Abstract
Objectives
Altered mental status (AMS) is a common presentation in the emergency department (ED). A previous study revealed 78% electroencephalogram (EEG) abnormalities, including nonconvulsive seizure (NCS; 5%), in ED patients with AMS. The objective of this study was to assess the effect of EEG on clinical management and outcomes of ED patients with AMS.
Methods
This was a randomized controlled trial at two urban teaching hospitals. Adult patients (>18 years old) with AMS were included. Excluded patients had immediately correctable AMS (e.g., hypoglycemia) and admission before enrollment. Patients were randomized to routine care (control) or routine care plus EEG (intervention). Research assistants (RAs) used a scalp electrode set with a miniature, wireless EEG device (microEEG) to record standard 30-minute EEGs at presentation, and results were reported to the ED attending physician by an off-site epileptologist within 30 minutes. Primary outcomes included changes in ED management (differential diagnosis, diagnostic work-up, and treatment plan from enrollment to disposition) as determined by surveying the treating physicians. Secondary outcomes were length of ED and hospital stay, intensive care unit (ICU) requirement, and in-hospital mortality.
Results
A total of 149 patients were enrolled (76 control and 73 intervention). Patients in the two groups were comparable at baseline. EEG in the intervention group revealed abnormal findings in 93% (95% confidence interval [CI] = 85% to 97%), including NCSs in 5% (95% CI = 2% to 13%). Using microEEG was associated with change in diagnostic work-up in 49% (95% CI = 38% to 60%) of cases and therapeutic plan in 42% (95% CI = 31% to 53%) of cases immediately after the release of EEG results. Changes in probabilities of differential diagnoses and the secondary outcomes were not statistically significant between the groups.
Conclusions
An EEG can be obtained in the ED with minimal resources and can affect clinical management of AMS patients.
Since the first reports of human electroencephalographic (EEG) studies by Hans Berger in 1929,1 the EEG has become a valuable test for objectively assessing the functional status of the brain. However, the use of EEG in the emergency department (ED) setting is often restricted by limited availability of technical expertise, as well as practical, administrative, and financial constraints. A new miniaturized, digital, wireless EEG device (microEEG) has been designed to overcome some of these limitations and allow for more liberal use of EEG in the ED.2 The diagnostic accuracy of microEEG is comparable to that of standard EEG.3 We have previously shown that the sensitivity, specificity, and diagnostic odds ratios for standard EEG and microEEG in identifying EEG abnormalities in ED patients with altered mental status (AMS) are similar.3 The new microEEG also shortens the duration and amount of preparation needed to obtain an EEG without interfering with other ongoing diagnostic testing.4 The wireless aspect allows real-time reporting of EEG results, which has the potential to affect clinical care.
Many EEG studies have focused on the prevalence of various EEG patterns, including nonconvulsive seizure (NCS) and nonconvulsive status epilepticus (NCSE) in patients with AMS.5–8 Yet, few have evaluated the influence of these findings on the clinical management of the studied subjects or their clinical outcomes. In the absence of clear guidelines for the incorporation of EEG in the ED, it is difficult to objectively evaluate the utility of EEG against the additional professional and financial demands involved.
Proving the effect of the EEG on clinical decision-making and management of AMS patients would justify additional steps to introduce EEG in the ED. ED patients with AMS pose unique diagnostic dilemmas in obtaining reliable histories and physical examinations, which emergent EEG may help solve. We designed our study to assess the effect of incorporating microEEG in the ED work-up on clinical management and outcome of ED patients with AMS.
METHODS
Study Design
This was a randomized control trial of the work-up of ED AMS patients with and without a microEEG. The study was approved by the joint institutional review board, which waived the requirement for patient consent. However, a surrogate written consent was obtained when a legally authorized representative was available. The study was registered on a clinical trial registration website (ClinicalTrials.gov, NCT01671475).
Study Setting and Population
Patients were enrolled at two urban academic centers with annual ED censuses of 120,000 and 75,000, respectively. Both institutions operate in an underserved community in central Brooklyn, New York. The population consists mostly of African American and Caribbean American individuals.
