Abstract
Background and Purpose
We evaluate associations between the severity of magnetic resonance perfusion-weighted imaging abnormalities, as assessed by the hypoperfusion intensity ratio (HIR), on infarct progression and functional outcome in the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution Study 2 (DEFUSE 2).
Methods
Diffusion-weighted magnetic resonance imaging and perfusion-weighted imaging lesion volumes were determined with the RAPID software program. HIR was defined as the proportion of TMax >6 s lesion volume with a Tmax >10 s delay and was dichotomized based on its median value (0.4) into low versus high subgroups as well as quartiles. Final infarct volumes were assessed at day 5. Initial infarct growth velocity was calculated as the baseline diffusion-weighted imaging (DWI) lesion volume divided by the delay from symptom onset to baseline magnetic resonance imaging. Total Infarct growth was determined by the difference between final infarct and baseline DWI volumes. Collateral flow was assessed on conventional angiography and dichotomized into good and poor flow. Good functional outcome was defined as modified Rankin Scale ≤2 at 90 days.
Results
Ninety-nine patients were included; baseline DWI, perfusion-weighted imaging, and final infarct volumes increased with HIR quartiles (P<0.01). A high HIR predicted poor collaterals with an area under the curve of 0.73. Initial infarct growth velocity and total infarct growth were greater among patients with a high HIR (P<0.001). After adjustment for age, DWI volume, and reperfusion, a low HIR was associated with good functional outcome: odds ratio=4.4 (95% CI, 1.3–14.3); P=0.014.
Conclusions
HIR can be easily assessed on automatically processed perfusion maps and predicts the rate of collateral flow, infarct growth, and clinical outcome.
Keywords: brain infarction, magnetic resonance imaging, perfusion imaging
Brain infarction results from a focal decrease in cerebral blood flow. The severity and the duration of tissue hypoperfusion determine the fate of the ischemic tissue.1 Magnetic resonance (MR) perfusion imaging offers an estimate of cerebral hemodynamics. The time when the residue function reaches its maximum (TMax) is one of the more commonly evaluated parameters and has been used to estimate the volume and location of the penumbra in several clinical trials.2 Our group and others have attempted to clarify which TMax thresholds provide the most accurate estimate of penumbral tissue. In the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution Study (DEFUSE), TMax delays of >6 s (TMax >6 s) were optimal to predict final infarction in patients who did not experience early reperfusion and reperfusion of TMax >6 s lesions was associated with clinical recovery.3 Comparisons between MR perfusion and reference techniques such as positron emission tomography or Xenon computed tomography have shown that TMax >6 s delay maps are highly correlated with penumbral thresholds.4–6 Moreover, 2 groups have demonstrated a consistent relationship between the proportion of any TMax lesion (≥2 s) with a TMax delay of >6 s and collateral flow, baseline lesion volume, and infarct growth.7,8 Finally, the DEFUSE 2 demonstrated prospectively that patients with a target mismatch between the diffusion-weighted magnetic resonance imaging (dMRI) lesion and the TMax >6 s lesion had a high rate of clinical recovery after reperfusion.9 In patients who did not reperfuse, DEFUSE 2 demonstrated a strong correlation between baseline TMax >6 s lesion volume and final infarct volume.10 Therefore, TMax >6 s seems to be a reasonable surrogate for estimation of the penumbra in patients with acute stroke.
Severe hypoperfusion has been associated with irreversible necrosis of the ischemic lesion even after reperfusion. 11,12 TMax >6 s lesions include regions with variable degrees of hypoperfusion. Hence, TMax >6 s lesions sometimes contain a large fraction of tissue with delays >8 to 10 s. In the DEFUSE and EPITHET meta- analysis, large regions of severe delay (>10 s) have been associated with poor outcome after reperfusion.13 This finding suggests that higher TMax threshold may identify tissue with more severely reduced cerebral blood flow values which may have a substantial impact on the evolution of the acute ischemic lesion. The DEFUSE 2 confirms in a prospective cohort of patients with a large perfusion lesion that reperfusion was associated with an increased rate of clinical recovery among patients with a target mismatch but not among those with a malignant profile.9
Based on those premises, we evaluated the proportion of the TMax >6 s lesion with a TMax >10 s delay that we termed the hypoperfusion intensity ratio (HIR). We hypothesized that the HIR would be associated with other parameters that are relevant to the evolution of an acute ischemic lesion and could therefore become an independent predictor of the clinical outcomes. To test these hypotheses, we assessed the relationships between the HIR (measured on baseline MRI) and clinical and radiological outcomes in DEFUSE 2.
