substance, and the contents of senkyunolide A and Z-ligustilide were calculated according to relative correction factors by high-performance liquid chromatography. Meanwhile, the influence of sulfur-fumigation on these chemical components in Angelicae Sinensis Radix were evaluated and discriminated by chromatographic fingerprint and chemometrics.
Results:
There was no significant difference observed between the QAMS method and the external standard method. Furthermore, sulfur-fumigation reduced the contents of ferulic acid, senkyunolide A, and Z-ligustilide in Angelicae Sinensis Radix by some degree, and the sun-drying and sulfur-fumigation processing could be easily discriminated by chromatographic fingerprint and chemometrics.
Conclusion:
QAMS is a convenient and accurate approach to analyzing multi-component when reference substances are unavailable, simultaneously, chemometrics is an effective way to discriminate sun-dried and sulfur-fumigated Angelicae Sinensis Radix.
Keywords: Angelicae Sinensis Radix, chemometrics, quantitative analysis of multi-components by a single marker, relative correction factor, sulfur-fumigation
INTRODUCTION
A characteristic of traditional Chinese medicines (TCMs) is the inclusion of a multitude of herbal ingredients, such that quality is evaluated using multiple components and indices. However, separating and analyzing individual chemical markers in TCMs is difficult owing to the instability of monomers and the high cost. Quantitative analysis of multi-components by a single marker (QAMS) is a new methodological approach based on the principle that the effective components in TCMs should maintain their internal function and proportional relations.[1] Therefore, determining the composition of one component (a reference substance) will allow simultaneous monitoring for the remaining ingredients. This has led to the development of innovative multi-component analysis techniques that can address the shortcomings of existing chemical marker analysis approaches for quality control in TCMs. In recent years, researchers have successfully applied the QAMS method to analyze the contents of different medicinal herbs.[2,3,4,5,6,7] In addition, the QAMS standard of Rhizoma Coptidis has been adopted by Chinese Pharmacopoeia (2010 eds.).[8] Nevertheless, analysis using QAMS in Angelicae Sinensis Radix, one of the oldest and most frequently used herbs in TCMs, has never been reported in the literature.
The theory of QAMS: Within a certain linear range, the quantity and concentration of chemical component is proportional to the response of the detector. In the multi-index valuation model (s, a, b…, i…,), taking a typical active ingredient of herbs (or medicines) as the internal reference substance (IS) and developing the relative correction factor (RCF, fsa, fsb, fsc, …) between the IS with other components, is calculated as follows:

As is the peak area of IS, Cs is the concentration of IS, Ai is the peak area of the sample i, and Ci is the concentration of the sample i. From formula (1) we can export formula (2):

We can calculate the concentration of samples using formula (2).[9] In this study, ferulic acid was used as the IS and was measured using the external standard method. This reference was then used to calculate the quantity and concentration of senkyunolide A and Z-ligustilide by RCFs. Finally the accuracy of the method was assessed through the external standard method.
In addition to QAMS, fingerprint analysis methods, such as high-performance liquid chromatography (HPLC), has also been widely introduced as a useful multi-component approach for quality control of medicinal herbs as well as herbal products.[10] With regard to the ability to identify a particular herb from related species, fingerprint analysis methods have gained more attention than QAMS. Chemical pattern recognition is acknowledged as a more objective and effective quality evaluation method compared with determination of single or multiple markers in medicinal herbs. Chemometric analyses, especially similarity analysis (SA), hierarchical cluster analysis (HCA), and principal components analysis (PCA) are widely used for chemical classification and chromatographic profile aligning. In this study, the simultaneous determination of three compounds in Angelicae Sinensis Radix, before and after sulfur-fumigation, was achieved using QAMS to assess the feasibility and accuracy of which it can be applied in the quality control of Angelicae Sinensis Radix. Furthermore, chromatographic fingerprint and chemometrics methods were employed to assess chemical alterations in Angelicae Sinensis Radix before and after sulfur-fumigation.
Angelicae Sinensis Radix is derived from the root of Angelica sinensis (Oliv.) Diels. Pharmacological and clinical studies have demonstrated that Angelicae Sinensis Radix possesses various bioactivities, including tonifying blood, promoting blood circulation, regulating menstruation, alleviating pain, lubricating the bowels to relieve constipation, and has preventative effects on cardiovascular disease, chronic obstructive pulmonary disease, and bronchial asthma.[11,12] The major active components of Angelicae Sinensis Radix are volatile oils, organic acids, polysaccharides, and amino acids. In particular, volatile oils and organic acids are the most important components of Angelicae Sinensis Radix and are critical for its therapeutic effect in TCMs. Consequently, we chose three chemical components for the simultaneous multi-component analysis comprising an organic acid (ferulic acid) and two volatile oils (senkyunolide A, Z-ligustilide).