The target population for this study consisted of AMS patients whose cause of AMS was not immediately correctable. We included all adult (18 year and older) ED patients with AMS, defined as any alteration in level of responsiveness or alertness or arousability, presenting as lethargy, delirium, confusion, agitation, coma, disinhibition, labile/blunted affects, or unexpected psychosis.
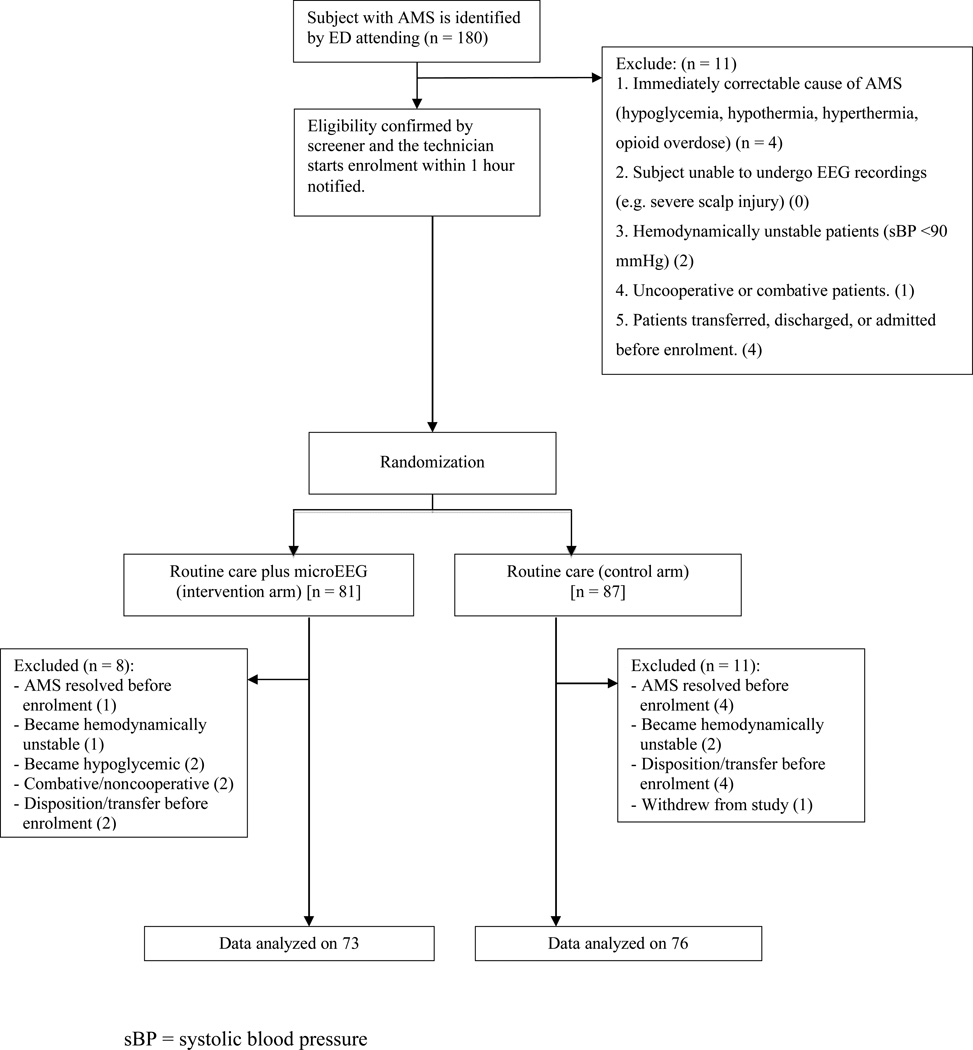
Exclusion criteria included patients with immediately correctable causes of AMS (including finger stick or serum glucose less than 60 mg/dL); hypothermia (body temperature below 35.0°C); hyperthermia, heat exhaustion, or heat stroke; opioid overdose responding to naloxone; patients who were unable to undergo EEG recordings (e.g., severe scalp injury); hemodynamically unstable patients (systolic blood pressure < 90 mm Hg); uncooperative or combative patients; and patients who were discharged, admitted, or transferred before enrollment. Patients who had overt seizures in the ED were only included if they experienced prolonged postictal periods (at the discretion of the ED attending physician).