Methods
Patients
The inclusion criteria, study design, and primary results of the DEFUSE 2 have been previously reported.9 Briefly, patients with an acute ischemic stroke in whom endovascular treatment was anticipated within 12 hours after stroke onset were enrolled. Patients underwent a baseline MRI before endovascular treatment. Reperfusion was assessed on a follow-up MRI performed within 12 hours after the revascularization procedure. Final infarct volume was measured on fluid attenuated inversion recovery (FLAIR) sequence 5 days after symptom onset. Clinical outcome was assessed by modified Rankin Scale (mRS) 90 days after the index event. Every patient with a detectable perfusion lesion with TMax >6 s was enrolled in this substudy. Approval for the study was obtained from local institutional review boards. A written informed consent was obtained from all the participants.
Definitions
The DEFUSE 2 MRI protocol has been previously reported.9 Perfusion (TMax >6, 8, 10 s) lesion volumes and the baseline diffusion-weighted imaging (DWI) volume were estimated by an automated software program (RAPID). Mismatch volume was calculated as the difference between the acute MR perfusion lesion (TMax >6 s) and the acute DWI lesion (apparent diffusion coefficient <600) volume. Mismatch ratio was calculated as the ratio between the acute perfusion lesion (TMax >6 s) and the acute DWI lesion volume. Target mismatch was defined by a mismatch ratio ≥1.8, a mismatch volume ≥15 mL, a DWI lesion volume <70 mL, and a perfusion lesion with TMax>10 s<100 mL. The malignant profile was defined by a DWI lesion volume ≥70 mL or perfusion lesion volume with TMax>10 s≥100 mL.
HIR was defined by the proportion of the TMax >6 s lesion with TMax >10 s. Patients were dichotomized into high and low HIR groups based on whether their baseline HIR values were above or below the median value for all patients. Patients were also divided into quartiles based on their HIR values. Angiographic recanalization rates and a collateral score were assessed on conventional angiography by a blinded reader (M.P.M). Only patients with a proximal vessel (internal carotid artery or M1) occlusion (defined by a thrombolysis in cerebral infarction score of 0–1) were included in this part of the analysis. Recanalization was assessed on the final angiogram using the arterial occlusive lesion score.14 Recanalization success was dichotomized into no/partial (0–2) versus complete (3) recanalization. The angiographic collateral score was assessed in patients with a proximal vessel occlusion (internal carotid artery or M1) by a 5-point scale, rated 0 (no collateral flow) to 4 (complete/rapid collaterals to entire ischemic territory). Analysis of this scale was dichotomized into poor flow (0–2) versus good flow (3–4).15
Early reperfusion on perfusion imaging was defined as a >50% reduction in the volume of the perfusion MRI (TMax >6 s) lesion between baseline and the early follow-up MRI. In patients with no early follow-up perfusion imaging, reperfusion was based on conventional angiography results and defined as a postprocedural thrombolysis in cerebral infarction score of 2b to 3. Final infarct volume was manually outlined on the 5-day follow-up fluid attenuated inversion recovery sequence.
The progression of the infarct volume was assessed at 2 different time points:
Initial infarct growth velocity was calculated as the acute DWI lesion volume on baseline MRI divided by the delay from symptom onset to baseline MRI in hours.
Total infarct growth was determined by the difference between 5-day fluid attenuated inversion recovery ischemic lesion volume and baseline DWI volume.
Functional outcome was assessed by mRS at 90 days. Good functional outcome was defined as an mRS ≤2 at 90 days.
