Abstract
Background:
Lycopene, a plant carotenoid, has potent effects against the various types of cancer cells. To date, the effect of lycopene in the free and encapsulated forms on the telomerase activity in human leukemia cell line K562 have not been investigated. The aim of the present study was to prepare a novel lycopene-loaded nanosphere and compare its anti-telomearse activity in K562 cell line with those of free lycopene.
Materials and Methods:
The lycopene-loaded nanospheres were prepared by nanoprecipitation method. The lycopene entrapment efficacy was measured by high-performance liquid chromatography (HPLC) method. The anti-proliferation effect of the lycopene in the free and encapsulated forms in the different times (0-72 h) and the different doses (0-100 μg/ml) on K562 cell line was studied using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. The changes of telomerase activity, following treatment with the lycopene in the free and encapsulated forms, were detected using the telomeric repeat amplification protocol-enzyme-linked immunosorbent assay.
Results:
The entrapment efficacy of lycopene was 78.5% ± 2. Treatment of the K562 cell line with lycopene, in particular in encapsulated form, resulted in a significant inhibition of the cell growth and increasing of percentage of apoptotic cells. It has also been observed that the telomerase activity in the lycopene-loaded nanospheres-treated cells was significantly inhibited in a dose and time-dependent manner.
Conclusion:
Our data suggest a novel mechanism in the anti-cancer activity of the lycopene, in particular in encapsulated form, and could be provided a basis for the future development of anti-telomerase therapies.
Keywords: Apoptosis, K562, lycopene, nanosphere, telomerase
INTRODUCTION
Telomerase is a cellular reverse transcriptase enzyme that can add nucleotide repeats to telomeres by using ribonucleic acid template.[1,2] Telomerase activity is closely dependent on the expression of subunit human telomerase reverse transcriptase (hTERT) that is widely expressed in many different cancers.[3,4,5,6,7,8] Many studies have indicated that the activation of telomerase and telomere stabilization is a crucial step in tumorigenesis.[3] Recent studies showed that the telomerase activity is present in the almost all tumor-derived human cell lines analyzed and in at least 85% of human tumor samples.[4,5] Additionally, many studies have demonstrated that the inhibition of telomerase triggers apoptotic death in various cell types.[6,7] In this case, many different synthetic chemical agents have been proposed for telomerase inhibition,[8,9] but most of these compounds have also influenced to the normal cell system with severe toxic side effects.[10] Extensive studies have been carried out in search of substances that are capable of inhibiting telomerase activity for retarding the growth of cancer cells without affecting normal cells.[11,12,13] In this field, certain plant-derived compounds have been found to be able to inhibit telomerase activity through transcriptional repression of hTERT leading to induction of cell death in several cancerous cell lines.[5,7,8,9,10,11,12,13]
Lycopene [Figure 1], a water insoluble carotenoids are found in some red fruits and vegetables, such as red carrots, papayas, and tomatoes.[14] Lycopene has an inhibitory effect on different kinds of cancer cells including colon, breast, endometrial, and prostate cancer cells.[15,16] However, the insolubility of the lycopene in the water has restricted its use in the biomedical research.[17]
Figure 1.
Lycopene chemical structure
Some studies showed that the encapsulation of plant-derived materials in the nanoparticles markedly alter their pharmacokinetics and compensated their water insolubility.[18,19] Nanoparticles as biodegradable and biocompatible polymeric submicron carriers have a general name to describe nanocapsules, nanospheres, and mixed micelles.[20,21] The nanospheres have a polymeric matrix and have been developed as drug targeting delivery system, using poly lactide, poly lactic acid-co-glycolid (PLGA) and other polymers.[22] PLGA is synthesized from two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid and used in drug delivery, owing to its biodegradability and biocompatibility.
