Abstract
Attachment to one's kin as an in-group emerges from a fundamental human motivation and is vital for human survival. Despite important recent advances in the field of social neuroscience, the neural mechanisms underlying family-related in-group perception remain obscure. To examine the neural basis of perceiving family-related in-group boundaries in response to written kinship scenarios, we used functional magnetic resonance imaging in 27 healthy adults and obtained self-report ratings of family-related entitativity, which measures to what degree participants perceive their family as a coherent and distinct group in society. We expected that activity in the subgenual cingulate cortex and septo-hypothalamic region would track individual differences in entitativity. Perceiving one's family as a distinct and cohesive group (high entitativity) was associated with increased subgenual cortex response to kinship scenarios. The subgenual cingulate cortex may represent a key link between kin-related emotional attachment and group perception, providing a neurobiological basis for group belongingness.
Keywords: Belongingness, Attachment, Entitativity, Subgenual cingulate cortex, fMRI
The need to belong to an in-group is a fundamental human motivation (Baumeister & Leary, 1995). The family is a primary in-group, and kin attachment increases cooperation and evolutionary fitness (Cornwallis, West, Davis, & Griffin, 2010). However, the neural mechanisms underlying family-related group perception and belongingness are still obscure. Social psychological research (Correll & Park, 2005) has highlighted a factor that determines whether individuals derive psychological comfort from group membership (Gaertner, Iuzzini, Witt, & Oriña, 2006): entitativity, or the degree to which individuals perceive their group as coherent and distinct. Higher entitativity heightens the “glow of inclusion” and the pain of rejection (Bernstein, Sacco, Young, Hugenberg, & Cook, 2010), as well as the sense of security and the value attached to group membership (Sacchi, Castano, & Brauer, 2009). To unveil the mechanisms underlying kin attachment in humans, it is thus crucial to understand the neural basis of family-related entitativity.
Research in humans and animal models has implicated a number of cortical and subcortical structures in social attachment mechanisms and prosocial behaviors (Insel & Young, 2001; Moll, Zahn, de Oliveira-Souza, Krueger, & Grafman, 2005; Nelson & Panksepp, 1998). Altruistic decisions, for instance, are promoted by affiliative emotions—such as compassion toward others—and charitable donations to societal causes engage the subgenual cortex (SGC, BA 25) (Moll et al., 2006). The SGC modulates affiliative behavior, in part through the effects of oxytocin and arginine vasopressin (McCall & Singer, 2012; Petrovic, Kalisch, Singer, & Dolan, 2008; Zink & Meyer-Lindenberg, 2012) in humans and other social animals. Furthermore, the SGC is activated when subjects view pictures of romantic partners or their own infants (Bartels & Zeki, 2004).
Neuroimaging studies have additionally suggested a role for hypothalamic, ventral striatal, ventral tegmental, medial prefrontal and cingulate regions, including the SGC, in social attachment to kin and other in-group members (Depue & Morrone-Strupinsky, 2005; Insel & Young, 2001; Strathearn, 2011). Affiliative scenarios activate the septal/anterior hypothalamic area, independent of emotional valence (Moll et al., 2012). Furthermore, in-group-related stimuli and perceived interpersonal similarity (Mobbs et al., 2009; Morrison, Decety, & Molenberghs, 2012) as well as stimuli evaluated as “self-related” (Northoff & Bermpohl, 2004) reliably engage hemodynamic responses in medial prefrontal regions encompassing the SGC. How the brain represents perceived family-related entitativity remains unexplored. Therefore, we used functional magnetic resonance imaging (MRI) in healthy adults and obtained self-report ratings of family-related entitativity. We hypothesized that individual differences in entitativity would be represented in the subgenual cingulate cortex and septo-hypothalamic region.