Statistics
Proportions were compared using Fisher exact test. We compared medians between 2 groups using the Mann–Whitney U test. We used Jonckheere–Terpstra test for ordered alternatives to test whether continuous variables increased or decreased with increasing HIR quartiles. Receiver operating characteristic curve analysis assessed the performance of the HIR to predict collaterals. Logistic regression analysis assessed the association of HIR with good functional outcome. This relationship was adjusted based on age, DWI, and TMax >6 s lesion volumes, reperfusion status, and mismatch profile; these variables were identified as predictors of outcome in the DEFUSE 2 main analysis.9 Interaction factors (TMax lesion volume, time to MR, time to treatment, reperfusion, and target mismatch status) were also assessed to identify whether associations of HIR with outcomes were dependent on reperfusion, imaging delay, or profile. All tests were 2 tailed and assumed significance at α <0.05. Statistical testing was performed with IBM SPSS Statistics software version 20.
Results
Baseline Characteristics
Among the 110 patients in the endovascular cohort of the DEFUSE 2, 99 (90%) had detectable TMax >6 s perfusion lesions and are included in this study. Baseline characteristics are listed in the Table. Median baseline National Institutes of Health Stroke Scale was 16 (interquartile range [IQR], 11–19), baseline DWI lesion volume was 15 mL (IQR, 7–32), TMax >6 s perfusion lesion volume was 80 mL (IQR, 45–115), and HIR was 39% (IQR, 22–51). HIR was dichotomized by its median value into low (<40%) and high HIR (≥40%). On baseline MRI, 81 patients (82%) had the target mismatch profile, 8 (8%) the malignant profile, and the remaining 10 (11%) a matched profile.
Table.
Baseline Characteristics
| Overall (n=99) | Low HIR (n=50) | High HIR (n=49) | P Value | |
|---|---|---|---|---|
| Age, mean (SD) | 65 (16) | 66 (19) | 65 (15) | 0.692 |
| Sex (female), n (%) | 50 (51) | 27 (54) | 23 (47) | 0.549 |
| SBP, mean (SD) | 147 (24) | 146 (25) | 148 (22) | 0.797 |
| DBP, mean (SD) | 80 (19) | 78 (19) | 83 (19) | 0.207 |
| Glucose, mean (SD) | 135 (50) | 134 (53) | 135 (46) | 0.952 |
| Hypertension, n (%) | 68 (69) | 37 (74) | 31 (65) | 0.382 |
| Diabetes mellitus, n (%) | 19 (19) | 7 (14) | 12 (25) | 0.206 |
| Hyperlipidemia, n (%) | 50 (51) | 25 (50) | 25 (52) | 0.843 |
| Atrial fibrillation, n (%) | 34 (35) | 16 (32) | 18 (38) | 0.672 |
| History of stroke/TIA, n (%) | 21 (21) | 7 (14) | 14 (29) | 0.086 |
| IV tPA, n (%) | 53 (54) | 24 (48) | 29 (59) | 0.316 |
| NIHSS, median (IQR) | 16 (11–19) | 13 (8–18) | 18 (14–21) | <0.001 |
| dMRI, mL, median (IQR) | 15 (7–32) | 9 (2–15) | 28 (15–52) | <0.001 |
| TMax >6 s, mL, median (IQR) | 80 (47–115) | 63 (41–81) | 112 (80–137) | <0.001 |
| TMax >10 s, mL, median (IQR) | 27 (12–55) | 12 (4–22) | 57 (41–76) | <0.001 |
| FLAIR 5-day infarct volume, mL, median (IQR) | 74 (25–138) | 32 (14–76) | 120 (68–203) | <0.001 |
| HIR, median (IQR) | 0.39 (0.23–0.51) | 0.23 (0.11–0.3) | 0.51 (0.45–0.63) | <0.001 |
| Target mismatch, n (%) | 81 (82) | 46 (92) | 35 (71) | 0.004 |
| Malignant, n (%) | 8 (8) | 0 (0) | 8 (16) | 0.009 |
| Matched, n (%) | 10 (10) | 4 (8) | 6 (12) | |
| Mismatch volume, mL, median (IQR) | 59 (31–85) | 46 (28–67) | 71 (42–94) | 0.011 |
| Time from symptom onset to MRI, h, median (IQR) | 4.5 (2.7–6.2) | 4.6 (4.1–7) | 3.9 (2.6–5.4) | 0.071 |
DBP indicates diastolic blood pressure; dMRI, diffusion-weighted magnetic resonance imaging; FLAIR, fluid attenuated inversion recovery; HIR, hypoperfusion intensity ratio; IQR, interquartile range; IV tPA, intravenous tissue-type plasminogen activator; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; TIA, transient ischemic attack; and TMax, time when the residue function reaches its maximum.