Study Protocol
Enrollment occurred 24 hours a day, 7 days a week. AMS patients were identified by ED attending physicians and were referred for enrollment (convenience sample) if there were no immediately correctable causes of AMS (e.g., hypoglycemia). Enrollment was carried out by research assistants (RAs; medical students with no previous EEG experience) who were recruited and specifically trained to collect data for this study, apply physician surveys, perform EEG using microEEG (intervention arm only), and follow up the patients during their hospital courses for determining the outcomes. Each RA underwent approximately 20 hours of training (didactic and hands-on). Their ability to record high-quality EEGs was supervised and approved by the study epileptologists (AGC, GC); each RA was required to have at least 10 complete EEGs approved by the study epileptologists.
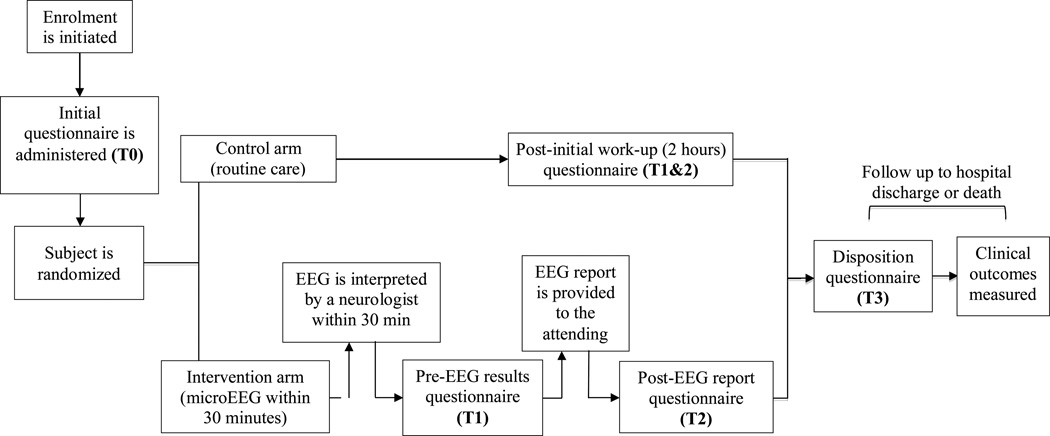
Patients were randomized to routine care (control arm) or routine care plus EEG (intervention arm) using randomization software after qualification for enrollment was confirmed. Neither RAs nor the treating physicians had any control of the randomization or allocation process. The emergency physician (EP) has the discretion of ordering standard EEGs for patients assigned to the control arm. A questionnaire was filled out by ED attending caring for each enrolled patient at the following time points (Figures 1 and 2):
Figure 1.
Schematic for study protocol and various assessment time points. EEG = electroencephalogram.
Figure 2.
Schematic for enrollment (inclusion/exclusion) process. AMS = altered mental status; EEG = electroencephalogram.
Intervention Arm
T0 was when enrollment was initiated; T1, EEG completed but results not revealed (pre-EEG survey); T2, immediately following revealing the EEG results to the EP (post-EEG survey); and T3, disposition (documentation of disposition in patient’s medical record regardless of patient’s physical location).
Control Arm
T0 was when enrollment was initiated; T1 and T2, approximately 2 hours after enrollment, corresponding to completion of initial ED work-up (corresponding to completion of work-up including EEG in intervention arm); and T3, disposition. The 2-hour work-up period cutoff for T1 and T2 was selected based on the median time for enrollment to completion of EEG using microEEG in our previous study.4
The questionnaire contained questions about the top differential diagnoses at each time point. It also asked questions pertaining to change in diagnostic management, therapeutic management (both arms), and effect of microEEG on establishing the diagnosis (intervention arm only).