Sulfur-fumigation processing provides many benefits for storage and curing of medicinal materials, such as mold resistance, pest control, curtailing the drying duration, and maintaining a better appearance.[13] However, this process leaves large amount residues of sulfur dioxide and harmful heavy metal residues in the medicinal materials, which are harmful to human health, and reduces its curative effects and medicinal quality.[14] In preliminary studies, we found that sulfur-fumigation led to changes in the quality and quantity of main active ingredients in both crude and prepared drugs.[15,16,17,18] Moreover, previous studies have also found that the sulfur-fumigation process severely damages chemical components in Fritillaria thunbergii Miq. (Zhebeimu) and White Ginseng.[19,20] Thus, after the simultaneous determination by QAMS, we also adopted chromatographic fingerprint and chemometrics methods to assess the influence of sulfur-fumigation on chemical components in Angelicae Sinensis Radix, which can supply scientific evidence for quality control.
MATERIALS AND METHODS
Materials and reagents
The reference substance, ferulic acid, was purchased from the National Institute for the Control of Pharmaceutical and Biological Products. Senkyunolide A and Z-ligustilide were purchased from Sichuan Weikeqi Biological Technology Co., Ltd. The chemical structures of these three compounds were confirmed by 1H, 13C nuclear magnetic resonance (NMR) spectroscopy and mass spectrum, and purities, assessed using HPLC analysis, were all over 98%. The chemical structures of these three components are shown in [Figure 1]. HPLC-grade methanol was purchased from Tedia (Ohio, USA). Analytical-grade formic acid and phosphoric acid were purchased from Nanjing Chemical Reagent Co., Ltd. Purified water was purchased from Wahaha (Hangzhou Wahaha Group).
Figure 1.
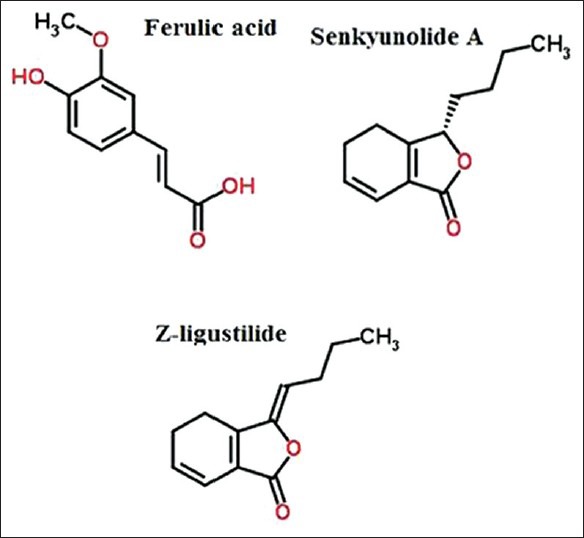
Chemical structures of ferulic acid, senkyunolide A, and Z-ligustilide
A total of thirty-three Angelicae Sinensis Radix was acquired from different regions and pharmacies in China. All samples were tested using acid distillation iodine titration method (GB/T 5009.34) for presence of sulfur-fumigation processing. Thirteen of them were found to be sun-dried (samples 1-13), twenty of them were found to be sulfur-fumigated (samples 14-33). Sample number 34 was prepared in the laboratory from sample number 2 by sulfur-fumigation as comparison. All samples were authenticated by an expert in the field.
Instruments and HPLC conditions
Analyses were performed using HPLC system Varian 920-LC, including Prostar 240 quatpump, Prostar 410 automatic sampler, Prostar 335 DAD, Galaxie Chemstation station (USA Varian); Agilent 1100, including G1312A Binary Pump, G1322A Degasser, G1367A WPLS, G1315B DAD (USA Agilent); Waters 515-2487, including Waters 515 HPLC Pump, 717 Plus Autosampler, Waters 2487 Dual λ Absorbance Detector, Empower station (USA Waters). Columns used included Kromasil C18 (250 mm × 4.6 mm, 5 μm), Symmetry C18 (250 mm × 4. 6 mm, 5 μm), Eclipse Plus C18 (250 mm × 4. 6 mm, 5 μm), and Hypersil ODS C18 (250 mm × 4. 6 mm, 5 μm). The flow rate of each column was set at 1.0 mL/min. The injection volume was 20 μL and the column temperature was maintained at 35°C. Mobile phase was composed of: (A) 0.05% phosphoric acid in water and (B) methanol using a gradient elution of 70%→40% A at 0 ~ 20 min, 40%→15% A at 20 ~ 50 min, and 15%→0% A at 50 ~ 55 min. Detection wavelength was set at 270 nm.
Preparation of sample solutions
Powdered Angelicae Sinensis Radix were precisely weighed (0.5 g), and transferred into dark brown calibrated flasks. They were extracted with 25 mL of methanol: formic acid (95:5) in an ultrasonic bath for 40 min. Additional methanol was added to compensate for any loss during this process. The supernatants were filtered through a 0.45-μm membrane prior to injection.