Associations Between Baseline HIR and Other Parameters
As shown in the Table, baseline National Institutes of Health Stroke Scale, lesion volumes on DWI, TMax >6 s, and TMax >10 s, as well as the total mismatch volume were higher in patients with higher HIR quartiles. Forty-six (57%) of the target mismatch patients had a low HIR compared with none of the 8 malignant profile patients (P=0.002).
Sixty-eight patients (69%) had a baseline proximal vessel (internal carotid artery or M1) occlusion documented on conventional angiography and a detectable TMax >6 s lesion on baseline MRI. Patients with a proximal internal carotid artery occlusion tended to have a higher HIR than patients with M1 occlusion: 0.45 (0.35–0.52) versus 0.38 (0.22–0.50); P=0.053.
Forty patients (67%) achieved complete recanalization. The median HIR before endovascular therapy did not differ in patients who eventually achieved recanalization compared with those who did not (P=0.592).
The collateral score could be assessed in 56 (83%), and scores were dichotomized into poor (0–2) versus good flow (3–4). Receiver operating characteristic curve analysis demonstrated that the HIR predicted poor collateral flow with an area under the curve of 0.73; HIR >40% had a sensitivity of 66% and a specificity of 70% for predicting poor collateral flow.
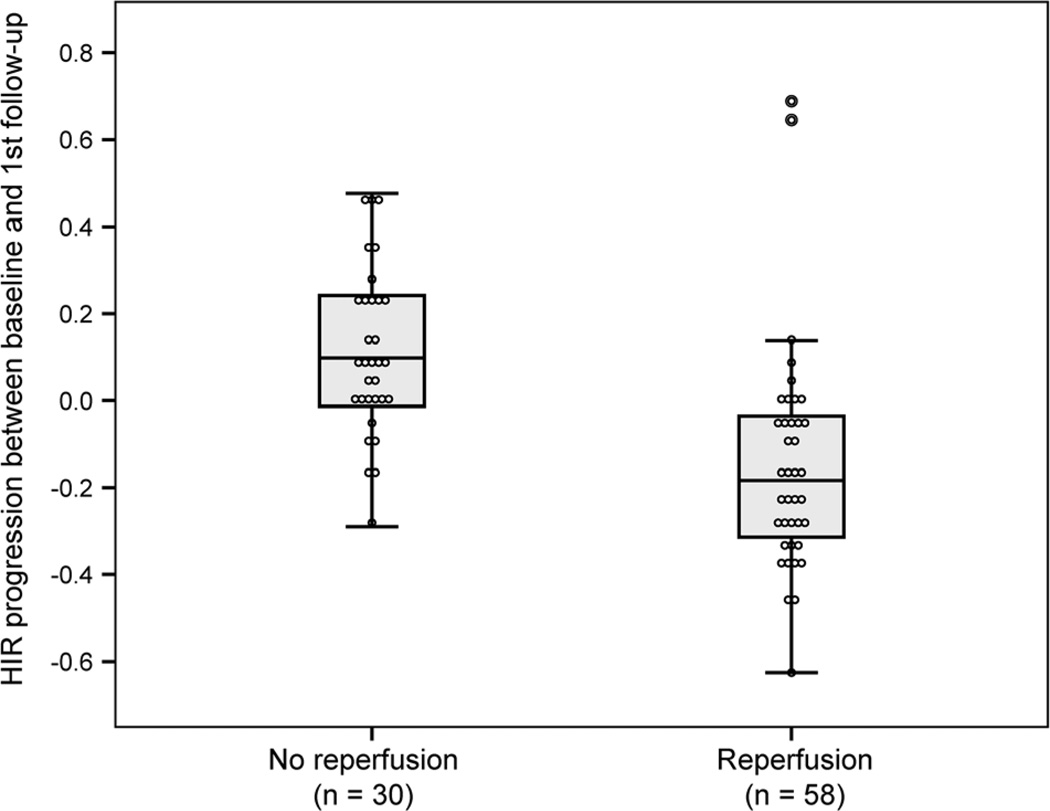