A 30-minute EEG was obtained for patients allocated to the intervention arm (within 30 minutes of presentation) using microEEG (Bio-Signal Group, Brooklyn, NY) and a commercially available FDA-approved cap (Hydrodot EzeNet, HydroDot Inc., Westford, MA) placed according to the international 10–20 system. The microEEG is an FDA-approved portable, wireless, battery-operated EEG device that has previously shown to have comparable diagnostic accuracy to that of standard EEG. The unique characteristics of the ED-friendly microEEG device has been described elsewhere.2 When the recording was completed, the EEG was transferred to a secure hospital server for review by an on-call epileptologist who reported the EEG findings to the ED attending over the phone within 30 minutes from completion of recording. At a later time, each EEG recording was reviewed by one of the study board-certified epileptologists (AGC or GC), who also had access to initial clinical information similar to the on-call epileptologists, but was blinded to the EEG interpretation reported by the first epileptologist. The second interpretation was used to estimate the reliability of the EEG interpretation.
For both the control and the intervention (EEG) arms, each subject’s age, sex, vital signs (including fingerstick glucose), past medical history (including history of seizure), evidence of head injury, anticonvulsive medications given in the field or in the ED, physical examination findings (including neurological examination), results of computed tomography (CT) of head (when applicable), and disposition (discharge, admission to the regular ward, or admission to intensive care unit [ICU]) were also collected and recorded at the time of enrollment.
Outcome Measures
Primary outcomes included effect of EEG on changing clinical management (diagnostic and therapeutic) for the intervention arm only and change in differential diagnosis throughout the ED stay (comparison between control and intervention arms). Secondary outcomes (compared between arms) were length of ED stay (hours), length of hospital stay (days), admission requirement, ICU requirement, and in-hospital mortality.
Method of Determination of Outcomes
The study arms consisted of the control arm (routine, unprotocolized care) and the intervention arm (routine care plus microEEG; Figure 1). The effect of EEG on changing management was measured by surveying EPs at different time points during ED work-up (from enrollment to disposition). EPs were asked whether the patient’s management (diagnostic and therapeutic) was changed from one time point to another. Change in differential diagnosis was also recorded in the same fashion. EPs were asked to list their top differential diagnoses at each time point. They were also asked to determine the subjective probability of each diagnosis (0%–100%) with the total probability for the selected diagnoses amounting to 100%. The diagnoses were classified into 10 categories: 1) metabolic (e.g., uremia); 2) respiratory (e.g., hypoxia, hypercarbia); 3) vascular (e.g., thrombotic thrombocytopenic purpura); 4) neurologic (nonseizure, e.g. stroke, brain tumor); 5) neurologic (seizure only, including status epilepticus); 6) trauma (e.g., traumatic brain injury); 7) toxicologic (e.g., drug overdose or withdrawal); 8) infection (e.g., sepsis); 9) psychological (e.g., acute psychosis); and 10) unknown/undifferentiated/other. The physician surveys were developed by a group of experts consisting of EPs, neurologists, and a statistician (study investigators as well as study consultants).
Secondary Outcomes
ED length of stay was measured by calculating the time interval (in hours) between patient’s arrival to the ED (triage time) to the point of disposition (when a disposition order was documented in the patient’s chart, regardless of the physical location of the patient). Hospital length of stay was defined as the time interval between the patient’s arrival to the ED (triage time) to the point of discharge from hospital or death (when discharge order or death time was documented in patient’s medical chart, regardless of the physical location of the patient). Admission and ICU requirements were documented at the time of the patient’s disposition. In-hospital mortality was measured by following the patient throughout the hospitalization course (up to 30 days).
Data Analysis
Continuous variables are presented as medians and interquartile ranges (IQRs; 25% to 75%). Rates are presented as percentages with 95% confidence intervals (CIs), calculated by the method of Agresti and Coull.9 EEG interpretations were reported in the following categories: normal, status epilepticus, seizure, interictal epileptiform discharges, and slowing.
Comparisons between the control and intervention arms were performed using Fisher’s exact test for categorical data and the Mann-Whitney U test for continuous variables (except for changes in differential diagnosis, described below). Interrater agreement was calculated using the two separate interpretations of each EEG. Unweighted Cohen’s kappa was used to measure interrater agreement.