To date, little is known about the molecular mechanism involved in the anti-cancer effect of lycopene on K562 cells,[23] and there is no report regarding the inhibitory effect of lycopene in the free and loaded forms on telomerase activity in this cell line. The aim of present study was therefore, to investigate the molecular mechanism involved in induction of apoptosis by lycopene in the free form and loaded in PLGA nanospheres in human leukemic cell line K562 with special emphasis on their role in inhibiting telomerase activity.
MATERIALS AND METHODS
Materials
Fetal bovine serum (FBS), Roswell Park Memorial Institute medium 1640 (RPMI)-1640, penicillin, streptomycin, and trypan blue were purchased from Gibco BRL (Gaithersburg, MD, USA). Lycopene (purity ≥ 90%), Hoechst 33342, PLGA, mineral oil, sorbitan monostearate, and tetrahydrofuran were obtained from Sigma (St. Louis, MO, USA). Annexin V-propidium iodide (PI) apoptosis Detection Kit and TeloTAGGG telomerase polymerase chain reaction (PCR)-enzyme-linked immunosorbent assay (ELISA) Plus kit were purchased from Roche (Applied Science, Germany). Methanol, methyl-tert-butyl ether and ammonium acetate were purchased from Merck (Darmstadt, Germany).
Preparation of lycopene-loaded nanospheres
The lycopene-loaded nanospheres were prepared by nanoprecipitation method, as described previously.[22] Briefly, the nanospheres suspension was prepared by dissolving the PLGA (l g), lycopene (1 g), and the sorbitan monostearate (0.766 g) in acetone (270 ml). This organic phase was added with moderate magnetic stirring into an aqueous solution (530 ml) containing the polysorbate 80 (0.766 g). Empty nanoparticles were prepared in a similar manner omitting the lycopene.
Characterization of nanospheres
The content of lycoene in nanospheres was determined by high-performance liquid chromatography (HPLC) as previously described.[24] Briefly, 20 mg of prepared nanospheres were dissolved in 0.5 ml of dimethyl sulfoxide (DMSO) and mixed with 0.98 ml of methanol: Water (50:50 vol./vol.) solution. Then, the 50 μl of mixture was injected into the HPLC column. In the HPLC analysis, a C30 column (3 μm, 150 × 4.6 mm, YMC, Wilmington, NC, USA) was used. The mobile phase for the determination of the lycopene was methanol/methyl-tert-butyl ether/water (83:15:2, v/v/v, with 1.5% ammonium acetate in the water; solvent A and methanol/methyl-tert-butyl ether/water (8:90:2, v/v/v, with 1% ammonium acetate in the water; solvent B at a flow rate of 1.2 ml/min and then the percentage of lycoene loading was then calculated as:
Amount of lycoene in nanospheres × total volume tested × 100/total sample volume × initial amount of nanospheres.
The particle size, zeta-potential, and polydispersity index of the lycopene-loaded nanospheres were evaluated using Malvern Zetasizer apparatus (Malvern Instrument, Worcestershire, UK), as reported previously.[22] Briefly, the particle size and zeta-potential of nanospheres were measured at angles of 90° and 120° at 25°C, respectively. These experiments were done in triplicate.
Cell culture
Human chronic myelogenic leukemia cell line K562 was purchased from Pasteur Institute of Iran (Tehran, Iran). The cells were maintained in RPMI-1640 medium supplemented with 10% heat inactivated FBS along with penicillin (100 units/ml) and streptomycin (100 μg/ml) and were grown at 37°C in a humidified atmosphere of 5% CO2. Lycopene was dissolved in the tetrahydrofuran to obtain a 100 mg/ml stock solution.[25] All subsequent dilutions were made in the RPMI medium.
Cell proliferation assay
Cell proliferation was assessed using the 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay as described previously.[26] Briefly, 5 × 103 cells were incubated in 96 well plates in the presence of different concentration of lycopene in the free and encapsulated forms (0, 10, 25, 50, 75 and 100 μg/ml) for 24, 48, and 72 h in a final volume of 200 μl. At the end of the treatment, 20 μl of MTT (5 mg/ml in phosphate buffered solution [PBS]) was added to each well and then incubated for an additional 2 h at 37°C. The purple-blue MTT formazan precipitate was dissolved in 200 μl of DMSO and the activity of the mitochondria that reflects cellular growth and viability was evaluated by measuring the optical density at 570 nm on microtiter plate reader. Three replicates of each concentration of lycopene in the free and encapsulated forms were performed.