Early reperfusion could be assessed in all but one patient (baseline and follow-up TMax >6 s perfusion lesions were compared in 71 patients, and angiographic thrombolysis in cerebral infarction scores were used in 17 patients). Fifty-eight patients (59%) experienced early reperfusion. The median HIR at baseline was not different between patients who achieved early reperfusion and nonreperfusers, P=0.33. In the absence of early reperfusion, the HIR increased between baseline and early follow- up by 0.10 (IQR, −0.01 to 0.25) which corresponds to 33% (IQR, −3 to 57). In contrast, among patients who reperfused, the HIR decreased by 0.18 (IQR, 0.03–0.32), corresponding to a 60% reduction (IQR, 12–100; P<0.001; Figure 1).
Figure 1.
Hypoperfusion intensity ratio (HIR) progression between baseline and first follow-up among reperfusers and nonreperfusers. Evolution of the HIR between baseline and follow-up perfusion-weighted magnetic resonance imaging according to the occurrence of an acute reperfusion.
Infarct Progression
Initial Infarct Growth Velocity
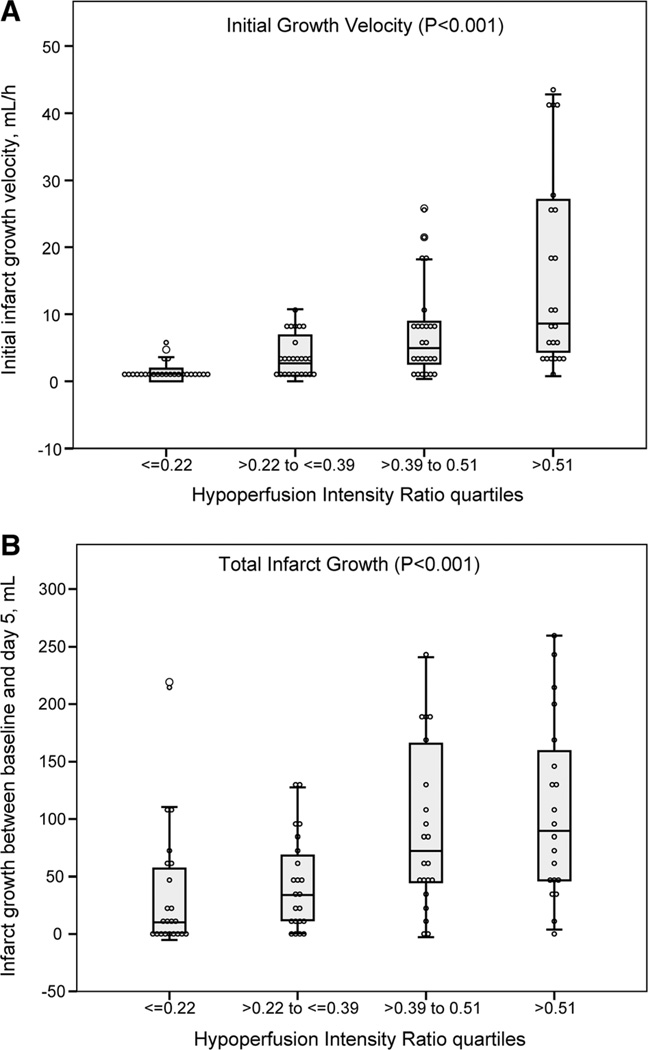
The initial infarct growth velocity could be determined in 98 (99%) patients. The median initial infarct growth velocity was 3.2 mL/h (IQR, 1.2–8.4). Initial infarct growth velocity increased in association with HIR quartiles (P<0.001; Figure 2A). Patients with a high HIR had faster initial infarct growth than patients with a low HIR: 7.2 mL/h (IQR, 2.9–17.8) versus 1.6 mL/h (IQR, 0.1–3.3); P<0.001. This relationship held for both the 80 patients with a baseline target mismatch profile, 5.0 mL/h (IQR, 2.9–8.8) versus 1.7 mL/h (IQR, 0.2–3.3; P<0.001), as well in the 18 patients who did not have the target mismatch profile, 23.6 mL/h (IQR, 7.9–40.4) versus 0.4 mL/h (IQR, 0.05–7.1; P=0.011).