RESULTS
Twenty-seven healthy participants saw written family-related affiliative scenarios (Moll et al., 2012). Perceiving one's family as a distinct and cohesive group (high entitativity) was associated with increased SGC activity (Figure 1). The effects of entitativity in the SGC survived family-wise error (FWE) correction (10-mm radius sphere; p = .04), using an a priori region of interest (ROI) centered at Montreal Neurological Institute (MNI) coordinates x = 0, y = 26, z = −5 derived from averaging local maxima in two independent functional MRI (fMRI) studies of prosocial feelings (Moll et al., 2006; Zahn, Moll et al., 2009). This analysis yielded a cluster with a peak-voxel coordinate MNI: x = 0, y = 20, z = −8 (Table 1), suggesting a bilateral effect within the SGC. Importantly, supporting analyses demonstrated that this effect was selective for the affiliative condition, as no parametric responses were observed within the SGC in the non-affiliative condition even at lenient thresholds (p < .1). Additional regions emerged in this analysis, but none survived an FWE-corrected threshold of p = .05 across the whole brain. The subgenual cluster survived correction for multiple comparisons using an independent a priori ROI (FWE-corrected p = .04).
Figure 1.
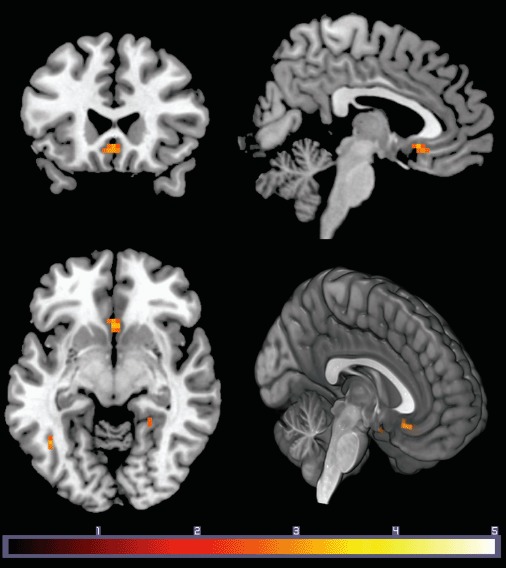
Subgenual cingulate cortex activation for affiliative versus non-affiliative stimuli was associated with stronger perception of one's family as a distinct group (entitativity). Results are displayed at p < .005 uncorrected, k = 10. Effects in the SGC survived FWE corrected over an a priori ROI (p = .04; 10 mm ROI). Color bar indicates t value.
TABLE 1.
Brain regions where activation differences in the affiliative versus non-affiliative contrast correlate with family-related entitativity ratings (significance level at p < .005 uncorrected; minimum cluster size = 10)
| MNI local maxima |
||||||
|---|---|---|---|---|---|---|
| Region | Cluster size | z | p (unc) | x | y | z |
| Middle temporal gyrus | 18 | 4.45 | .001 | 57 | −4 | −20 |
| Parahippocampal gyrus | 49 | 3.98 | .001 | 24 | −34 | −14 |
| Parahippocampal gyrus | 29 | 3.87 | .001 | −39 | −28 | −20 |
| Inferior frontal gyrus | 52 | 3.58 | .001 | 54 | 14 | 25 |
| Dorsal anterior cingulate | 14 | 3.54 | .001 | −18 | 35 | 25 |
| Posterior parahippocampal gyrus | 30 | 3.50 | .001 | −24 | −43 | −14 |
| Subgenual cingulate cortex | 28 | 3.24 | .001 | 0 | 20 | −8 |
| Fusiform/middle temporal gyrus | 49 | 3.23 | .001 | −45 | −55 | −2 |
| Septal area | 17 | 3.10 | .001 | −12 | 5 | −20 |
| Cerebellum | 12 | 3.05 | .001 | −18 | −67 | −26 |
Notes: The subgenual cluster survived correction for multiple comparisons using an independent a priori ROI (FWE-corrected p = .04). None of the regions survived an FWE-corrected threshold of p = .05 across the whole brain.
There was no difference between positive and negative affiliative subconditions in SGC activation (affiliative positive: r = .53; affiliative negative: r = .48; z = .23, p = .4), indicating that emotional valence had no influence on SGC hemodynamic response to individual differences in entitativity.
