Abstract
Background:
Ferns are a group of plants that have been little explored from a chemical and biological perspective but that have interesting potential, occurring in various parts of the world.
Objective:
This work investigates the chemical profile and the biological effects of ferns from Brazil.
Materials and Methods:
Analyses were performed using rapid performance liquid chromatography (RP-LC) with a diode array detector (DAD). Extracts were tested for their in vitro antioxidant activity, by the total reactive antioxidant potential method and for their antichemotactic potential, by the Boyden chamber method. Cytotoxic effects were assessed by lactate dehydrogenase levels, while the monoamine oxidase (MAO) assay was carried out using a fluorescence-based method.
Results:
Different chemical compositions were found for the studied ferns, such as Asplenium gastonis, in which hesperidin was identified in its extract, while A. serra showed the presence of xanthone mangiferin. The most samples with highest antioxidant activity were the Asplenium serra, Lastreopsis amplissima and Cyathea dichromatolepis extracts, at 10 μg/mL. High antichemotactic activity was found for A. serra (94.06%) and Didymochlaena truncatula (93.41%), at 10 μg/mL. The extracts showed no cytotoxicity at the highest concentration. Against MAO-A, D. truncatula (82.61%), Alsophila setosa (82.21%), Cyathea phalerata (74.07%) and C. delgadii (70.32%) were the most active extracts (100 μg/mL).
Conclusion:
The hypothesis was considered that phenolics and triterpenes are responsible for these pronounced activities.
Keywords: Antichemotactic, antioxidant, ferns, liquid chromatography, MAO inhibition, non-cytotoxic
INTRODUCTION
Plants are an important source of biological active natural products, which present a wide variety of structure, physical/chemical and biological properties. Within the plant kingdom division, Pteridophyta represent a group of vascular plants, presenting 13,600 species, of which many are tree ferns found in the tropical and subtropical regions of the world.[1]
Few studies have demonstrated the presence of some secondary metabolites in ferns. The most commonly reported are flavonoid glycosides, mainly kaempferol, quercetin, luteolin and apigenin derivatives. Other compounds such as xanthones, spiropyranosyl derivatives and triterpenoids have also been reported. For Brazilian species, the number of studied ferns is still low. The extracts of these plants appear as complex mixtures of substances, requiring the use of sensitive techniques that enable separation of compounds for further identification.
The biological activities of some of these plants are described in the literature. Recent studies on the beneficial properties of ferns include: antibacterial activity of Dryopteris crassirhizoma;[2] antimicrobial activity for some Adiantum species;[3] cytotoxic and apoptotic capacity against liver cancer cells,[4] as well as, antinociceptive and anti-inflammatory activities for Cheilanthes farinosa.[5]
A significant number of fern species are commonly used in Brazilian folk medicine. An example is Cyathea phalerata Mart, which is used as an expectorant and for kidney diseases. This species is also used for several inflammatory diseases and in southeastern Brazil, is reported for the treatment of varicose veins and hemorrhoids.[6] It is well known that medicinal plants are widely used in the folk medicine of many countries to treat different pathological conditions. However, for many of the plants in use, the efficacy and the relevant active principles are unknown.
Therefore, the aims of this study were to investigate the in vitro antioxidant and antichemotactic activities of different species of ferns from Southern and Southeastern Brazil and to evaluate the inhibitory effects displayed by ferns extracts on monoamine oxidase subunits A (MAO-A) and B (MAO-B) obtained from rat brain mitochondria. Chemical analysis was also carried out, by rapid performance liquid chromatography (RP-LC), on some active fern extracts that have still be little studied. This study sought to characterize the biological properties of these plants, correlating them with the chemical profiles found.
MATERIALS AND METHODS
Chemicals and reagents
Caffeic acid and 3-D-galactoside-quercetin were obtained from Fluka Chemie (Buchs, Switzerland). Hesperidin and rutin were purchased from Merck (Darmstadt, Germany). Apigenin was obtained from Extrasynthese (Genay Cedex France) and chlorogenic acid from MP Biomedicals (Illkirch, France). Mangiferin was obtained as a secondary standard metabolite from Mangifera indica L. Methanol (HPLC grade) was purchased from Tedia (Fairfield, USA). Trifluoroacetic acid (analytical grade) was obtained from Vetec (Rio de Janeiro, Brazil). Luminol (5-amino-2,3-dihydro-1, 4-phthalazinedione), quercetin, glycogen from oyster (type II), bovine albumin, heparin, kaempferol, flavone and LPS (Lipopolysaccharide from Escherichia coli) were all purchased from Sigma Chemicals (St. Louis, MO, USA). AAPH (2,2’-azobis-2-methyl-propanimidamide, dihydrochloride) and trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxilic acid) were purchased from Aldrich Chemical (Milwaukee, WI, USA). Glycine was purchased from Nuclear (Diadema, Brazil). All other chemicals were of reagent grade.
Plant material
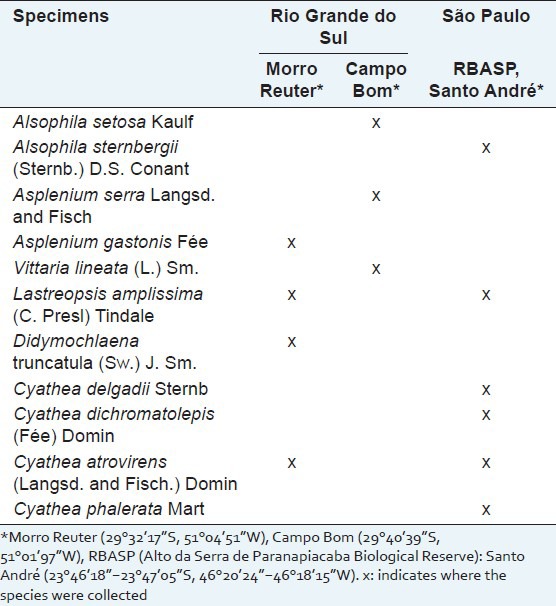
Seven ferns were collected in the regions of Morro Reuter (29°32’17”S, 51°04’51”W) and Campo Bom (29°40’39”S, 51°01’97”W), Rio Grande do Sul state (RS), Brazil, in September 2010 and received botanic identification by Maria Angélica Kienling-Rubio M.Sc. Voucher specimens were deposited at the Herbarium of the Instituto de Ciências Biológicas, Universidade Federal do Rio Grande do Sul, Brazil (ICN/UFRGS).