Figure 2.
Relationship between hypoperfusion intensity ratio (HIR) quartiles and (A) initial infarct growth velocity (baseline diffusion-weighted magnetic resonance imaging [dMRI] lesion volume/delay from onset to baseline MRI in hours) and (B) final infarct growth (final infarct volume on 5-day fluid attenuated inversion recovery baseline diffusion-weighted imaging volume).
Final Infarct Volume and Total Infarct Growth
Final infarction volume was assessed in 86 patients (87%) after a median (IQR) delay of 121 hours (97–132). Final infarction volume increased in association with HIR quartiles (P<0.001; Figure 2B) and was larger among patients with a high versus low HIR: 120 mL (IQR, 65–207) versus 32 mL (IQR, 14–76); P<0.001.
Median infarct growth was 49 mL (IQR, 11–106). There was a significant increase in infarct growth associated with the HIR quartiles (P<0.001; Figures 2B and 3). Patients who had high HIR experienced more infarct growth (83 mL [IQR, 45–166]) than those with low HIR (20 mL [4–67]); P<0.001. This association was present in both the 54 patients who experienced early reperfusion (P=0.001) and the 31 who did not (P=0.029).
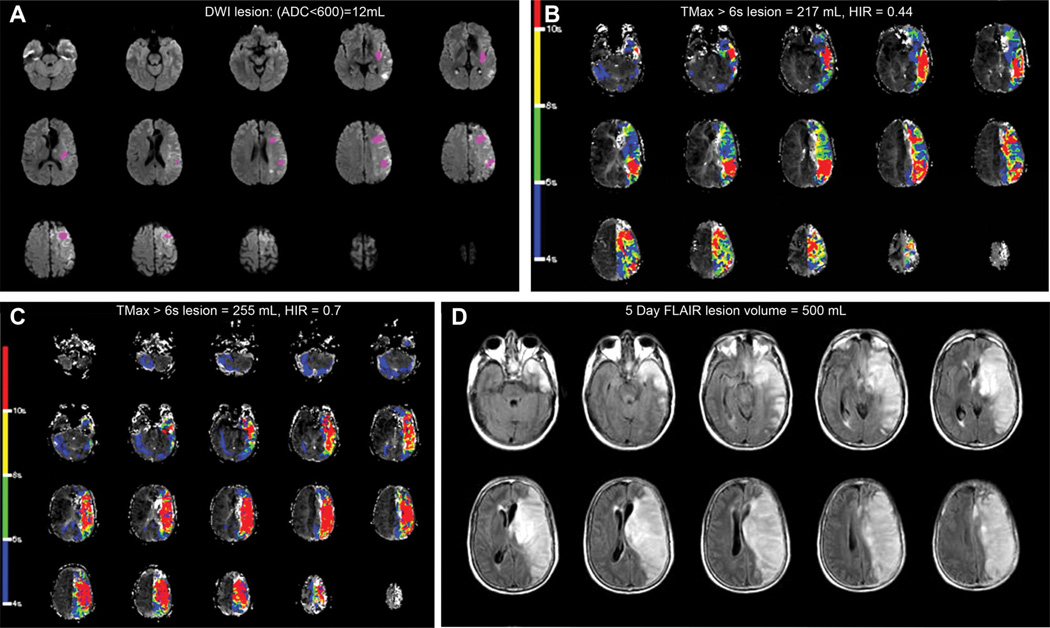
Figure 3.