DISCUSSION
Our findings support the prediction that the SGC enables the association of complex social contexts with affiliative emotions (Zahn, de Oliveira-Souza, & Moll, 2011), which is in agreement with its selective engagement in altruistic donations (Moll et al., 2006), social exclusion (Cristofori et al., 2013) and guilt elicitation depending on trait individual differences in empathic concern (Zahn, de Oliveira-Souza, Bramati, Garrido, & Moll, 2009). This effect is further consistent with the involvement of ventromedial prefrontal regions in compassion for pain felt by in-group members (Decety, 2011) and with the effect of in-group similarity in economic games (Mobbs et al., 2009). Our findings may also bear impact on two other fields: first, given the overlapping SGC responses in normal attachment and in depressive disorders (Drevets, Öngür, & Price, 1998; Mayberg et al., 2005), dysfunctional in-group perception may contribute to insecure attachment and altered subgenual activity in depression; second, altered in-group perception may mediate the increases in SGC activity induced by the “social neuropeptide” oxytocin in trust and in infant–parent bonding conditions (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008; Zak, Kurzban, & Matzner, 2005).
A previous study on human affiliation (Moll et al., 2012) has demonstrated that affiliative experience can be dissociable from general hedonic experience (e.g., positive and negative emotional valence). Our findings are consistent with that result, as SGC effects on group perception did not differ between positive and negative emotional valence. Some caveats and limitations should be emphasized. Participants were neither aware nor explicitly informed about the group perception aspect when performing the fMRI task, which only required them to evaluate each social scenario as emotionally positive or negative. Because most imaging studies using social group manipulation employed explicit tasks (e.g., Mobbs et al., 2009; Morrison et al., 2012), direct comparisons to our findings should be made with caution. In addition, overall familiarity or experience with one's own family could play a role in the expression of brain responses to different levels of family entitativity. The exact relationship between individual differences in entitativity and life experiences with one's own family is a complex issue that deserves to be investigated in the future.
In summary, our study demonstrates that SGC response to kin-related affiliative stimuli is associated with perceiving one's family as a distinct and coherent group. By linking emotional attachment to in-group perception the SGC may serve as a “social gate” that enables more complex forms of prosocial behavior, group identification and cooperation.
METHODS
Subjects
From 34 adult healthy right-handed participants, 7 datasets were discarded due to excessive signal dropouts yielding a final dataset of 27 subjects (14 females; age M = 29.5, SD = 6.1 years; education M = 16.8, SD = 2.2 years). Participants had no history of psychiatric or neurological disorders, and were not taking centrally active medications. The study, which comprises a secondary data analysis of a recent study on affiliative emotions (Moll et al., 2012), was approved by the D'Or Institute Ethics and Scientific Committees. Written informed consent was obtained from all participants.
fMRI task
The stimulus set comprised written social scenarios belonging to (i) affiliative negative or positive scenarios associated with kin-based attachment (e.g., “You taught your son to ride the bike and he came to thank you with a hug”); (ii) non-affiliative positive or negative scenarios devoid of kin-based attachment (e.g., “You work for a company that made great profits and received a good salary raise”) and (iii) emotionally neutral social scenarios (e.g., “You participated in a business meeting and then went back to your office”). Positive and negative valence were matched between affiliative and non-affiliative conditions (details in Moll et al. (2012)).
Image acquisition and analysis
Functional images were acquired with a 3T Achieva scanner (Philips Medical Systems, Cleveland, OH, USA) using a T2*-weighted echoplanar sequence (TR = 2000 ms, TE = 22 ms, Matrix = 96 × 96, FOV = 240 mm, flip angle = 90°, slice thickness = 3 mm, 39 slices; 287 volumes per run, 4 runs). Total functional scanning time was approximately 41 minutes. A SENSE factor of 1.5 and “dynamic stabilization” were additionally employed. These parameters were used to maximize temporal signal-to-noise in brain regions that normally suffer from magnetic susceptibility effects (Bellgowan, Bandettini, van Gelderen, Martin, & Bodurka, 2006; Bodurka, Ye, Petridou, Murphy, & Bandettini, 2007). Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8) and the general linear model were used for image analysis (Friston, Frith, Turner, & Frackowiak, 1995; Worsley & Friston, 1995).