Preparation of standard solutions
The standard stock solutions of ferulic acid (0.2 mg/mL), senkyunolide A (0.3 mg/mL), and Z-ligustilide (1.1 mg/mL) were prepared in methanol and stored at 4°C. The calibration curves were prepared at seven different concentration levels, and the diluting factors were 1, 2, 4, 8, 16, and 32 times, respectively.
Data analysis
Chromatographic fingerprint analysis and similarity analysis (SA) were performed using professional computer software recommended by the State Food and Drug Administration (SFDA), entitled the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine. This software was used in the SA of chromatographic and spectral patterns, and also to calculate the similarities of fingerprint profiles. The 3D model in [Figure 2] was produced by Origin 8.0. The HCA and PCA were performed on the characteristic chromatographic peaks in the HPLC fingerprints using SPSS 16.0 (IBM). In HCA, a statistical method called average linkage between groups was applied and the squared Euclidean distance was used as the metric.
Figure 2.
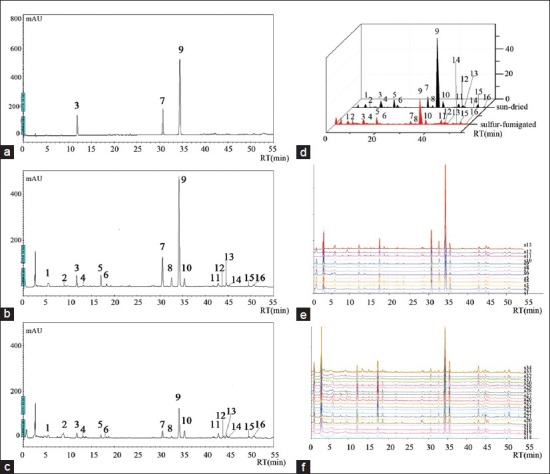
Representative chromatograms and chromatographic fingerprints. (a) HPLC chromatogram of reference substances. (b) HPLC chromatogram of sun-dried Angelicae Sinensis Radix. (c) HPLC chromatogram of sulfur-fumigated Angelicae Sinensis Radix. Ferulic acid (3), senkyunolide A (7), Z-ligustilide (9). (d) 3D representation of sun-dried and sulfur-fumigated samples. (e) The chromatographic fingerprint of sun-dried Angelicae Sinensis Radix. (f) The chromatographic fingerprint of sulfur-fumigated Angelicae Sinensis Radix
RESULTS AND DISCUSSION
Optimization of extraction conditions
To obtain optimal chromatograms, effects on extraction rate of three components in Angelicae Sinensis Radix with methanol, 70% methanol, and methanol: formic acid (95:5) were investigated. The results indicated that the contents of ferulic acid in methanol and 70% methanol were higher in accordance with the report in literature.[21] Coniferyl ferulate in methanol solution was easily decomposed and a small quantity was converted into ferulic acid. As a result, this led to an inaccurate calculation of ferulic acid and coniferyl ferulate quantities. This may be the reason why the quantity of ferulic acid was higher in these two solutions. However, when they were examined in the methanol-formic acid, these three components were more stable and possessed a higher extraction rate. Furthermore, in comparison with ultrasonic method and reflux method, the result indicated that ultrasonic method had a higher extraction rate and operated easily. Extraction time was also investigated. We tested at 20 min, 40 min, and 60 min, and found that the highest extraction rate occurred at 40 min. Therefore, methanol: formic acid (95:5) in an ultrasonic bath for 40 min was determined to be optimal parameters to extract sample solutions.
HPLC method validation
Calibration curves
The injection volume of the mixed reference standard solutions No. 1 - No. 7 was 20 μL precisely. Every concentration was repeated three times, and an average value was calculated. Calibration curves were then drawn and the regression equations were calculated via partial least squares. The results showed that ferulic acid, senkyunolide A, and Z-ligustilide in Angelicae Sinensis Radix had good linear relationships in the ranges of 0.003125 - 0.2 mg/mL, 0.0046874 - 0.3 mg/mL, 0.0171875 - 1.1 mg/mL, respectively. The regression equations were Y = 67.11 X − 82.56, Y = 91.7 X + 4.6381, Y = 1076.40 X + 32.58, respectively, with the correlation coefficients (r2) of 0.9998, 0.9995, 0.9999, respectively.
Precision
The precision test was conducted by replicating the injection of the standard solution six times, following the above procedure. Peak areas were measured and relative standard deviations (RSDs) were calculated. The RSDs of relative peak areas of ferulic acid, senkyunolide A, and Z-ligustilide were 1.20%, 1.11%, and 1.35%, respectively. Given that these values did not exceed 5%, the instrument had high precision.