Fifty-year-old patient, baseline National Institutes of Health Stroke Scale was 22, (A) baseline magnetic resonance imaging (MRI) showed a 12-mL diffusion-weighted imaging (DWI; apparent diffusion coefficient [ADC] <600) lesion and (B) a 217-mL perfusion-weighted imaging lesion with an hypoperfusion intensity ratio (HIR) of 0.44. Cerebral angiogram demonstrated a left internal carotid artery occlusion with poor collaterals, and recanalization was not achieved. HIR increased to 0.7 on the follow-up MRI performed 3 hours later (C). The infarct grew to 500 mL at day 5, and the 90-day modified Rankin Scale was 3 (D). FLAIR indicates fluid attenuated inversion recovery; and TMax, time when the residue function reaches its maximum.
Clinical Outcome
Forty-two patients (42%) had a good functional outcome (mRS ≤2) at 90 days. Their median HIR was lower than the patients with an mRS ≥3 at 90 days (30% [IQR, 19–46] versus 43% [IQR, 30–55]; P=0.006). Time to MR had no significant impact on outcome (P=0.43). After adjustment for age, baseline DWI volume, time to MR, and the occurrence of early reperfusion, the odds ratio for good outcome associated with a low HIR was 4.3 (95% CI, 1.3–14.1), P=0.016. TMax >6 s volume was not a significant independent predictor of functional outcome. However, there was a significant interaction between perfusion-weighted imaging lesion volume and HIR with functional outcome. At low perfusion-weighted imaging volumes, the severity of the relationship between HIR and clinical outcome is diminished.
Discussion
Our results indicate that in the DEFUSE 2 cohort, the HIR (defined as the TMax 10/6 s ratio and assessed within 4.9 [±2.6] hours after stroke onset) is associated with the progression of the acute ischemic lesion as well as functional outcome. As previously demonstrated using lower TMax thresholds (TMax 6/2 s ratio),7 the TMax 10/6 s ratio may predict collateral flow. HIR is associated with the severity of stroke at baseline (larger ischemic lesion and National Institutes of Health Stroke Scale) and tended to be more severe among patients with a proximal vessel occlusion.7,8 In addition, our results indicate that a low HIR is associated with slower infarct growth and small final infarct volumes. Finally, the comparison between baseline and follow-up perfusion- weighted imaging revealed that the HIR worsens over time in patients who do not experience reperfusion.
Altogether, these results support the concept that the HIR may provide an estimate of collateral blood flow and that the failure of collateral flow correlates with infarct growth.16 Furthermore, a high HIR on baseline MRI predicts more rapid growth of the ischemic core, which could limit the therapeutic potential of reperfusion therapies. Multivariate analyses showed that HIR was an independent prognostic factor that did interact with perfusion lesion volume. Hence, for patients with a similar perfusion lesion volume, a high HIR seems to predict a less favorable outcome than a low HIR. Furthermore, this relationship is most potent for patients with larger baseline perfusion lesions.
We defined HIR as a clinical parameter that can be easily and quickly determined from automated perfusion maps. We prespecified that this parameter would be defined by the TMax thresholds used to estimate critical hypoperfusion (>6 s) and the malignant MRI profile (>10 s) in DEFUSE 2. It is probable that the use of different blood flow parameters (cerebral blood volume, cerebral blood flow, mean transit time) or alternative TMax thresholds to define the HIR would produce different results. Our results apply to the highly selected patient population enrolled in the DEFUSE 2. This population of patients is characterized by a high proportion of patients with small DWI lesions and large perfusion lesions. In addition, the final infarct volume was assessed 5 days after onset which is a time point when edema may result in overestimation of infarct volume.9 Therefore, our conclusions are limited to the DEFUSE 2 cohort and the results may not be applicable to other populations.