Another six fern specimens (two of them from the same southern species collected) were collected in the Reserva Biológica Alto da Serra de Paranapiacaba (23°46’18”-23°47’05”S, 46°20’24”- 46°18’15”W), Santo André, São Paulo state (SP), Brazil, in August 2010. The botanical identification of the specimens was performed by Dr. Jefferson Prado. A voucher specimen was deposited at the Herbarium of the Universidade de São Paulo (SPF/USP). The species collected in different locations of Southern and Southeastern Brazil are described in Table 1.
Table 1.
Species of ferns collected from different locations in the states of Rio Grande do Sul and São Paulo, Brazil
Extraction method
The air-dried and powdered aerial parts of ferns were extracted with ethanol in a turboextraction system, using a IKA T50 homogenizer Ultra Turrax apparatus. The resulting extracts were filtered and evaporated under reduced pressure, on a rotary evaporator at 40-45°C.
Chromatographic analysis
Liquid chromatographic conditions
The analyses were performed using a Waters 2695 Separation Module, consisting of a pump, a column heater, an auto sampler, a degasser and a DAD (UV/VIS Waters 996) photodiode array detector; equipment control, data acquisition and integration were managed with Waters Empower software (Waters, Milford, MA, USA). A C18 reversed-phase column (Phenomenex, 150 × 4.6 mm × 5 μm) was used, with a C18 Security Guard cartridge (C18) operating at a temperature of 25 ± 2°C.
The samples were dissolved in methanol (LC grade) to obtain final concentration extracts of 10 mg/mL. These extracts were filtered through a 0.45 μm pore size membrane (Millipore, Bedford, USA) before injection into the liquid chromatographic (LC) system. Elution of samples was performed using a linear gradient system and the mobile phases consisted of a mixture of water:trifluoroacetic acid (100:0.025; v/v) (A) and methanol (100; v) (B).
The gradient profile was: 0-10 min from 5 to 17% of B, 10-15 min from 17 to 30% of B, 15-40 min from 30 to 50% of B, 40-45 min from 50 to 100% B, 45-70 min 100% of B. At the end of each analysis, 7 min of 95% A was used to restore the initial conditions. The flow-rate was 0.6 mL/min and the injection volume was 10 μL. The detection was at 254 nm in the DAD (UV/VIS Waters 996) photodiode array detector. All the sample analyses were compared with pure commercial standards and with a secondary standard substance (mangiferin) injected under the same conditions.
Biological activities
Antioxidant activity by the total reactive antioxidant potential method
The total reactive antioxidant potential (TRAP) is widely used to estimate the antioxidant capacity of samples in vitro. This method is based on the quenching of luminol-enhanced chemiluminescence (CL) derived from the thermolysis of 2,20-azo-bis (2-amidinopropane) dihydrochloride (AAPH) as the free radical source.[7] A 96 cell-plate was used in the assay. The CL emission was monitored for 3000s, in a luminescence counter (MicroBeta TriLu× 1450 LSC, PerkinElmer).
The dried ethanolic fern extracts were dissolved in DMSO (dimethylsulfoxide) and then diluted with glycine buffer to their final concentrations. The extracts were evaluated at concentrations of 0.1, 1.0, 10.0, 50.0 and 100.0 μg/mL. The highest concentration of DMSO in samples was 1%. Quercetin and mangiferin were also tested at concentrations of: 1.0, 10.0 and 100 μM. All samples were tested in triplicate and the results were expressed as the area under curve (AUC) versus concentration (μg/mL).
Antichemotactic assay
The antichemotaxic assay was performed using a 48-well plate, as described by Boyden[8] with minor modifications introduced by Andrade et al.[9] The dried ethanolic extracts dissolved in DMSO (final concentration of up to 1%) were assayed at concentrations of 0.01, 0.10, 1.0 and 10.0 μg/mL. Mangiferin was tested at concentrations of 0.1, 1.0, 10.0, 50.0 and 100.0 μM and indometacin (50 μM) was used as the reference drug. All the samples were dissolved in Hanks’ balanced salt solution (HBSS, pH 7.4) to obtain final concentrations for the test.
To obtain the rat polymorphonuclear neutrophils, 20 mL of sterile 1% glycogen (w/v) were injected into the peritoneum of Wistar rats and four hours later, the animals were killed by decapitation and the leukocytes collected. The cell pellets were washed, suspended in HBSS, in order to obtain a leukocyte density of about 1.5 × 106 cells mL-1 and kept on ice until use. Chemotactic migration of leukocytes was measured using the micrometer on a fine-focus knob of a Nikon Alphaphot-2 YS2 microscope. The distance from the top of the filter to the plane of the farthest focus containing only two cells in ten microscopic fields, allowed leukocyte migration to be determined. Samples were tested in duplicate.
Measurement of the cytotoxicity of extracts against leucocytes
The cytotoxic effects of ferns on polymorphonuclear neutrophils (PMN) were determined by the release of the cytosolic lactate dehydrogenase, LDH (EC1.1.1.27), according to Selloum et al.[10] PMNs (1.5 × 107 cells/mL) were incubated with a concentration of 10 μg/mL of fern extract, for 30 min at 37°C. The LDH activity in the supernatant was measured at 492 nm (EnVision 2104, PerkinElmer) using a commercial kit (Doles). All the concentrations given are the final ones. The values for the samples were obtained using a standard curve.
Monoamine oxidase inhibition assay
MAO inhibition assays were carried out with a fluorescence-based method (end-point reading), according to van Diermen et al.,[11] with some minor modifications introduced by Dos Santos Passos et al.[12] The substrate used for the assay was kynuramine, which is non-fluorescent until it undergoes oxidative deamination by MAO, resulting in the fluorescent metabolite 4-hydroxyquinoline. Product formation was quantified by comparing the fluorescence emission of the samples to that of known amounts of the authentic metabolite, 4-hydroxyquinoline.