All datasets underwent registration and 12-parameter affine normalization and were transformed into standard MNI space using a 2 × 2 × 2 isotropic resolution. Data were smoothed using an 8 mm full width at half maximum Gaussian kernel. High-pass filtering and cubic detrending were employed (Macey, Macey, Kumar, & Harper, 2004) and statistical effects were calculated on the second level using a random-effects model. Categorical contrasts were created by condition-specific events with a canonical hemodynamic response function (Zarahn, Aguirre, & D'Esposito, 1997). The entitativity score of each participant was entered as a between-subject covariate of interest on the contrast affiliative versus non-affiliative condition.
Emotional valence SGC analysis was performed by extracting parameter estimates from affiliative negative versus neutral and affiliative positive versus neutral contrasts, using the local maxima coordinate obtained in the previous analysis step (parametric effect of entitativity on the affiliative vs. non-affiliative contrast, MNI:0,20,−8). These parameter estimates were subsequently correlated with individual entitativity scores and r values were compared using Fisher's r to z transformation.
Self-report measure of entitativity
Perceived entitativity of one's family as a distinct and coherent group in society was measured by adapting a previously validated measure of perceived group entitativity (Rüsch et al., 2009). The family entitativity scale comprised four items, each ranging between 1 and 9, with a higher mean score indicating higher perceived entitativity. In our sample, family entitativity had a mean value of 5.7, SD = 1.7, and the scale showed good internal consistency (Cronbach's alpha = .72). Regarding the individual items, participants rated whether they thought their family (i) is not a group at all or is very much a group (from 1 to 9), (ii) had many characteristics in common (from 1, not at all, to 9, very much), (iii) shared a common fate and common goals (from 1, not at all, to 9, very much) and (iv) could be recognized as a distinct group within a larger society (from 1, not at all, to 9, very much).
REFERENCES
- Bartels A., Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Baumeister R. F., Leary M. R. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Baumgartner T., Heinrichs M., Vonlanthen A., Fischbacher U., Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bellgowan P. S. F., Bandettini P. A., van Gelderen P., Martin A., Bodurka J. Improved BOLD detection in the medial temporal region using parallel imaging and voxel volume reduction. NeuroImage. 2006;29(4):1244–1251. doi: 10.1016/j.neuroimage.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Bernstein M. J., Sacco D. F., Young S. G., Hugenberg K., Cook E. Being “in” with the in-crowd: The effects of social exclusion and inclusion are enhanced by the perceived essentialism of ingroups and outgroups. Personality & Social Psychology Bulletin. 2010;36(8):999–1009. doi: 10.1177/0146167210376059. [DOI] [PubMed] [Google Scholar]
- Bodurka J., Ye F., Petridou N., Murphy K., Bandettini P. A. Mapping the MRI voxel volume in which thermal noise matches physiological noise—implications for fMRI. NeuroImage. 2007;34(2):542–549. doi: 10.1016/j.neuroimage.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwallis C. K., West S. A., Davis K. E., Griffin A. S. Promiscuity and the evolutionary transition to complex societies. Nature. 2010;466(7309):969–972. doi: 10.1038/nature09335. [DOI] [PubMed] [Google Scholar]
- Correll J., Park B. A model of the ingroup as a social resource. Personality and Social Psychology Review. 2005;9(4):341–359. doi: 10.1207/s15327957pspr0904_4. [DOI] [PubMed] [Google Scholar]
- Cristofori I., Moretti L., Harquel S., Posada A., Deiana G., Isnard J., Sirigu A. Theta signal as the neural signature of social exclusion. Cerebral Cortex. 2013;23(10):2437–2447. doi: 10.1093/cercor/bhs236. [DOI] [PubMed] [Google Scholar]
- Decety J. The neuroevolution of empathy. Annals of the New York Academy of Sciences. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- Depue R. A., Morrone-Strupinsky J. V. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. The Behavioral and Brain Sciences. 2005;28(3):313–350. doi: 10.1017/S0140525X05000063. discussion 350–395. [DOI] [PubMed] [Google Scholar]
- Drevets W. C., Öngür D., Price J. L. Reduced glucose metabolism in the subgenual prefrontal cortex in unipolar depression. Molecular Psychiatry. 1998;3(3):190–191. doi: 10.1038/sj.mp.4000380. [DOI] [PubMed] [Google Scholar]