In conclusion, the HIR can be assessed rapidly and easily by automated software following bolus contrast perfusion imaging. This variable correlates with the degree of collateral circulation and is associated with the rate of infarct growth and clinical outcome. Further data are required to clarify whether HIR is useful for optimizing the selection of patients for stroke treatments.
Supplementary Material
Acknowledgments
Statistical analyses were conducted by Dr Mlynash.
Sources of Funding
The study was funded by grants from the National Institute for Neurological Disorders and Stroke (NINDS; R01 NS03932505 [Dr Albers], K23 NS051372 [Dr Lansberg]) and the Stanford Medical Scholars Fellowship Program.
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.113.003857/-/DC1.
Disclosures
Dr Albers has equity interest in iSchemaView and has worked as a consultant for Covidien, Codman, and Stryker. Dr Bammer has equity interest in iSchemaView. Dr Zaharchuk receives modest research funding support from GE Healthcare. The other authors report no conflicts.
References
- 1.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 2.Calamante F, Christensen S, Desmond PM, Ostergaard L, Davis SM, Connelly A. The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke. 2010;41:1169–1174. doi: 10.1161/STROKEAHA.110.580670. [DOI] [PubMed] [Google Scholar]
- 3.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takasawa M, Jones PS, Guadagno JV, Christensen S, Fryer TD, Harding S, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke. 2008;39:870–877. doi: 10.1161/STROKEAHA.107.500090. [DOI] [PubMed] [Google Scholar]
- 5.Olivot JM, Mlynash M, Zaharchuk G, Straka M, Bammer R, Schwartz N, et al. Perfusion MRI (Tmax and MTT) correlation with xenon CT cerebral blood flow in stroke patients. Neurology. 2009;72:1140–1145. doi: 10.1212/01.wnl.0000345372.49233.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke. 2010;41:2817–2821. doi: 10.1161/STROKEAHA.110.594432. [DOI] [PubMed] [Google Scholar]
- 7.Bang OY, Saver JL, Alger JR, Starkman S, Ovbiagele B, Liebeskind DS UCLA Collateral Investigators. Determinants of the distribution and severity of hypoperfusion in patients with ischemic stroke. Neurology. 2008;71:1804–1811. doi: 10.1212/01.wnl.0000335929.06390.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoli F, Lafaye de Micheaux P, Girard N. Perfusion-weighted imaging-derived collateral flow index is a predictor of MCA M1 recanalization after i.v. thrombolysis. AJNR Am J Neuroradiol. 2013;34:107–114. doi: 10.3174/ajnr.A3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. DEFUSE 2 study investigators. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler HM, Mlynash M, Inoue M, Tipirneni A, Liggins J, Zaharchuk G, et al. DEFUSE 2 Investigators. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke. 2013;44:681–685. doi: 10.1161/STROKEAHA.111.000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrera E, Jones PS, Alawneh JA, Kl.rke Mikkelsen I, Cho TH, Siemonsen S, et al. Predicting infarction within the diffusion-weighted imaging lesion: does the mean transit time have added value? Stroke. 2011;42:1602–1607. doi: 10.1161/STROKEAHA.110.606970. [DOI] [PubMed] [Google Scholar]
- 12.Guadagno JV, Jones PS, Aigbirhio FI, Wang D, Fryer TD, Day DJ, et al. Selective neuronal loss in rescued penumbra relates to initial hypoperfusion. Brain. 2008;131(pt 10):2666–2678. doi: 10.1093/brain/awn175. [DOI] [PubMed] [Google Scholar]
- 13.Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, et al. DEFUSE-EPITHET Investigators. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke. 2011;42:1270–1275. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T IMS-I Investigators. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 15.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012;33:576–582. doi: 10.3174/ajnr.A2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. EPITHET Investigators. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1168–1172. doi: 10.1038/jcbfm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.