Reactions were performed in black, flat bottom 96-well plates, using a final volume of 200 μL. The mitochondrial fraction of MAO enzyme, obtained from brain homogenized of male Wistar rats (180-220 g), was prepared to obtain a final protein concentration of 1.0 mg/mL, in the assay mixture.[13] The samples were tested at a concentration of 100 μg/mL. The dried ethanolic extracts were dissolved in DMSO (at a final concentration of 1%) and then diluted in potassium phosphate buffer (0.1M, pH 7.4, made isotonic with KCl). Quercetin and mangiferin were also tested at concentrations of 10.0 and 100.0 μM.
As positive control for MAO activity (without inhibition), 40% DMSO solution (in phosphate buffered saline - PBS) was used in place of the inhibitor, in the absence of samples. As positive control for MAO-A and MAO-B activities, 40% DMSO solution (in PBS) and pargyline 10 μM (or clorgyline 10 μM) were used in the absence of the samples. Fluorescence emission at 380 nm was measured with a 96-well microplate fluorescent reader (EnVision 2104, PerkinElmer). Measurements by extracts, quercetin and mangiferin were performed in triplicate. The results of this assay were expressed as % of inhibition ± standard deviation means (SDM).
Statistical analysis
For the antioxidant assay (TRAP), the results were expressed as mean ± standard deviation mean (SDM) of the mean. In the antichemotactic assay, the data of leucocyte migration distances obtained from these experiments were analyzed as mean values ± S.D. of the mean (in micrometers) and expressed as a percentage of the maximal chemotaxis in relation to the reference chemoattractant (LPS). For the monoamine oxidase inhibition assay, values were expressed as the percentage inhibition of the enzymatic activity with standard deviation means (SDM).
A one-way analysis of variance (ANOVA) followed by Tukey's test was used for the comparison of means. A difference was considered statistically significant when P < 0.05. Data analyses were performed using Prism 5.0 (GraphPad Software, Inc., CA, USA).
RESULTS
Chromatographic analysis
The majority of the plant extracts proved to be highly complex, with many peaks in both, polar, medium and non-polar regions of the chromatograms. The LC of Asplenium gastonis [Figure 1] mostly presented eight compounds, with greater affinity for 30% to 50% of mobile phase B. In comparison with the chromatograms and UV spectra of the standards tested in this study, it was found that the sample presented some compounds with quercetin-related structures (peak at 20.3 min, UVmax : 253.4 and 343.1 nm) and hesperidin (47.56 min, UVmax : 281.9nm) as chemical compounds of the extract.
Figure 1.
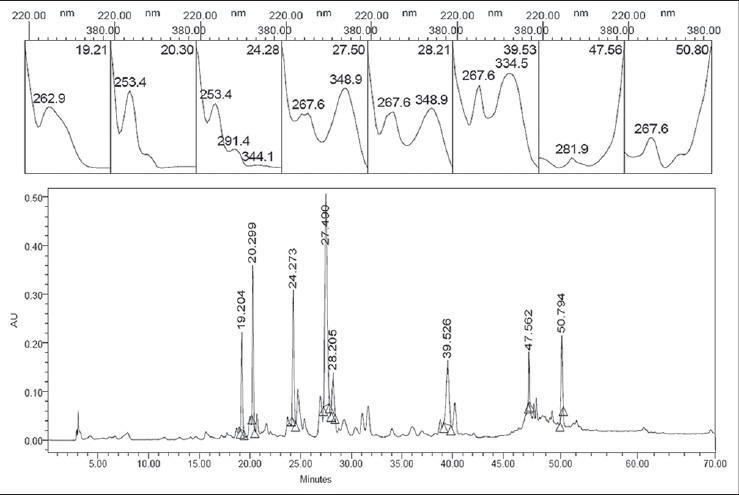
Chromatogram and ultraviolet spectra of major compounds of Asplenium gastonis. The selected peak is from hesperidin on the chromatogram
Another species of the Aspleniaceae family tested was Asplenium serra. In the chromatogram, this species [Figure 2a] presented mangiferin as the main compound. The retention time of mangiferin was 21.95 min (UVmax : 258.1, 315.3 and 361.8 nm). The identification of the compound was confirmed by comparing the chromatogram and UV spectra of the peak with those of the isolated substance [Figure 2b].
Figure 2a.
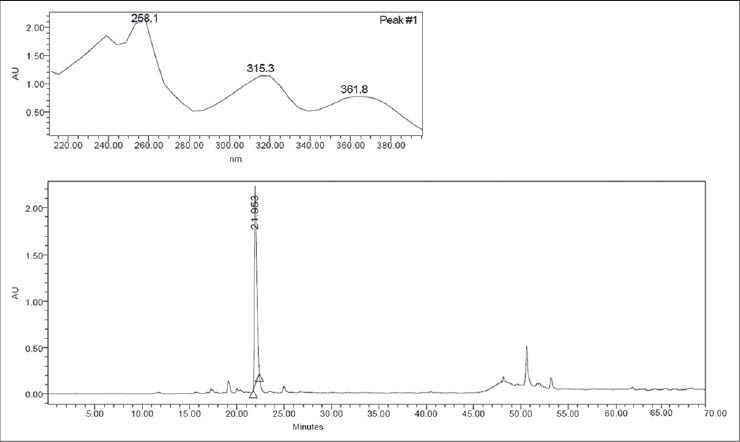
Chromatogram and ultraviolet spectra (UV) of Asplenium serra extract
Figure 2b.