Repeatability
Repeatability was tested by injecting six independently prepared samples. Samples were prepared using the method outlined in section “Preparation of sample solutions.” The RSDs of relative peak areas of ferulic acid, senkyunolide A, and Z-ligustilide were 2.78%, 3.21%, and 2.56%, respectively, which indicated that the experiment had high repeatability.
Stability
Stability was determined with one sample solution that was analyzed at 0 h, 2 h, 4 h, 8 h, 10 h, 12 h, and 24 h. The RSDs of relative peak areas of ferulic acid, senkyunolide A, and Z-ligustilide were 2.79%, 3.36%, and 2.50%, respectively. Therefore, samples were stable within 1 day.
Average recovery
Recovery tests were carried out to investigate the accuracy of the method by spiking known amounts of the mixed reference standard solutions to approximately 0.25 g of the testing sample. The resulting recoveries of ferulic acid, senkyunolide A, and Z-ligustilide were 101.1%, 93.26%, and 100.91%, respectively, and the RSDs were 2.33%, 3.01%, and 1.78%, respectively.
Selection of internal reference substances
Ferulic acid is readily accessible and inexpensive; moreover, it is one of the most pharmacologically active components, showing anti-platelet aggregation and inhibitory effects on platelet serotonin release. Owing to its widespread application and cost-effectiveness, it is the most commonly used substance for determining standard of quality in Angelicae Sinensis Radix (Chinese Pharmacopoeia 2010 eds.). In our previous investigation, it was also found that the retention time error of ferulic acid was minimal in diverse instruments and chromatographic columns. Therefore, ferulic acid was chosen as the IS for our study.
Calculation of relative calibration factor
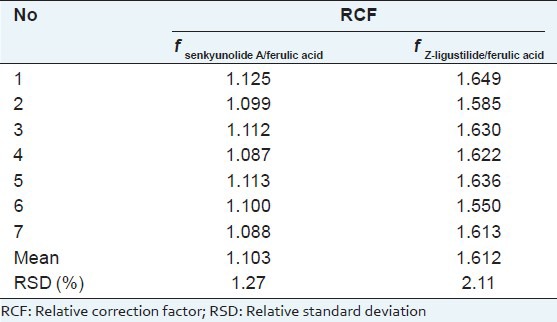
RCF was calculated according to formula (1) outlined in section of “INTRODUCTION.” The IS used was ferulic acid. Relative calibration factors of senkyunolide A and Z-ligustilide are shown in [Table 1]. From [Table 1], we can see that there were no significant differences between these seven concentration samples.
Table 1.
Relative correction factors of ferulic acid, senkyunolide A, and Z-ligustilide in Angelicae Sinensis Radix
Comparison of the external standard method and QAMS
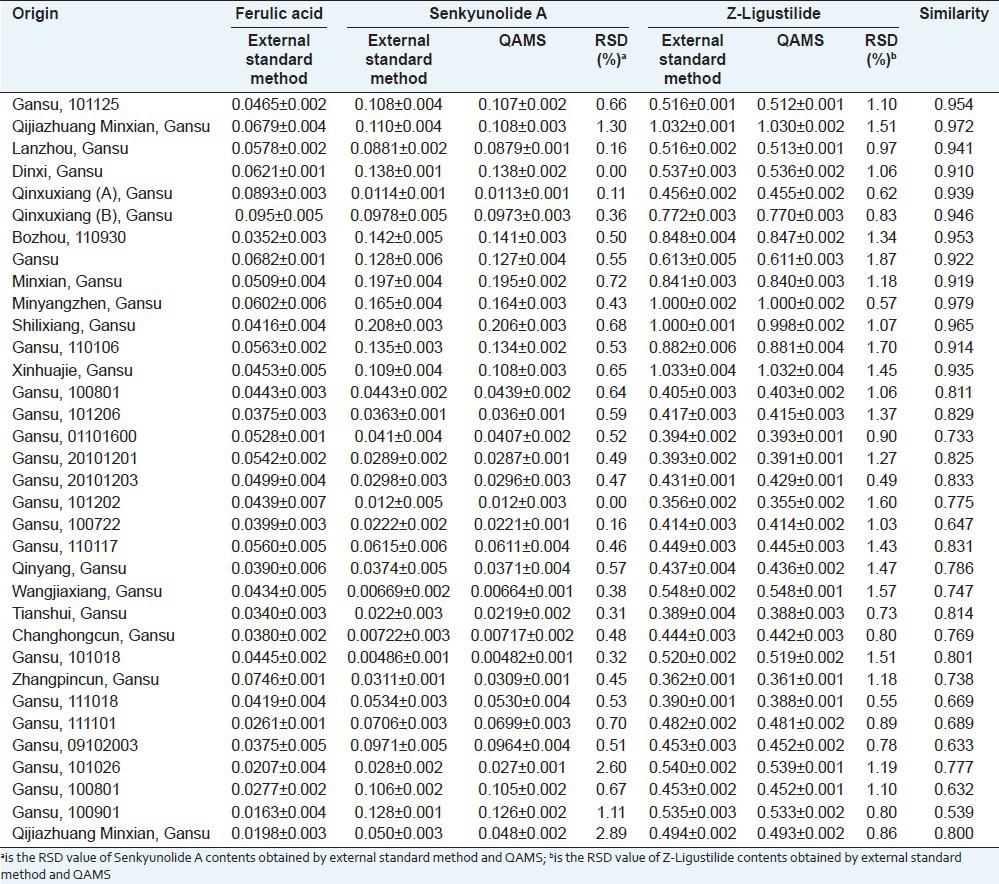