Changes in Differential Diagnosis
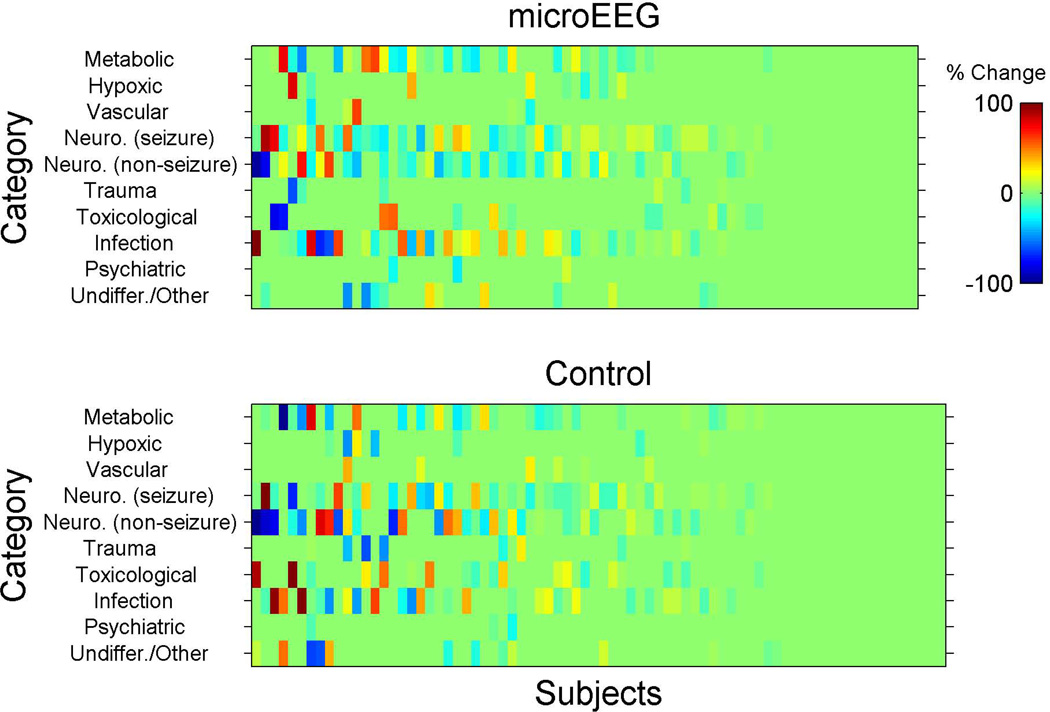
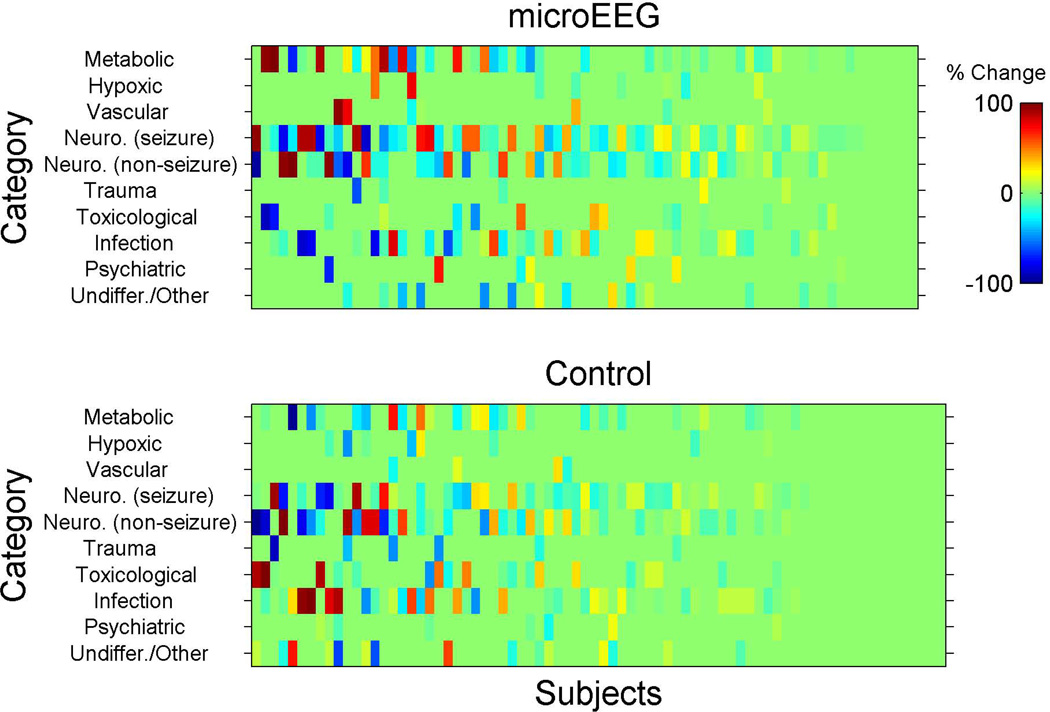
To our knowledge, measuring the outcome as a change in differential diagnosis is without precedent. In accordance with our a priori analysis plan, we considered any change of any kind (in either the diagnoses listed or their subjectively estimated probabilities) in the differential diagnosis list between one time point and another. Fisher’s exact test was used to perform between-arm comparisons. Once we collected these data it became apparent that the physicians selected some categories with high frequency and high probability while other categories were selected with low or zero probability. In addition, this method appeared to be too sensitive and picked up even non–clinically meaningful changes in probabilities. Therefore, this approach was not ideal for estimating the change in the differential diagnosis. Accordingly, an additional and novel analysis plan was chosen post hoc after reviewing the distribution of the data, which are presented in raw form in Figures 3–5. This analysis provides with the reader with the opportunity to visually examine the data as well.
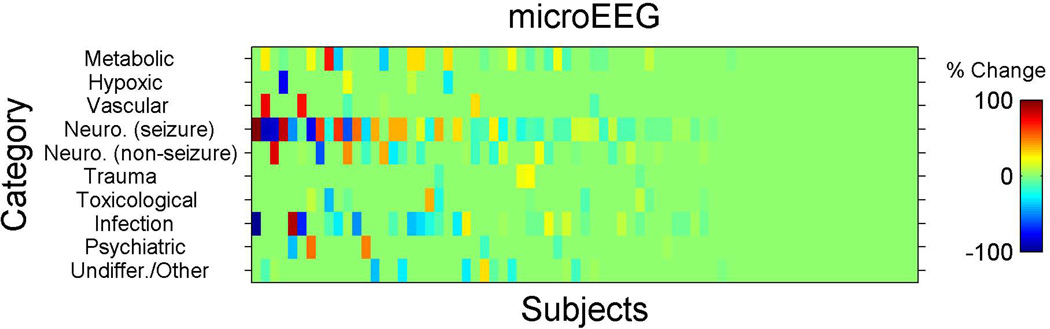
Figure 3.
Schematic for enrollment (inclusion/exclusion) process. EEG = electroencephalogram.
Figure 5.
Schematic for enrollment (inclusion/exclusion) process. EEG = electroencephalogram.
For this additional analysis, we calculated for each subject the differences in probabilities for the selected differential diagnoses for each category and between the following time points: T0–T1 (from enrollment to completion of work-up but before microEEG), T0–T3 (from enrollment to disposition), and T1–T2 (in the intervention arm only, before and after the microEEG). These changes are shown in Figures 3, 4, and 5. Each subject was assigned a score for the overall change in differential diagnosis. The score was calculated as the standard deviation (SD) of the probability changes (for that subject) across all 10 diagnostic categories. For example, if a physician assigned the same probabilities to all the categories on two occasions then the SD change score would be zero. However, if the physician assigned the same 10 probability values, but to different categories, then the change score would be nonzero and grow as a function of the number of diagnostic categories that were changed. The probability changes represent the change in subjective confidence in each diagnosis of the treating physician. In other words, what is measured here is the SD of the probability change scores from time X to time Y. For example, at time X a patient’s 10 differential diagnosis probability scores (as assessed subjectively by the clinician) are X1 to X10. At time Y those scores are Y1 to Y10. We now compute Y1–X1, Y2–X2, etc., to generate 10 change scores. The SD of those 10 change scores is the variable of interest.
Figure 4.