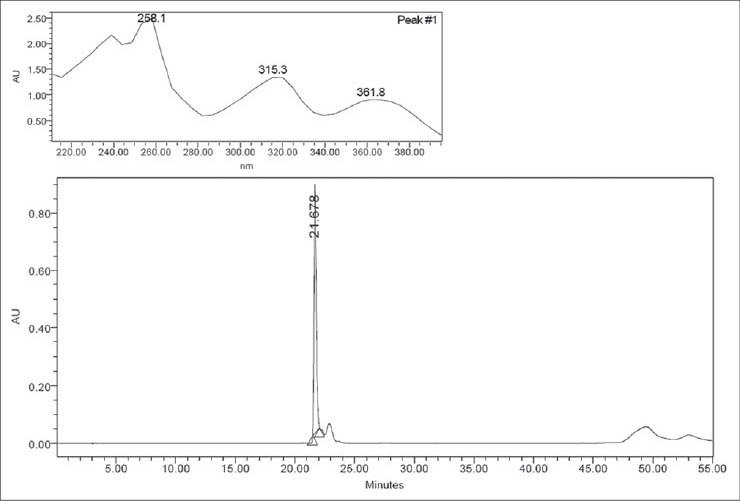
Chromatogram with UV spectra of mangiferin
The chromatogram of Cyathea phalerata presented six majority compounds, five of which showed similar UV spectra. For this sample, two distinct regions were found where compounds were eluted; one of them relatively more polar and the other non-polar. Three kaempferol-derivative glycosides were characterized in the extract of this fern species (not shown).
The species Lastreopsis amplissima was the only one collected both regions of Brazil (south and southeast), in order to compare the chemical composition. Four of the five majority peaks of L. amplissima (RS) presented very similar maximum UV (215.5 and 277.1 nm, or just 277.1 nm) to those found for coumarin and its derivatives. For L. amplissima (SP) the same UVmax were found for four compounds, and another four non-polar substances, eluted with 100% B, was observed in this chromatogram (results not shown).
Biological activities
The AOA of extracts from ferns was measured using the total reactive antioxidant potential (TRAP) assay. A strong antioxidant effect was verified for all the samples at concentrations of 100.0, 50.0 (data not shown) and of 10.0 μg/mL, with no significant differences between the values for the same samples (P < 0.05) and higher than the standard Trolox (200 nm). Figure 3 shows the radical scavenging activity of fern extracts at 10 μg/mL.
Figure 3.
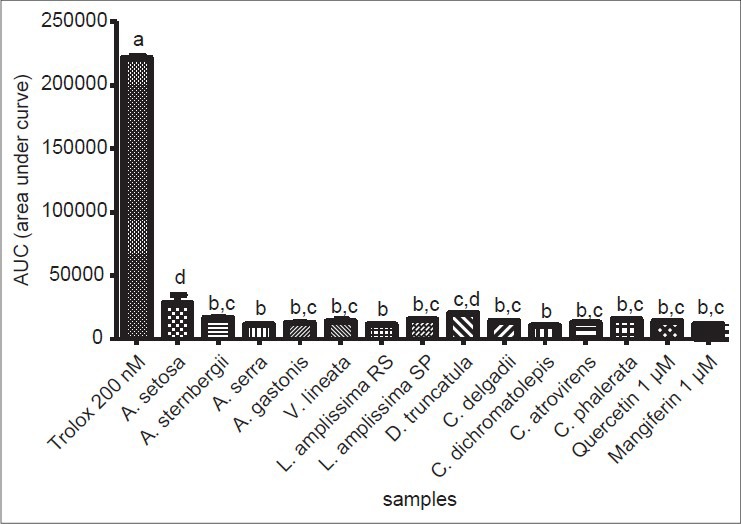
Total reactive antioxidant potential (TRAP) from fern extract samples at 10 μg/mL. Trolox at 200nM was used as standard antioxidant. Bars represent mean ± standard mean deviation (SDM). Statistically significant differences are represented by different letters in the graph (P < 0.05). (a) Statistically different from all the tested samples; (b) statistically different from A. setosa, D. truncatula; (c) statistically different from Trolox, A. setosa, A. serra, L. amplissima from Rio Grande do Sul (RS), C. dichromatolepis; (d) statistically different from Trolox, A. sternbergii, A. serra, A. gastonis, V. lineata, L. amplissima (RS), L. amplissima from São Paulo (SP), C. delgadii, C. dichromatolepis, C. atrovirens, C. phalerata, quercetin and mangiferin
The highest antioxidant capacities were obtained for the extracts of Asplenium serra, Lastreopsis amplissima (RS) and Cyathea dichromatolepis, although their values did not differ significantly from those of other samples (P < 0.05). Quercetin and mangiferin, even at the lowest concentration of 1 μM, presented good AOA. Trolox, the positive control at 500nM, showed longer lasting and superior activity when compared to 200nM [Figure 4a], indicating sensitivity of the system to variations in the concentration and its potential antioxidant capacity, even at very low concentrations.
Figure 4.
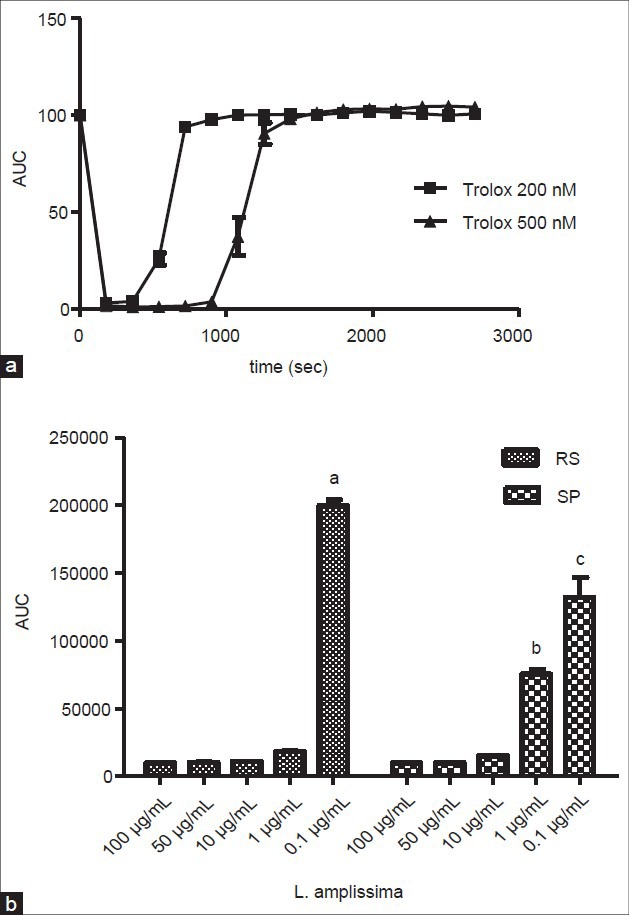
Antioxidant activity by total reactive antioxidant potential (TRAP) assay. A) Comparison of the kinetic activity from positive control (Trolox) at concentrations of 200 and 500nM. (B) Comparison of samples from Lastreopsis amplissima, from the states of Rio Grande do Sul (RS) and São Paulo (SP), at five different concentrations. (a), (b) and (c) Indicates values that are statistically different from all the other samples; P < 0.05
A comparison between the same species (Lastreopsis amplissima) collected at two different regions in Brazil demonstrated that both presented potent AOA at concentrations of 100, 50 and 10 μg/mL. At the concentration of 1 μg/mL, the sample from the State of Rio Grande do Sul (RS) showed a high AOA, compared to that exhibited by the highest concentrations tested, which did not occur for the sample collected in the State of São Paulo (SP). These samples, at this concentration, presented statistically different values (P < 0.05), where the Southern region sample was more active than that from the Southeast region [Figure 4b].