The HPLC method was applied to the simultaneous determination of ferulic acid, senkyunolide A, and Z-ligustilide compounds in Angelicae Sinensis Radix. Under these conditions, well-separated and reproducible chromatograms were produced [Figure 2]. Ferulic acid, contained in samples of Angelicae Sinensis Radix, before and after sulfur-fumigation were determined by the external standard method. Senkyunolide A and Z-ligustilide were calculated according to their RCFs. The contents of these three components, in samples, were determined with the external standard method to verify the applicability and accuracy of QAMS. There was no significant difference between these two methods, and RSDs were below 5% [Table 2]. Therefore, QAMS is a technique both feasible and accurate in the simultaneous determination of chemical compounds in Angelicae Sinensis Radix.
Table 2.
Comparing average determination of ferulic acid, senkyunolide A, and Z-ligustilide by the external standard method and QAMS (n=3) as well as the similarities in chromatograms of 34 samples. Values indicate sample mean and standard deviation from the mean and the similarities in chromatograms of 34 samples
Evaluation of durability and system suitability of QAMS
Effects of different instruments, different column temperature, and different flow rates on RCFs
The experiment was carried out in different labs. A Kromasil C18 (250 mm × 4. 6 mm, 5 μm) was used. The results showed that three different HPLC instruments, including Varian 920-LC, Agilent 1100, and Waters 515-2487, had little influence on RCFs. The effects of different column temperatures and different flow rates on RCFs were analyzed using Varian 920-LC and Kromasil C18. The RSDs were all below 5% [Table 3].
Table 3.
Effects of different instruments, different column temperatures and different flow rates on relative correction factors
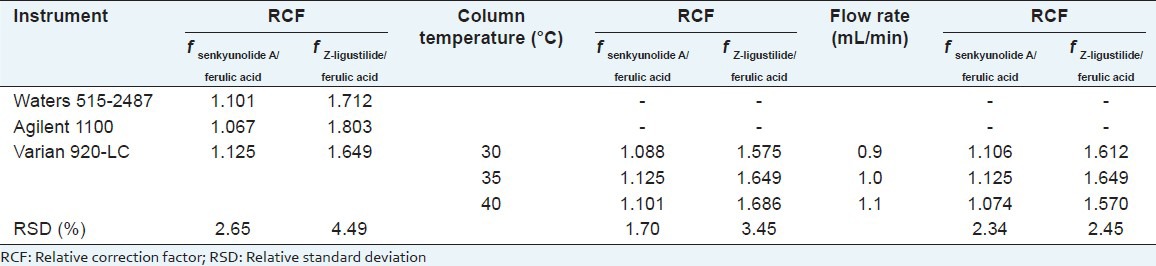
Effects of different chromatographic columns on RCFs and position of analyte peaks
Agilent 1100 and Varian 920-LC were used to investigate effects of four chromatographic columns on RCFs. As there were no other reference substances except for the IS, we only judged correct analyte peak positions by relative retention value, that is, knowing the IS together with retention time and peaks shape. The results indicated that the RSDs were all below 5% [Table 4].
Table 4.