Nuclear staining with Hoechst 33342
The K562 cells were incubated with the lycopene in the free and encapsulated forms (0, 10, 25, 50, 75, and 100 μg/ml) for 24, 48 h and were washed with PBS buffer. After fixation in freshly prepared ice-cold paraformaldehyde (0.1%) for 10 min, the cells were stained with Hoechst 33342 (50 μg/ml) for 1 min in the dark and its morphologic changes were observed under the fluorescent microscope. Subsequently, the percentage of apoptotic cells was determined after counting for at least 100 cells per treatment groups. This experiment was repeated in triplicate.
Flow cytometric analysis
The K562 cells were treated with varying concentrations of the lycopene in the free and encapsulated forms (0, 10, 25, 50, 75, and 100 μg/ml) in the complete medium for 24 and 48 h. At the end of each treatment, the cells were collected and quantitative apoptotic death assay was performed by Annexin V and PI staining following manufacturer's protocol and apoptotic cells were analyzed immediately by flow cytometry.
Telomerase activity assay
The cells were first treated with different concentrations of the lycopene in the free and encapsulated forms for 48 h. In order to investigation of the effect of different incubation time on telomerase activity, concentrations of the lycopene in the free and encapsulated forms at 100 μg/ml dose was selected for different time (0-72 h) incubation. At the end of each treatment, the cells were harvested and washed twice with washing buffer and lysed in the lysis buffer. The protein concentration was assay using Bardford method.[27] The telomerase activity was determined using the TeloTAGGG telomerase PCR-ELISA plus Detection Kit according to the manufacturer's protocol (Roche Applied Science, Germany). Briefly, the cell extracts were incubated in presence of biotin-labeled primers for 30 min and the telomeric repeats were built by cell-extracted telomerase. Subsequently, the elongated products as well as especial internal standard were amplified by PCR and then, the PCR products were denatured and split in two aliquots and each separately hybridized to a digoxigenin-labeled detection specific probes and was then allowed to bind to a streptavidin coated 96-well plate and the biotin-labeled PCR products were detected using peroxidase-conjugated antibody. Finally, the absorbance of developed blue color was measured at 450 nm by STAT-FAX 3200 ELISA reader (Awareness Technology, Inc., USA). The telomerase activity was measured in triplicate. As the negative control, each extract was heated at 95°C for 10 min prior to the PCR step. Relative telomerase activity (RTA) of each sample was calculated according to the instruction of TeloTAGGG Telomerase PCR-ELISA PLUS kit.
Statistical analysis
Data were presented as percentage of control. The results were represented as means ± standard deviation (SD). The analysis of variance was performed on data for all comparisons and the differences with P < 0.05 were considered statistically significant.
RESULTS
Characterization study
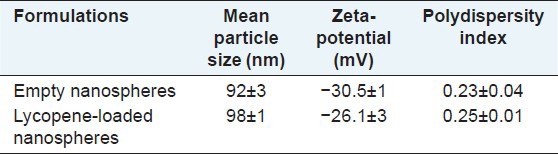
The results showed that the percentage of lycopene entrapment efficacy was 78.5% ± 2. Table 1 shows mean particle size, zeta-potential, and polydispersity index of empty and lycopene-loaded nanospheres. Size homogeneity of empty and loaded nanospheres suggested that lycopene was entrapped into nanoparticle core, according to previous studies.[28,29] In this work, the results from vesicle size distribution showed a monomodal pattern. Furthermore, zeta-potential and polydispersity index of the lycopene-loaded nanospheres revealed that nanoparticles have appropriate stability in aqueous dispersion, as reported previously.[30]
Table 1.