Chemotaxis plays an important role in leukocyte recruitment and therefore constitutes a target for anti-inflammatory drug discovery, the objective of which is to develop methods to control inflammation by modulating or blocking leukocyte chemotaxis to the site of inflammation.[14] Given that the suppression of neutrophil functions can control inflammatory responses and is one of the mechanisms of action of certain nonsteroidal anti-inflammatory drugs,[15] 16 samples from different ferns were investigated for their effects on leukocyte chemotaxis, using the Boyden chamber in vitro assay.
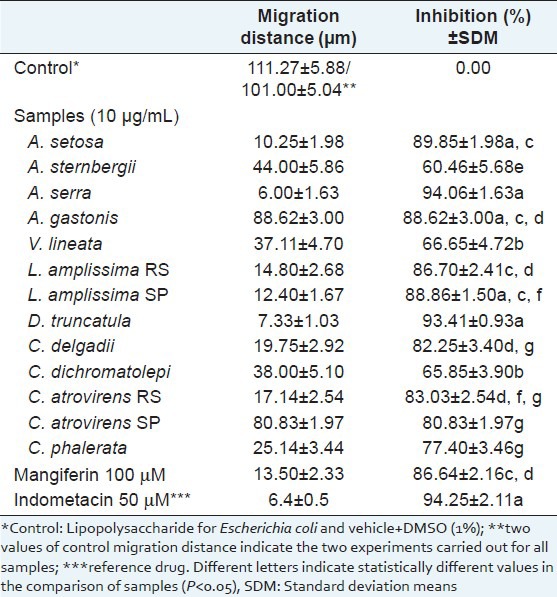
The values of migration distances (μm) and percentages of inhibition for samples at 10 μg/mL and mangiferin at 100 μM are presented in Table 2. All samples showed more than 54% leukocyte migration inhibition towards the chemotactic factor (LPS). The most active samples were Asplenium serra (94.06 ± 1.63%), Didymochlaena truncatula (93.41 ± 0.93%) and Alsophila setosa (89.85 ± 1.98), where their activity was compared to another two fern extracts tested (P < 0.05).
Table 2.
Effect of sample extracts of ferns (10 μg/mL) and of mangiferin (100 μM) on the in vitro chemotaxis of polymorphonuclear neutrophils towards lipopolysaccharide
The citotoxicity analysis, using the LDH method, measures cell death due to damage to the plasma membrane, and is based on the increase of the LDH activity in culture supernatant proportional to the number of lysed cells. Under the assay conditions, cell toxicity was not observed for any of the assayed samples. All the extracts, at 100 μg/mL, exhibited values compared to the control (P < 0.05), indicating total membrane cell viability [Figure 5]. Triton X at 1% was used as positive control, demonstrating cell death.
Figure 5.
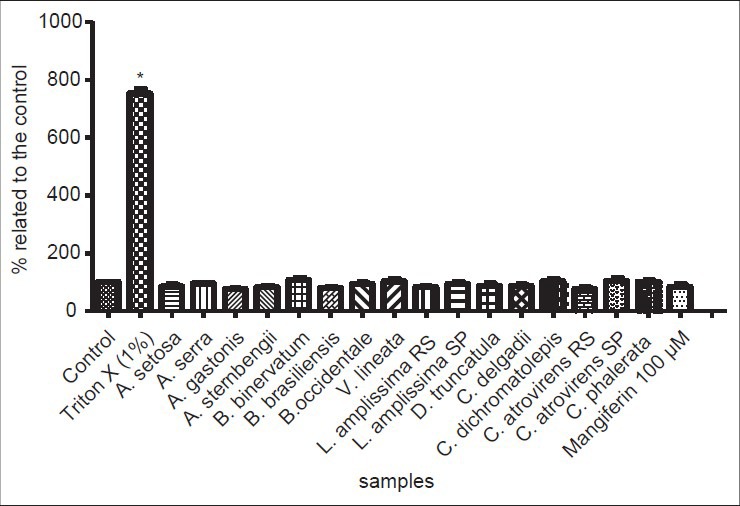
Results of lactate dehydrogenase (LDH) assay for cellular viability. Values of 100% indicate total cellular viability (control data). Triton X (1%) exhibited cell death, and was used as positive control. *indicates significant differences between means (P < 0.05)
The results for inhibitory activity displayed by fern extracts on MAO are shown in Table 3. The samples that inhibited MAO-A more strongly were Didymochlaena truncatula (82.61%), Alsophila setosa (82.20%), Cyathea phalerata (74.07%) and C. delgadii (70.32%), without significant differences among the values (P < 0.05). The pure substance, quercetin (100 μM), showed comparable activity against MAO-A (94.46%) to the positive control (98.98%) (P < 0.05), and also high inhibitory activity on MAO-B (63.76%), which demonstrates low selectivity for the different monoamine oxidase subunits.
Table 3.