Effects on four chromatographic columns for relative correction factors using Agilent 1100 and Varian 920-LC and the relative retention values of different instruments and chromatographic columns
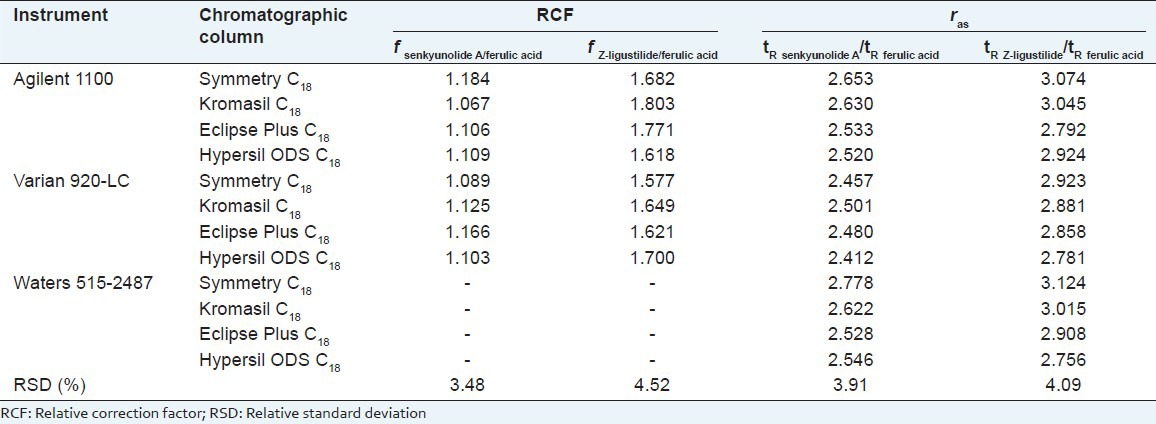
To assess the accuracy, validity, and scientific value of QAMS, we compared the measured value via the external standard method with the calculated value using QAMS. By examining the impact of RCFs for various chromatographic columns and HPLC systems, the results demonstrated that there was no significant difference between these two methods (RSDs <5%). The accuracy of RCFs were also relatively high. Therefore, QAMS is suitable for quantifying the multi-component in Angelicae Sinensis Radix, when authenticated standard substances are unavailable.
Comparison of ferulic acid, senkyunolide A, and Z-ligustilide contents in sun-dried and sulfur-fumigated Angelicae Sinensis Radix
From [Figure 2], we can see that, in the same coordinate, the peak height of sulfur-fumigated Angelicae Sinensis Radix was distinctly lower than that received from the sun-drying processing. Analysis of ferulic acid, senkyunolide A, and Z-ligustilide content using the external standard method and QAMS both found reduced levels in all three components after sulfur-fumigation. One-way analysis of variance (ANOVA) analysis, comparing sun-dried to sulfur-fumigated samples, found the differences were significantly different (P < 0.05).
The contents of senkyunolide A and Z-ligustilide decreased more than ferulic acid following sulfur-fumigation. This is due to the sulfur-fumigation process changing the condition of these two components from being lactone to acidic. During sulfur-fumigation, the lactonic ring is opened and reacts with oxygen. As a result, the double bond in the structure is oxidized, which brings about a reduction in content.
Ferulic acid, senkyunolide A, and Z-ligustilide provide important functions, such as platelet aggregation as well as antithrombotic and tracheal smooth muscle effects. These three substances are the main medicinal components in Angelicae Sinensis Radix. Our results indicated that sulfur-fumigation had a negative impact on the effective chemical components in Angelicae Sinensis Radix, leading to a decline in quality. This brings to question whether the practice of sulfur-fumigation should replace sun-drying processing, at least in the preparation of medicinal herbs.
HPLC fingerprint of Angelicae Sinensis Radix and similarity analysis
The thirty-four samples studies were processed by analytical instrument association (AIA) style and imported into the Similarity Evaluation System for Chromatographic Fingerprint of TCM software. Analyses confirmed the presence of 16 common peaks and identified the three chemical compounds through the reference substances. Peaks 3, 7, and 9 were identified as ferulic acid, senkyunolide A, and Z-ligustilide, respectively. The chromatographic fingerprint profiles showed abundant diversity of chemical constituents in sun-dried and sulfur-fumigated Angelicae Sinensis Radix [Figure 2]. Similarities of each chromatogram from the thirty-four samples were calculated with the reference fingerprint, which was the median of all chromatograms. Similarities of sun-dried samples (1-13) varied from 0.914 to 0.979, while chromatograms of sulfur-fumigated samples (14-34) ranged from 0.539 to 0.833 [Table 2]. These results implied that the chemical constituents of sun-dried and sulfur-fumigated Angelicae Sinensis Radix differ greatly.
Quality assessment by hierarchical clustering analysis
Hierarchical clustering analysis is a multivariate analysis method that is used to evaluate the resemblance and differences in these two groups of samples. The areas of the 16 common peaks of samples were calculated; the areas of the 16 constituents of samples 1-34 formed a matrix of 34 × 16. Clustering analysis, using average linkage (between groups) and square euclidean distance, was performed to differentiate and classify the thirty-four samples. The results of the hierarchical clustering analysis showed that samples could be divided into two main groups (I and II) [Figure 3a]. Samples 1-13 (sun-dried) were in cluster I and the remaining samples 14-34 (sulfur-fumigated) were in cluster II, indicating that the two processing methods result in significant differences in quality and illustrating that the sulfur-fumigation processing of Angelicae Sinensis Radix significantly alters chemical components than the sun-drying processing.
Figure 3.