Schematic for enrollment (inclusion/exclusion) process. EEG = electroencephalogram.
Using the SD has numerous advantages: it provides an unbiased indicator of the overall probability change, since each category contributes in proportion to its own probability differential. In addition, this SD is mathematically equivalent to the Euclidean (straight line) distance in the 10-dimensional differential diagnosis space between the two questionnaires at the starting and ending times, which provides an intuitive metric for the change of probability between those two time points. We also repeated the calculation by using the Mahalanobis distance10[CE1] instead of the Euclidean distance and obtained results that are qualitatively similar to those reported here. Mahalanobis distance naturally takes into account the correlations of the probability changes in the calculation of the distance and reduces to the Euclidean distance when all probability changes are uncorrelated and have the same variance. In other words, the Euclidean metric assumes that the 10 dimensions have uniform variance and are mutually independent. The Mahalanobis does not.
The distributions of probability SDs over the subjects were highly nonnormal. Therefore, we chose the two-sided two-sample Kolmogorov-Smirnov test, a nonparametric and conservative estimator of significance that is suitable for such distributions, to compare the intervention arm’s distribution with that of the control arm. The effect sizes are presented as differences in mean of SDs of probability for differential diagnoses with 95% CIs (calculated using Hodges-Lehmann method). Standardized between-arm effect sizes (measured in SD units) were estimated by dividing difference between study arms of mean change by pooled SD. Data analyses were performed with SPSS software (Version 20.0, IBM, Armonk, NY).
Sample Size Analysis
Ziai et al.11 showed that EEG assisted in ED diagnosis in 51% of the cases in their patient cohort. Therefore, we used this number as the basis for change in diagnosis in our study for the intervention arm. Our group decided that 25% change in diagnosis for the control arm (due to information obtained from other tests or new information) was clinically reasonable. Based on these assumptions, the study would need 65 patients in each arm to reach a power of 80% (α = 5%, two-sided Fisher exact test).
RESULTS
Enrollment occurred from August 1, 2012, to December 15, 2012. During the study period, a total of 180 patients were screened. The final analysis was performed on 149 patients (73 EEG and 76 controls). The process of patient selection and reasons for exclusions are presented in Figure 2. The characteristics of the enrolled subjects were similar at baseline (Table 1).
Table 1.
Baseline Characteristics of Patients Included in the Study
| Variables | Control Group (n = 76), n (%) 95% CI |
Treatment Group (n = 73), n (%) 95% CI |
|---|---|---|
| Age in years, median (IQR) | 68 (53–81) | 62 (47–74) |
| Sex (male) | 41 (54) 43–65 | 43 (59) 47–69 |
| History of seizure | 23 (30) 20–40 | 27 (37) 27–48 |
| Seizure in the field | 22 (29) 20–40 | 20 (27) 18–39 |
| Seizure in the ED | 11 (14) 8–20 | 10 (14) 7–24 |
| High risk for seizure* | 30 (39) 29–51 | 35 (48) 37–59 |
| Anticonvulsive medications in field | 8 (11) 5–20 | 11 (7) 8–25 |
| Anticonvulsive medications in ED† | 35 (46) 35–57 | 43 (59) 47–69 |
| Abnormal neurologic findings | 17 (22) 14–33 | 18 (25) 16–36 |
| Acute head injury | 7 (9) 4–18 | 4 (5) 2–14 |
| Acute head CT findings | 14 (20) 10–29 | 13 (19) 11–28 |
IQR = interquartile range.
Consisted of patients with history of seizure or seizure in the field or seizure in the ED.
Includes medications given for sedation (e.g., benzodiazepines).
At baseline (T0), the most common differential diagnoses for the control arm were neurologic (seizure only), 63%; neurologic (nonseizure), 42%; and metabolic, 41%. In the intervention arm, the most common diagnoses were similar to those of the control arm, with different distribution: neurologic (seizure only) in 75%, metabolic in 38%, and neurologic (nonseizure) in 47% of cases. Sixty-eight of 73 EEGs (intervention arm only) were interpreted as abnormal (93%, 95% CI = 85% to 97%). Four patients (5%, 95% CI = 2% to 13%) had NCS. Frequency of EEG interpretations is presented in Table 2.