Values of monoamine oxidase inhibition (expressed as a %) by ethanolic extracts from ferns (100 μg/mL) and of mangiferin and quercetin (100 μM, 10 μM). Standard deviation mean (SDM) of the values of activities against total monoamine oxidase, monoamine oxidase subunit A (MAO-A) and subunit B (MAO-B) are presented after each mean
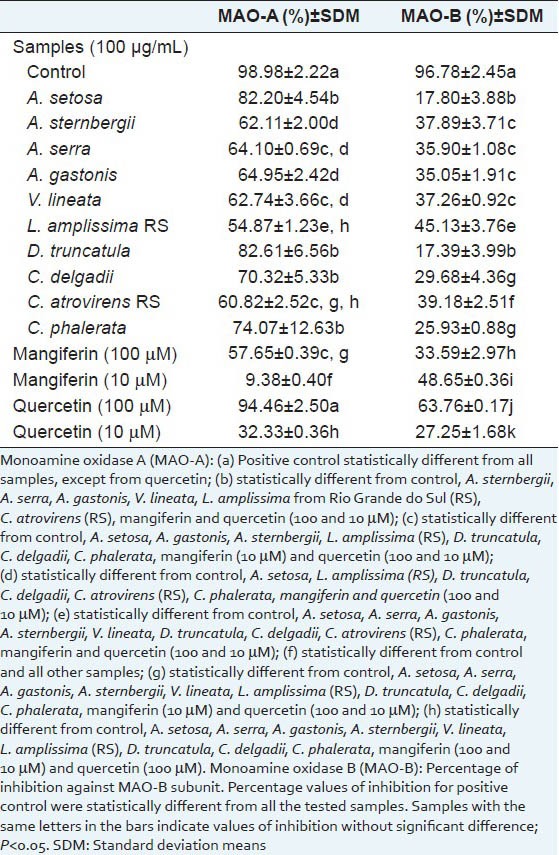
With regards to MAO-B inhibition, all fern samples were less active against this MAO subunit, since none resulted in more than 50% inhibition. The samples that were most active against MAO-A presented the lowest inhibitions of MAO-B, indicating a high degree of selectivity for the isoform A of the enzyme.
DISCUSSION
Chromatographic analysis
Polyphenols, from different classes, such as mangiferin (xanthone), hesperidin (flavonoid) and coumarin derivatives, were found in the fern extracts analyzed. The HPLC system proved good option for separating the compounds of these Pteridophytes from the South and Southeast regions of Brazil. Yet the chemical composition of these plants had not been investigated but the results of this study gave an interesting starting point for the chemical analysis of ferns.
Mangiferin is a well-known antioxidant and analgesic compound,[16] with antidiabetic activity, particularly in type 2 diabetes.[17] It also exhibits cardioprotective and antigenotoxic properties[18] among others. Some authors have previously reported the presence of xanthones as mangiferin and its analogue (isomangiferin) in Asplenium species such as Asplenium montanum[19] and Asplenium adiantum-nigrum.[20] Matsumoto et al.,[21] in their study, found the presence of flavonoids in hybrids of Asplenium normale, being apigenin and luteolin glycosides the detected mainly compounds.
Triterpenes, phenolic acids and flavonoids, whose aglicone is frequently kaempferol, were found in some records of phytochemical Brazilian studies of the genus Cyathea.[22] Pizzolatti et al.,[23] showed the presence of a glucopyranosyl caffeic acid, a glucopyranosyl p-coumaric acid, as well as cyathenosin A, in their study with Cyathea phalerata. The isolation of kaempferol and its glucopyranoside, as well as a glucopyranosyl caffeic acid, was described in the studies of C. phalerata by Brighente et al.[24]
Many factors are involved in the composition differences observed for plants of the same species, collected in different locations. Different weather and soil conditions of southern and southeastern Brazil regions are the main reasons for the differences in the compounds found in the same sample plant, obtained from these different regions. The age and plant development, as well as, different plant organs, are also of considerable importance and may influence not only the total amount of metabolites produced, but also the relative proportions of the mixture (reviewed in[25]).
Biological activities
With regard to the antioxidant methods, there are several in vitro and in vivo methods for evaluating the AOA of complex substance mixtures or even single compounds. Each method provides different results, expressed in different ways and units. Comparison of the results of AO, measured by different methods is often impossible. In the current study, the total reactive antioxidant potential (TRAP) method was used, characterized as a simple and fast method providing reliable results that are expressed as the area under the curve (AUC). This method is particularly useful for extract samples that contain multiple ingredients and have complex reaction kinetics and it is used for any antioxidant compound (or complex mixture) independent of the kinetic emission profile. A strong antioxidant effect was verified, using this method, for all samples at the concentration of 10.0 μg/mL [Figure 3].
Talukdar et al.,[26] reviewed pteridophyte antioxidants, demonstrating a total of thirty-six species with AOA and eight species in which the isolation of antioxidant compounds was performed. From the species reported in their studies, only Cyathea phalerata was also tested here. In the present study, this fern demonstrated a strong AOA, compared with that exhibited for the most active ferns (P < 0.05).
In another study by Hort et al.,[22] the antioxidant and hepatoprotector potential of the crude hydroalcoholic extract and of fractions obtained from C. phalerata was investigated. The authors noted that the ethyl acetate fraction of the crude extract displayed the best AOA, especially as a scavenger of the OH· radical, and was also the best for inhibiting lipid peroxidation by in vitro tests. For the in vivo tests, the same extract decreased thiobarbituric acid reactive substance levels, DNA damage and carbonyl protein contents, and also increased catalase and glutathione S-transferase activities. They also concluded that the flavonoids contained in this fraction may be responsible for these activities.
Brighente et al.,[24] determined the AOA of extracts and fractions of six vegetal species from the Brazilian Atlantic Forest, among them Cyathea phalerata. The hydroalcoholic extract of the leaves of this plant presented IC50 of 9.0 μg/mL in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, demonstrating high free radical sequestering activity. The authors also found that there was no parallelism between AOA and phenolic compound content for this species tested. Ding et al.,[27] analyzed 31 species of ferns, focusing on their phenolic composition and AOA. The results showed that most of the extracts had strong antioxidant activities, as well as high total phenolic contents.
It is well-established that free radical stress is involved in the etiology of several degenerative and non-degenerative disorders such as cancer,[28] diabetes,[29] inflammation,[30] neurological disease[31] and vascular disease,[32] among others. Thus, antioxidant agents act in the prevention and cure of many human diseases. The discovery of new sources of AO is of extreme interest to scientists, as it increases the available treatment options for patients.