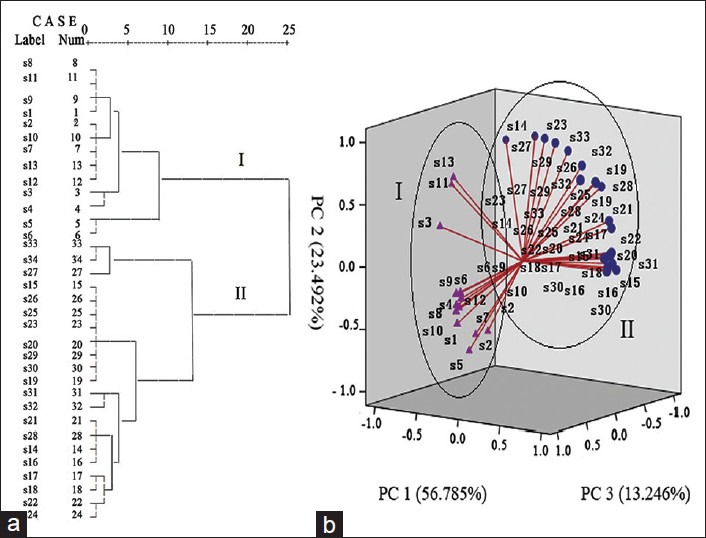
Chemometric figures of sun-dried and sulfur-fumigated Angelicae Sinensis Radix. (a) Result of hierarchical clustering analysis. (b) Loading plot obtained by principal components analysis
Discrimination of Angelicae Sinensis Radix using principal components analysis
To analyze further differences in Angelicae Sinensis Radix before and after sulfur-fumigation, PCA was used to independently discriminate each chemical component. A 34 × 16 data matrix was created. The first two principal components (PC 1 and PC 2) accounted for more than 80% of variance, 56.785% and 23.491%, respectively [Figure 3b]. Similar to HCA, samples 1-13 (sun-dried) were categorized differently from samples 14-34 (sulfur-fumigated), further illustrating that alterations in chemical components occur as a result of different post-harvest processing methods.
CONCLUSIONS
In this study, we successfully applied QAMS for the simultaneous determination of ferulic acid, senkyunolide A, and Z-ligustilide and chemometrics for the discrimination in sun-dried and sulfur-fumigated Angelicae Sinensis Radix. Our findings indicated that through the validation of the methodology and the verification of durability and system suitability, QAMS possesses high accuracy and feasibility. Furthermore, QAMS analyses showed that sulfur-fumigation processing had an obvious impact on the quantity of three crucial chemical compounds in Angelicae Sinensis Radix. The results of decreased quantity of the main effective components in sulfur-fumigated Angelicae Sinensis Radix tested by QAMS and chemometric discrimination in sun-dried and suflur-fumigated Angelicae Sinensis Radix concluded that sulfur-fumigation could significantly change the quality of Angelicae Sinensis Radix. The method developed in this study will be useful for providing a more efficient and feasible quality assessment of Angelicae Sinensis Radix, as well as application in evaluating other medicinal herbs.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (No. 81173546, No. 30940093, and No. 81202918), the Natural Science Foundation of Jiangsu Province, China (No. BK2009495), the International Science and Technology Cooperation Project of Jiangsu Province, China (No. BZ2011053), the Project of Science Technology Department of Zhejiang Province, China (No. 2012D60SA1C0065, and No. 2012D60SA1C0066), the Open Project of National First-Class Key Discipline for Science of Chinese Materia Medica, Nanjing University of Chinese Medicine (No. 2011ZYX2-001, and No. 2011ZYX2-006), the Chinese Medicine Research Program of Zhejiang Province, China (No. 2008ZA002), the Science Foundation of Zhejiang Chinese Medical University (No. 2011ZY25, and No. 7211093), the Project of Science and Technology for Chinese Medicine of Zhejiang Province, China (No. 2013KYB183), the Project of Science Technology Department of Hangzhou, China (No. 20130533B68), and the Fund of Zhejiang Modernization of Traditional Chinese Medicine Item (No. [2008]436, and No. [2012]680).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wang ZM, Gao HM, Fu XT, Wang WH. Multi-components quantitation by one marker new method for quality evaluation of Chinese herbal medicine. China J Chin Mater Med. 2006;31:1925–8. [PubMed] [Google Scholar]
- 2.Xie YC, Zhang QM, Jin SH. Quantitative determination of Huperzine A tablets by a new HPLC method of substitute for reference substance. Chin Pharm J. 2008;43:217–21. [Google Scholar]
- 3.Kuang YH, Zhu JJ, Wang ZM, Zhang QW. Simultaneous quantitative analysis of five alkaloids in Rhizoma of Coptis chinensis by multi-components assay by single marker. Chin Pharm J. 2009;44:390–4. [Google Scholar]
- 4.Cai HX, Chen J, Li P. Simultaneous assay of four isoflavonoids in Astragali Radix by QAMS. China J Chin Mater Med. 2010;35:2712–5. [PubMed] [Google Scholar]