The particle size, zeta-potential, and polydispersity index of the empty and lycopeneloaded nanospheres
Proliferation study
The effect of the lycopene in the free and encapsulated forms on the cell growth was examined in the K562 cells by MTT assay. The results showed that the cell proliferation was inhibited in the K562 cells in a dose- and time-dependent manner [Figure 2]. In all conditions, the lycopene-loaded nanospheres were more effective than those of free lycopene on K562 cell growth. As shown in Figure 2, the extent of inhibition increased significantly at 24 h with 10 μg/ml of lycopene in the free and encapsulated forms which is continued to increase up to 48 h and 72 h with its maximum concentration (100 μg/ml).
Figure 2.
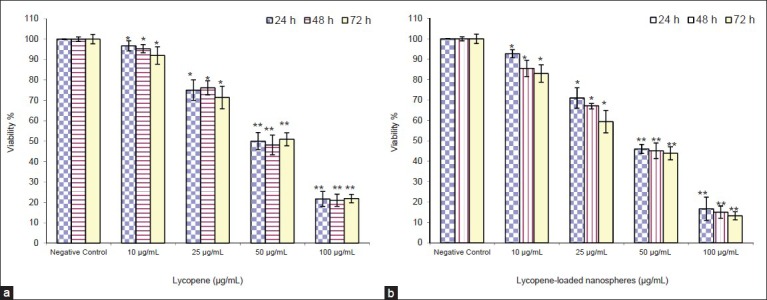
Dose- and time-dependent inhibition of K562 cell growth by the lycopene in the free (a) and encapsulated (b) forms. The cells were incubated with increasing concentration of lycopene in the free and encapsulated forms and then the cell survival was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Data were expressed as mean ± standard deviation from three independent experiments (*P < 0.05 and **P < 0.001)
Hoechst 33342 staining study
The staining of the lycopene-treated cells after 24 and 48 h under a fluorescence microscope showed characteristic apoptotic features such as chromatin condensation and nuclear fragmentation. For each experiment, 100 cells were scored at random under a fluorescence microscope and classified into apoptotic and non-apoptotic cells based on above characteristics. In particular, in the presence of lycopene-loaded nanospheres the percentage of the apoptotic cells was increased in a dose- and time-dependent manner [Figure 3].
Figure 3.
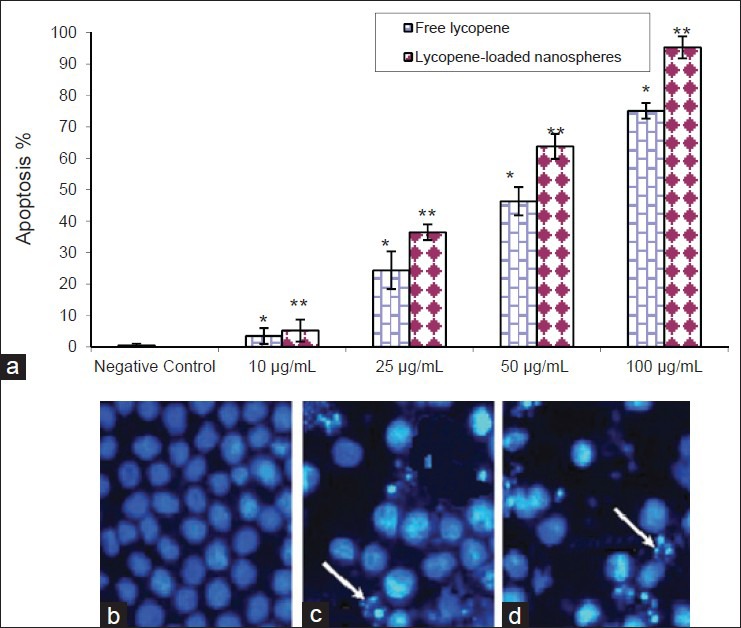
The K562 cells were incubated for 48 h without lycopene or with lycopene in the free and encapsulated forms (100 μg/ml) (a) and nuclear morphological changes observed under the fluorescent microscope after staining with hoechst 33342. As compared to control cells (b) fragmented or condensed nuclei indicative of apoptosis could be observed in the lycopene-treated as arrows indicated. The rate of apoptotic K562 cells after treatment with the lycopene in the free (c) and encapsulated (d) forms are presented in the. Data represent as mean ± standard deviation from three independent experiments (*P < 0.05 and **P < 0.001)
Flow cytometric study
As shown in the representative Fluorescence Activated Cell Sorting (FACS) analysis scatter-grams, control cells showed a large viable cell population, in contrast to the treated cells with lycopene, in particular in the loaded form, at 10, 25, 50, 75, and 100 μg/ml doses for 48 h resulted in a strong shift from live cells to the early and late apoptotic cells with a little change in the necrotic cell population [Figure 4].