The plant Asplenium serra demonstrated strong antioxidant and antichemotactic activities. It has been previously demonstrated that the antioxidant properties of some substances found in plants may be partially responsible for some of the therapeutic properties attributed to them, such as their anti-inflammatory activity.[33] Schinella et al.,[34] proposed that the anti-inflammatory activity of plant extracts may be due to the synergistic effect of pro-inflammatory enzyme inhibitors, free radical scavenging activities or corticoid-like effects. Therefore, the knowledge of chemical composition of this species enables further understanding of biological mechanisms of action from this plant extract.
Several phenolics are considered as antioxidant and anti-inflammatory agents, such as flavonoids, phenolic acids and xanthones, among others. Also, natural triterpenes, widely distributed in plants are known to be potential anti-inflammatory agents acting, for example, on the inflammatory mediators and enzymes.[35] In this study, the presence of mangiferin, hesperidin, glycoside derivatives of quercetin and coumarin derivates was identified. Triterpenes were not identified in the analyzed samples, as they present low adsorption in the UV region. The results may support the hypothesis that these compounds are responsible of the prominent antioxidant and antichemotactic activities observed. More specific studies are needed to confirm these hypotheses.
Monoamine oxidase (MAO) is an enzyme that oxidizes a variety of monoamine neurotransmitters and neuromodulators. MAO-A preferentially degrades biogenic amines such as epinephrine, norepinephrine and serotonin[36] and is very sensitive to the MAO-A inhibitor chlorgyline. In contrast, MAO-B is the main enzyme implicated in the metabolism of dopamine and is more sensitive to the MAO-B inhibitor, pargyline.[37]
MAO-A inhibitors have proven to be effective in the pharmacological treatment of depression.[38] Several studies have shown that MAO-B is involved in aging-related neurodegenerative diseases, such as Parkinson's disease[39] and in the formation of plaque-associated astrocytes present in the brains of patients suffering from Alzheimer's disease.[40] This division is questionable, since selective and reversible MAO-A inhibitors, such as moclobemide, may also be used for the treatment of Parkinson's disease, demonstrating improvements in psychomotor and long-term memory.[41]
In this context, some fern extracts that showed potential selective activity against MAO-A, such as Didymochlaena truncatula (82.61%), Alsophila setosa (82.20%), Cyathea phalerata (74.07%) and C. delgadii (70.32%), can be considered as antidepressant or anti-neurodegenerative candidates or even sources of active molecules against these diseases. Complementary studies are needed to confirm these hypotheses, as this is the first study to report such activity for fern extracts, demonstrating high relevance in pharmacological sciences.
CONCLUSIONS
In the chromatogram for Asplenium gastonis, hesperidin was identified. In Asplenium serra, mangiferin was identified as the main compound of the extract. Lastreopsis amplissima seems to present coumarin derivatives in its extract, with the sample from the Southeast Brazil exhibiting a more complex composition. Some Brazilian ferns present antioxidant, antichemotactic and MAO inhibitory activities, corroborating with previous research findings and contributing to the rational use of these plants in folk medicine.
Phenolics are suggested as being responsible for its important activities. It is well-established that free radical stress is involved in the etiology of several human disorders and inflammatory injuries affect people all over the world. Furthermore, MAO inhibitors are known to be important candidates for the treatment of neurodegenerative diseases. Thus, the compiled data indicates that ferns may be considered as important tools in the search for new substances that will contribute to the improvement of various human injuries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moran RC. Ranker TA, Haufler CH. Biology and Evolution of Ferns and Lycophytes. Cambridge: Cambridge University Press; 2008. Diversity, biogeography and floristics; pp. 417–61. [Google Scholar]
- 2.Kwon DY, Kang OH, Choi JG, Lee YS, Oh YC, Chae HS, et al. Antibacterial effect of Dryopteris crassirhizoma against methicillin-resistant Staphylococcus aureus. Fitoterapia. 2007;78:430–3. doi: 10.1016/j.fitote.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Singh M, Singh N, Khare PB, Rawat AK. Antimicrobial activity of some important Adiantum species used traditionally in indigenous systems of medicine. J Ethnopharmacol. 2008;115:327–9. doi: 10.1016/j.jep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Radhika NK, Sreejith PS, Asha VV. Cytotoxic and apoptotic activity of Cheilanthes farinosa (Forsk.) Kaulf. against human hepatoma, Hep3B cells. J Ethnopharmacol. 2010;128:166–71. doi: 10.1016/j.jep.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Yonathan M, Asres K, Assefa A, Bucar F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J Ethnopharmacol. 2006;108:462–70. doi: 10.1016/j.jep.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Korbes CV. Brazil: Studies Association, Guidance and Assistance Rural; 1995. Medicinal plants; p. 32. [Google Scholar]
- 7.Dresch MT, Kappel VD, Rossato SB, Biegelmeyer R, Mayorga P, Zuanazzi JA, et al. Optimization and validation of an alternative method to evaluate Total Reactive Antioxidant Potential (TRAP) Anal Biochem. 2009;385:107–14. doi: 10.1016/j.ab.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med. 1962;115:453–66. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade JM, Aboy AL, Apel MA, Raseira MC, Pereira JF, Henriques AT. Phenolic composition in different genotypes of Guabiju fruits (Myrcianthes pungens) and their potential as antioxidant and antichemotactic agents. J Food Sci. 2011;76:C1181–7. doi: 10.1111/j.1750-3841.2011.02375.x. [DOI] [PubMed] [Google Scholar]
- 10.Selloum L, Bouriche H, Tigrine C, Boudoukha C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol. 2003;54:313–8. doi: 10.1078/0940-2993-00260. [DOI] [PubMed] [Google Scholar]