- 5.Kong JJ, Zhu JJ, Wang ZM, Zhang QW, Gao HM, Yan LH. Quantitative analysis of polytype components in Forsythia suspensa by QAMS method. Chin Pharm J. 2010;45:1301–4. [Google Scholar]
- 6.Zhou JL, Li P, Li HJ, Jiang Y, Ren MT, Liu Y. Development and validation of a liquid chromatography/electrospray ionization time-of-flight mass spectrometry method for relative and absolute quantification of steroidal alkaloids in Fritillaria species. J Chromatogr A. 2008;1177:126–37. doi: 10.1016/j.chroma.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Wang ZM, Zhang QW, Feng WH. A quantitative method for simultaneous assay of five ingredients with one marker in Salvia miltiorrhiza. China J Chin Mater Med. 2011;36:2372–9. [PubMed] [Google Scholar]
- 8.2010. Vol. 1. Beijing: China Medical Science Press; 2010. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China; p. 285. [Google Scholar]
- 9.Wang ZM, Qian ZZ, Zhang QW, Zhu JJ, Gao HM, Wang ZT. Technical guidelines of developing QAMS. China J Chin Mater Med. 2011;36:657–8. [Google Scholar]
- 10.Liu XJ, Hu J, Li ZY, Qin XM, Zhang LZ, Guo XQ. Species classification and quality assessment of Chaihu (Radix Bupleuri) based on high-performance liquid chromatographic fingerprint and combined chemometrics methods. Arch Pharm Res. 2011;34:961–9. doi: 10.1007/s12272-011-0613-2. [DOI] [PubMed] [Google Scholar]
- 11.2010. Vol. 1. Beijing: China Medical Science Press; 2010. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China; pp. 124–5. [Google Scholar]
- 12.Du JR, Bai B, Yu Y, Wang CY, Qian ZZ. The new progress of the study about volatile oil of the Angelica. China J Chin Mater Med. 2005;30:1400–6. [PubMed] [Google Scholar]
- 13.Vol. 2. Shanghai: Shanghai Scientific and Technical Publishers; 1986. Jiangsu New Medical College. Traditional Chinese Medicine Dictionary; p. 2075. [Google Scholar]
- 14.Yang ZH, Song L, Qiao RX, Luo DQ. Study of extrinsic harmful residue-sulfur dioxide in traditional Chinese medicine. Chin J Pharm Anal. 2010;30:2246–50. [Google Scholar]
- 15.Liu JJ, Liu X, Cai H, Li SL, Cai BC. Further investigation on the reasons for contents of paeoniflorin in commercial Radix Paeoniae Alba of prepared Chinese crude drug lower than the standard of Chinese pharmacopeia. Chin J Pharm Anal. 2010;30:1817–21. [Google Scholar]
- 16.Liu JJ, Cai H, Liu X, Ma XQ, Li SL, Zong DQ, et al. Analysis of harmful heavy metals, sulfur and main trace elements from Paeoniae Radix Alba before and after sulfur-fumigated process by ICP-AES method. China J Chin Mater Med. 2011;36:1790–3. [PubMed] [Google Scholar]
- 17.Ma XQ, Cai H, Liu X, Liu JJ, Li SL, Zong DQ, et al. Analysis on the influence of essential oils from Hangzhou White Chrysanthemum before and after sulfur-fumigated process by GC-MS. J Chin Mass Spectro Society. 2011;32:374–9. [Google Scholar]
- 18.Lou YJ, Cai H, Liu X, Pei K, Ma XQ, Li SL, et al. Quick identification of sun-dried and sulfur-fumigated Angelicae Sinensis Radix by Fourier transform infrared spectroscopy. China J Chin Mater Med. 2012;37:1127–32. [PubMed] [Google Scholar]
- 19.Duan BZ, Huang LF, Chen SL. Study on the destructive effect to inherent quality of Fritillaria thunbergii Miq. (Zhebeimu) by sulfur-fumigated process using chromatographic fingerprinting analysis. Phytomedicine. 2012;19:562–8. doi: 10.1016/j.phymed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Li SL, Shen H, Zhu LY, Xu J, Jia XB, Zhang HM, et al. Ultra-high-performance liquid chromatography–quadrupole/time of flight mass spectrometry based chemical profiling approach to rapidly reveal chemical transformation of sulfur-fumigated medicinal herbs, a case study on White Ginseng. J Chromatogr A. 2012;1231:31–45. doi: 10.1016/j.chroma.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 21.Lu GH, Chan K, Leung K, Chan CL, Zhao ZZ, Jiang ZH. Assay of free ferulic acid and total ferulic acid for quality assessment of Angelica sinensis. J Chromatogr A. 2005;1068:209–19. doi: 10.1016/j.chroma.2005.01.082. [DOI] [PubMed] [Google Scholar]