Figure 4.
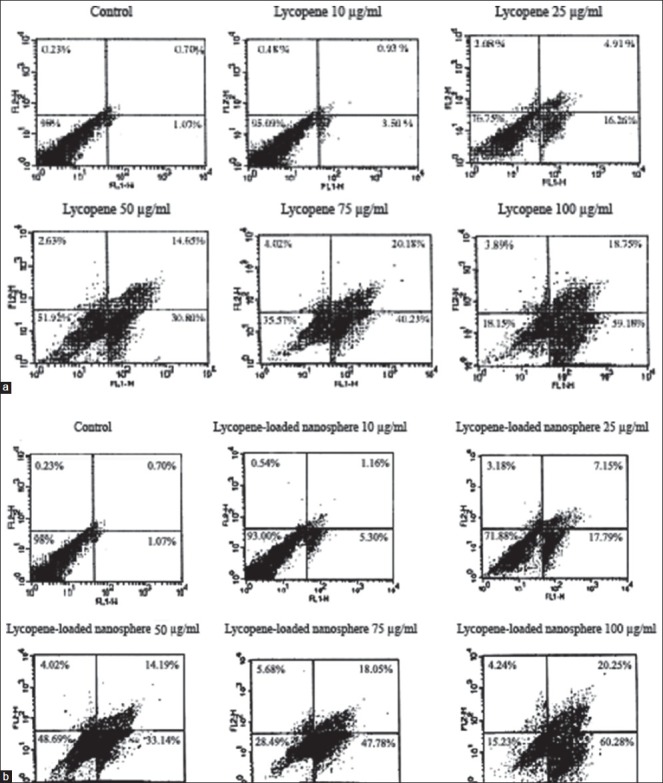
Lycopene, in particular in encapsulated forms caused strong apoptotic death on the K562 cell line. Representative Fluorescence Activated Cell Sorting analysis scatter-grams of Annexin V/propidium iodide stained 10, 25, 50, 75, and 100 μg/ml lycopene in the free (a) and loaded (b) forms treatment showed four different cell populations (*P < 0.05 and **P < 0.01)
Telomerase activity
The RTA of K562 cells, compared to untreated cells, was significantly suppressed after treatment with lycopene, in particular in the encapsulated form [Figures 5 and 6]. As compared to control group, results revealed that the RTA of K562 cells treated with 100 μg/ml lycopene in the free and loaded forms at 48 h was reduced to 23.5% ± 1.5 and 10.5% ± 1, respectively [Figure 5]. Similarly, as shown in Figure 6, RTA of K562 treated with lycopene in the free and loaded forms as compared to control group (100 μg/ml) was reduced to 19% ± 1.35 and 14% ± 1.1 at 48 h, respectively. Furthermore, there was no significant difference between the results obtained at 48 and 72 h (P > 0.05).
Figure 5.