- Friston K., Frith C. D., Turner R., Frackowiak R. S. J. Characterizing evoked hemodynamics with fMRI. NeuroImage. 1995;2(2):157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Gaertner L., Iuzzini J., Witt M. G., Oriña M. M. Us without them: Evidence for an intragroup origin of positive in-group regard. Journal of Personality and Social Psychology. 2006;90(3):426–439. doi: 10.1037/0022-3514.90.3.426. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Young L. J. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2(2):129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Macey P. M., Macey K. E., Kumar R., Harper R. M. A method for removal of global effects from fMRI time series. NeuroImage. 2004;22(1):360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Mayberg H. S., Lozano A. M., Voon V., McNeely H. E., Seminowicz D., Hamani C., Kennedy S. H. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McCall C., Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nature Neuroscience. 2012;15(5):681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Yu R., Meyer M., Passamonti L., Seymour B., Calder A. J., Dalgleish T. A key role for similarity in vicarious reward. Science. 2009;324(5929):900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Bado P., de Oliveira-Souza R., Bramati I. E., Lima D. O., Paiva F. F., Zahn R. A neural signature of affiliative emotion in the human septo-hypothalamic area. The Journal of Neuroscience. 2012;32(36):12499–12505. doi: 10.1523/JNEUROSCI.6508-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., de Oliveira-Souza R., Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Zahn R., de Oliveira-Souza R., Krueger F., Grafman J. Opinion: The neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6(10):799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Morrison S., Decety J., Molenberghs P. The neuroscience of group membership. Neuropsychologia. 2012;50(8):2114–2120. doi: 10.1016/j.neuropsychologia.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Nelson E. E., Panksepp J. Brain substrates of infant-mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neuroscience & Biobehavioral Reviews. 1998;22(3):437–452. doi: 10.1016/S0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Northoff G., Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Kalisch R., Singer T., Dolan R. J. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. The Journal of Neuroscience. 2008;28(26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch N., Corrigan P. W., Wassel A., Michaels P., Olschewski M., Wilkniss S., Batia K. Ingroup perception and responses to stigma among persons with mental illness. Acta Psychiatrica Scandinavica. 2009;120(4):320–328. doi: 10.1111/j.1600-0447.2009.01403.x. [DOI] [PubMed] [Google Scholar]
- Sacchi S., Castano E., Brauer M. Perceiving one's nation: Entitativity, agency and security in the international arena. International Journal of Psychology: Journal international de psychologie. 2009;44(5):321–332. doi: 10.1080/00207590802236233. [DOI] [PubMed] [Google Scholar]
- Strathearn L. Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. Journal of Neuroendocrinology. 2011;23(11):1054–1065. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K. J., Friston K. J. Analysis of fMRI time-series revisited—again. NeuroImage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Zahn R., de Oliveira-Souza R., Bramati I., Garrido G., Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neuroscience Letters. 2009;457(2):107–110. doi: 10.1016/j.neulet.2009.03.090. [DOI] [PubMed] [Google Scholar]
- Zahn R., de Oliveira-Souza R., Moll J. The neuroscience of moral cognition and emotion. In: Decety J., Cacioppo J. T., editors. The Oxford handbook of social neuroscience. Oxford: Oxford University Press; 2011. pp. 477–490. [Google Scholar]
- Zahn R., Moll J., Paiva M., Garrido G., Krueger F., Huey E. D., Grafman J. The neural basis of human social values: Evidence from functional MRI. Cerebral Cortex. 2009;19(2):276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak P. J., Kurzban R., Matzner W. T. Oxytocin is associated with human trustworthiness. Hormones and Behavior. 2005;48(5):522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zarahn E., Aguirre G., D'Esposito M. A trial-based experimental design for fMRI. NeuroImage. 1997;6(2):122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- Zink C. F., Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopressin in social cognition. Hormones and Behavior. 2012;61(3):400–409. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