- 11.van Diermen D, Marston A, Bravo J, Reist M, Carrupt PA, Hostettmann K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J Ethnopharmacol. 2009;122:397–401. doi: 10.1016/j.jep.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Dos Santos Passos C, Soldi TC, Torres Abib R, Anders Apel M, Simões-Pires C, Marcourt L, et al. Monoamine oxidase inhibition by monoterpene indole alkaloids and fractions obtained from Psychotria suterella and Psychotria laciniata. J Enzyme Inhib Med Chem. 2013;28:611–8. doi: 10.3109/14756366.2012.666536. [DOI] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-Phenol reagents. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Entschladen F, Drell TL, Lang K, Masur K, Palm D, Bastian P. Analysis methods of human cell migration. Exp Cell Res. 2005;307:418–26. doi: 10.1016/j.yexcr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Rioja I, Ubeda A, Terencio MC, Guillen I, Riguera R, Quintela JM. An anti-inflammatory ditriazine inhibiting leukocyte functions and expression of inducible nitric oxide synthase and cyclo-oxygenase-2. Eur J Pharmacol. 2000;397:207–17. doi: 10.1016/s0014-2999(00)00243-0. [DOI] [PubMed] [Google Scholar]
- 16.Dar A, Faizi S, Naqvi S, Roome T, Zikr-ur-Rehman S, Ali M, et al. Analgesic and antioxidant activity of mangiferin and its derivatives: the structure activity relationship. Biol Pharm Bull. 2005;28:596–600. doi: 10.1248/bpb.28.596. [DOI] [PubMed] [Google Scholar]
- 17.Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Lal J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol. 2005;97:497–501. doi: 10.1016/j.jep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Viswanadh EK, Rao BN, Rao BS. Antigenotoxic effect of mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with cadmium chloride. Hum Exp Toxicol. 2010;29:409–18. doi: 10.1177/0960327110361752. [DOI] [PubMed] [Google Scholar]
- 19.Smith DM, Harborne JB. Xanthones in the Appalachian Asplenium complex. Phytochemistry. 1971;10:2117–9. [Google Scholar]
- 20.Imperato F. Xanthone 2,4-di-C-glycosides from Asplenium adiantum-nigrum. Phytochemistry. 1991;30:3839–40. [Google Scholar]
- 21.Matsumoto S, Iwashina T, Kitajima J, Mitsuta S. Evidence by flavonoid markers of four natural hybrids among Asplenium normale and related species (Aspleniaceae) in Japan. Biochem Syst Ecol. 2003;31:51–8. [Google Scholar]
- 22.Hort MA, Dalbó S, Brighente IM, Pizzolatti MG, Pedrosa RC, Ribeirodo-Valle RM. Antioxidant and hepatoprotective effects of Cyathea phalerata Mart. (Cyatheaceae) Basic Clin Pharmacol. 2008;103:17–24. doi: 10.1111/j.1742-7843.2008.00214.x. [DOI] [PubMed] [Google Scholar]
- 23.Pizzolatti MG, Brighente IM, Bortolluzi AJ, Schripsema J, Verdi LG. Cyathenosin A, a spiropyranosyl derivative of protocatechuic acid from Cyathea phalerata. Phytochemistry. 2007;68:1327–30. doi: 10.1016/j.phytochem.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Brighente MC, Dias M, Verdi LG, Pizzolatti MG. Antioxidant activity and total phenolic content of some Brazilian species. Pharm Biol. 2007;45:156–61. [Google Scholar]
- 25.Gobbo-Neto L, Lopes NP. Medicinal plants: Factors of influence on the content of secondary metabolites. Quim Nova. 2007;30:374–81. [Google Scholar]
- 26.Talukdar AD, Tarafdar RG, Choudhury MD, Nath D, Choudhury S. A review on pteridophyte antioxidants and their potential role in discovery of new drugs. Biol Environ Sci. 2011;7:151–5. [Google Scholar]
- 27.Ding ZT, Fang YS, Tai ZG, Yang MH, Xu YQ, Li F, et al. Phenolic content and radical scavenging capacity of 31 species of ferns. Fitoterapia. 2008;79:581–3. doi: 10.1016/j.fitote.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Lau AT, Wang Y, Chiu JF. Reactive oxygen species: Current knowledge and applications in cancer research and therapeutic. J Cell Biochem. 2008;104:657–67. doi: 10.1002/jcb.21655. [DOI] [PubMed] [Google Scholar]
- 29.Muhammad S, Bierhaus A, Schwaninger M. Reactive oxygen species in diabetes-induced vascular damage, stroke, and Alzheimer's disease. J Alzheimers Dis. 2009;16:775–85. doi: 10.3233/JAD-2009-0982. [DOI] [PubMed] [Google Scholar]
- 30.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolanos JP, Moro MA, Lizasoain I, Almeida A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: Therapeutic implications. Adv Drug Deliv Rev. 2009;61:1299–315. doi: 10.1016/j.addr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: Bellwether for vascular disease? Cardiovasc Res. 2007;75:679–89. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic Biol Med. 1997;22:749–60. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 34.Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Ríos JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002;70:1023–33. doi: 10.1016/s0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 35.Ríos JL, Recio MC, Máñez S, Giner RM. Natural triterpenoids as anti-inflammatory agents. Stud Nat Prod Chem. 2000;22:93–143. [Google Scholar]
- 36.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: From genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novaroli L, Reist M, Favre E, Carotti A, Catto M, Carrupt PA. Human recombinant monoamine oxidase B as reliable and efficient enzyme source for inhibitor screening. Bioorg Med Chem. 2005;13:6212–7. doi: 10.1016/j.bmc.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 38.Priest RG, Gimbrett R, Roberts M, Steinert J. Reversible and selective inhibitors of monoamine oxidase A in mental and other disorders. Acta Psychiatr Scand. 1995;386:40–3. doi: 10.1111/j.1600-0447.1995.tb05923.x. [DOI] [PubMed] [Google Scholar]
- 39.Magyar K, Szende B. (-)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicology. 2004;25:233–42. doi: 10.1016/S0161-813X(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 40.Saura J, Luque JM, Cesura AM, Da Prada M, Chan-Palay V, Huber G, et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994;62:15–30. doi: 10.1016/0306-4522(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 41.Youdim MB, Bakhle YS. Monoamine oxidase: Isoforms and inhibitors in Parkinson's disease and depressive illness. Br J Pharmacol. 2006;147:S287–96. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]