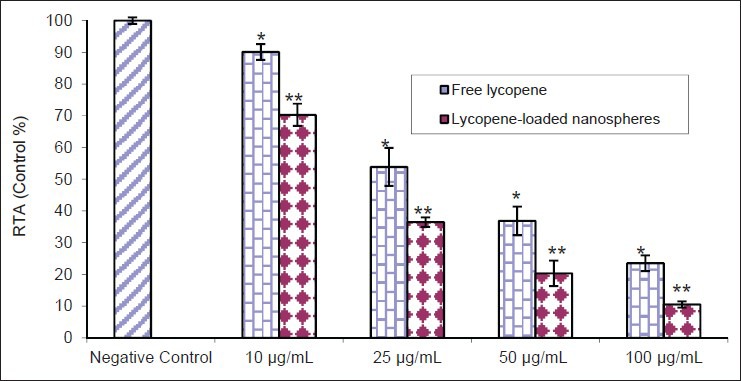
Dose-dependent inhibition of relative telomerase activity by the lycopene in the free and loaded forms as detected by telomeric repeat amplification protocol enzyme-linked immunosorbent assay after 48 h (*P < 0.05 and **P < 0.001). This experiment was done in triplicate
Figure 6.
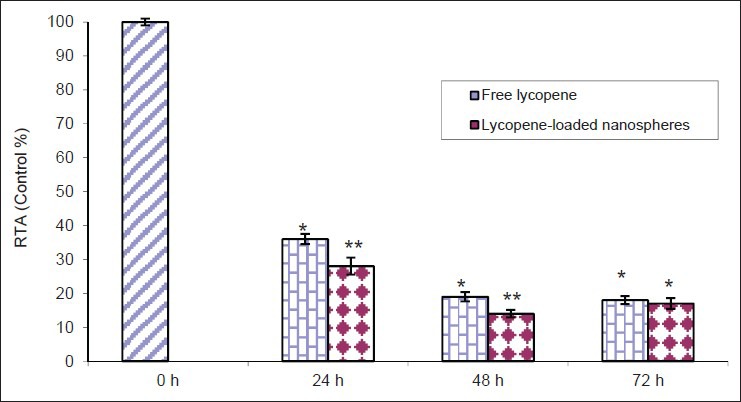
Time-dependent inhibition of relative telomerase activity by lycopene in the free and encapsulated forms (100 μg/ml) as detected by telomeric repeat amplification protocol enzyme-linked immunosorbent assay (*P < 0.05 and **P < 0.001). This experiment was done in triplicate
DISCUSSION
Lycopene, a carotenoid pigment and phytochemical, is naturally present in many vegetables and fruits such as tomatoes and pink grapefruit.[14] Evidence for lycopene's benefit was strongest for anti-oxidative, anti-tumor, anti-inflammatory, and chemo-preventive effects.[15,16,31] It is reported that plant-derived materials in the free and encapsulated forms that could suppress telomerase might be potential candidates as chemotherapeutic agents for cancer due to the fact that they have no side-effects.[7,8,9,10,11,12,13]
Telomerase activity occurred in a wide variety of cancer cells, including leukemia, and this phenomenon could enhance resistance to apoptosis through multiple mechanisms.[1,2,3,4,5] Telomerase has been proposed as an important marker for tumorigenesis and is a potentially highly selective target for the development of anti-proliferative agents and anti-tumor drugs.[1,2,3] The significant observation in this study was that the lycopene, in particular in the loaded form, inhibited telomerase activity in K562 cells with a dose as well as time dependent manner.
Our results showed that anti-telomerase activity of lycopene-loaded nanospheres in K562 cells was higher than those of free lycopene. Several hypotheses, including increased penetration of plant-derived materials into cells and water suspensibility may explain the mechanism of enhanced anti-cancer activities of this nanoparticulate formulation.[18,19]
The findings of this study showed a positive correlation between telomerase inhibition and induction of apoptosis. In this case, similar correlation between apoptosis and telomerase inhibition have been reported in the previous studies with other natural plant products such as silymarin,[32] berberin,[6] wagonin,[5] tea polyphenol,[7,8] curcumin,[9] gossypol,[10] butein,[11] Koran red ginseng extract,[12] and aqueous extract from Platy codon grantiflorum[13] in various cancer cell lines. Therefore, lycopene and other effective plant-derived agents in free and loaded forms could be considered as the promising strategy for developing the anti-telomerase drugs.
In conclusion, we have shown that the lycopene, in particular in the encapsulated form is a good candidate for inhibiting telomerase activity in leukemia. This is the first report regarding inhibition of telomerase activity by lycopene in the free and loaded forms in the K562. This strategy could be useful in supporting a rationale for clinical trial in leukemia patients.
ACKNOWLEDGMENT
This study was supported by Islamic Azad University, Borujerd Branch, Iran. The authors would like to acknowledge staffs of the university.
Footnotes
Source of Support: This study was supported by Islamic Azad University, Borujerd Branch, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Tian X, Chen B, Liu X. Telomere and telomerase as targets for cancer therapy. Appl Biochem Biotechnol. 2010;160:1460–72. doi: 10.1007/s12010-009-8633-9. [DOI] [PubMed] [Google Scholar]
- 2.Cheung AL, Deng W. Telomere dysfunction, genome instability and cancer. Front Biosci. 2008;13:2075–90. doi: 10.2741/2825. [DOI] [PubMed] [Google Scholar]
- 3.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–79. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann S, Martens UM. Telomeres and telomerase as targets for cancer therapy. Cell Mol Life Sci. 2007;64:906–21. doi: 10.1007/s00018-007-6481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang ST, Wang CY, Yang RC, Chu CJ, Wu HT, Pang JH. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine. 2010;17:47–54. doi: 10.1016/j.phymed.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Ji ZN, Ye WC, Liu GQ, Huang Y. Inhibition of telomerase activity and bcl-2 expression in berbamine-induced apoptosis in HL-60 cells. Planta Med. 2002;68:596–600. doi: 10.1055/s-2002-32896. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Hao MW, Dong K, Lin F, Ren JH, Zhang HZ. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Arch Pharm Res. 2009;32:1263–9. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- 8.Lin SC, Li WC, Shih JW, Hong KF, Pan YR, Lin JJ. The tea polyphenols EGCG and EGC repress mRNA expression of human telomerase reverse transcriptase (hTERT) in carcinoma cells. Cancer Lett. 2006;236:80–8. doi: 10.1016/j.canlet.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran C, Fonseca HB, Jhabvala P, Escalon EA, Melnick SJ. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002;184:1–6. doi: 10.1016/s0304-3835(02)00192-1. [DOI] [PubMed] [Google Scholar]
- 10.Moon DO, Kim MO, Choi YH, Lee HG, Kim ND, Kim GY. Gossypol suppresses telomerase activity in human leukemia cells via regulating hTERT. FEBS Lett. 2008;582:3367–73. doi: 10.1016/j.febslet.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Moon DO, Kim MO, Lee JD, Choi YH, Kim GY. Butein suppresses c-Myc-dependent transcription and Akt-dependent phosphorylation of hTERT in human leukemia cells. Cancer Lett. 2009;286:172–9. doi: 10.1016/j.canlet.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Park SE, Park C, Kim SH, Hossain MA, Kim MY, Chung HY, et0 al. Korean red ginseng extract induces apoptosis and decreases telomerase activity in human leukemia cells. J Ethnopharmacol. 2009;121:304–12. doi: 10.1016/j.jep.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Park DI, Lee JH, Moon SK, Kim CH, Lee YT, Cheong J, et al. Induction of apoptosis and inhibition of telomerase activity by aqueous extract from Platycodon grandiflorum in human lung carcinoma cells. Pharmacol Res. 2005;51:437–43. doi: 10.1016/j.phrs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Gerster H. The potential role of lycopene for human health. J Am Coll Nutr. 1997;16:109–26. doi: 10.1080/07315724.1997.10718661. [DOI] [PubMed] [Google Scholar]
- 15.Nahum A, Hirsch K, Danilenko M, Watts CK, Prall OW, Levy J, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001;20:3428–36. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 16.Narisawa T, Fukaura Y, Hasebe M, Ito M, Aizawa R, Murakoshi M, et al. Inhibitory effects of natural carotenoids, alpha-carotene, beta-carotene, lycopene and lutein, on colonic aberrant crypt foci formation in rats. Cancer Lett. 1996;107:137–42. doi: 10.1016/0304-3835(96)04354-6. [DOI] [PubMed] [Google Scholar]
- 17.Gouranton E, Yazidi CE, Cardinault N, Amiot MJ, Borel P, Landrier JF. Purified low-density lipoprotein and bovine serum albumin efficiency to internalise lycopene into adipocytes. Food Chem Toxicol. 2008;46:3832–6. doi: 10.1016/j.fct.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Singh M, Bhatnagar P, Srivastava AK, Kumar P, Shukla Y, Gupta KC. Enhancement of cancer chemosensitization potential of cisplatin by tea polyphenols poly (lactide-co-glycolide) nanoparticles. J Biomed Nanotechnol. 2011;7:202. doi: 10.1166/jbn.2011.1268. [DOI] [PubMed] [Google Scholar]
- 19.Ranjan AP, Mukerjee A, Helson L, Vishwanatha JK. Scale up, optimization and stability analysis of curcumin C3 complex-loaded nanoparticles for cancer therapy. J Nanobiotechnology. 2012;10:38. doi: 10.1186/1477-3155-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou XL, He JT, Du HJ, Fan YY, Wang Y, Zhang HX, et al. Pharmacokinetic and pharmacodynamic profiles of recombinant human erythropoietin-loaded poly (lactic-co-glycolic acid) microspheres in rats. Acta Pharmacol Sin. 2012;33:137–44. doi: 10.1038/aps.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Punfa W, Yodkeeree S, Pitchakarn P, Ampasavate C, Limtrakul P. Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol Sin. 2012;33:823–31. doi: 10.1038/aps.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gharib A, Faezizadeh Z, Mohammad Asghari H. Preparation and antifungal activity of spray-dried amphotericin B-loaded nanospheres. Daru. 2011;19:351–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Salman H, Bergman M, Djaldetti M, Bessler H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomed Pharmacother. 2007;61:366–9. doi: 10.1016/j.biopha.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Yeum KJ, Ahn SH, Rupp de Paiva SA, Lee-Kim YC, Krinsky NI, Russell RM. Correlation between carotenoid concentrations in serum and normal breast adipose tissue of women with benign breast tumor or breast cancer. J Nutr. 1998;128:1920–6. doi: 10.1093/jn/128.11.1920. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Wang Q, Neil B, Chen X. Effect of lycopene on androgen receptor and prostate-specific antigen velocity. Chin Med J (Engl) 2010;123:2231–6. [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Safdar A, Ma J, Saliba F, Dupont B, Wingard JR, Hachem RY, et al. Drug-induced nephrotoxicity caused by amphotericin B lipid complex and liposomal amphotericin B: A review and meta-analysis. Medicine (Baltimore) 2010;89:236–44. doi: 10.1097/MD.0b013e3181e9441b. [DOI] [PubMed] [Google Scholar]
- 29.Vyas SP, Gupta S. Optimizing efficacy of amphotericin B through nanomodification. Int J Nanomedicine. 2006;1:417–32. doi: 10.2147/nano.2006.1.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Suresh PK, Desmukh R. Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine. 2010;6:318–23. doi: 10.1016/j.nano.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–8. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 32.Faezizadeh Z, Mesbah-Namin SA, Allameh A. The effect of silymarin on telomerase activity in the human leukemia cell line K562. Planta Med. 2012;78:899–902. doi: 10.1055/s-0031-1298464. [DOI] [PubMed] [Google Scholar]