Table 2.
EEG Interpretations for Patients in the Intervention Group (n = 73)
| EEG interpretation | n (%) | 95% CI |
|---|---|---|
| NCS | 4 (5) | 2–13 |
| Status epilepticus | 3 (4) | 1–12 |
| Seizure without status epilepticus | 1 (1) | 0–8 |
| Interictal epileptiform waves | 6 (8) | 3.5–17 |
| Slowing | 58 (79) | 68–87 |
| Normal | 5 (7) | 3–15 |
EEG = electroencephalogram; NCS = nonconvulsive seizure.
In our initial (a priori) analysis, prevalence of change of any kind in differential diagnosis from T0 to T1 did not differ significantly between study arms (intervention 58 of 73, control 58 of 76, p = 0.696), nor from T0 to T3 (intervention 67 of 73, control 61 of 76, p = 0.059). In the intervention arm, introducing microEEG was associated with an immediate (i.e., T1 to T2) change in differential diagnosis in 73% of cases (95% CI = 61% to 82%).
Inspection of changes in differential diagnosis using the post hoc approach revealed no significant difference between study arms in change from T0 to T1 (mean [±SD] change score was 12 [±12] for intervention and 12 [±13] for the control group, Hodges-Lehman mean difference 0.00, 95% CI = –2.4 to 3.9, p = 0.869). Standardized between-arm effect size estimate was 0.00 SD units. The difference between study arms from T0 to T3 was more prominent, but still not statistically significant (mean [±SD] change score 19 [±14] for intervention and 15 [±14] for control), with a Hodges-Lehman mean difference of 4.1 (95% CI = 0.0 to 8.3, p = 0.066). Standardized between-arm effect size estimate was 0.29 SD units, i.e., a small effect. Incorporating EEG in the work-up of patients in the intervention arm (T1 to T2) was associated with an immediate and substantial mean (±SD) change score of 11 (±12) (95% CI = 7.7 to 13.5; Figures 3–5).
The physician-reported effect of EEG on establishing the diagnosis, change in diagnostic work-up, and change in treatment plan immediately after the EEG results and at disposition time are presented in Tables 3 and 4. Almost half of the patients were dispositioned (admitted or discharged) shortly after EEG results. Therefore, the time interval between T2 and T3 in most subjects in the intervention arm was negligible. Physician-reported changes in patient management specifically in the intervention arm at patient disposition is presented in Table 5.
Table 3.
Physician-reported Effect of MicroEEG on Patient Management in the Intervention Group
| Effect of MicroEEG | Upon Receiving EEG Result, n (%) 95% CI | At Final ED Disposition, n (%) 95% CI |
|---|---|---|
| Helped establish a diagnosis | 41 (56) 41–70 | 43 (59) 47–69 |
| Changed overall diagnostic workup | 36 (49) 38–60 | 36 (49) 38–61 |
| Changed the overall treatment plan | 31 (42) 31–53 | 31 (42) 31–53 |
n = 73.
EEG = electroencephalogram.
Table 4.
Physician-reported Changes in Patients’ Management in the Intervention and Control Arms at Patient Disposition
| Questionnaire at the Time of ED Disposition | n/N (%) 95% CI |
|---|---|
| Intervention group | |
| EEG helped establish a diagnosis | 43/73 (59) 47–69 |
| EEG affected pre-EEG diagnostic impression: | |
| a. Ruled out a diagnosis | 32/73 (44) 33–55 |
| b. Confirmed a diagnosis | 15/73 (21) 13–31 |
| c. No change | 26/73 (36) 26–47 |
| EEG changed the overall diagnostic work-up or disposition | 36/73 (49) 38–61 |
| EEG changed the overall treatment plan | 31/73 (42) 32–54 |
| Control group | |
| The diagnosis changed during the ED work-up | 15/76 (20) 12–30 |
| The overall diagnostic management changed since enrollment | 11/76 (14) 8–24 |
| The overall treatment plan changed because of a laboratory test or an imaging study | 35/76 (46) 35–57 |
| Would you have ordered an EEG, had you had it available? | 45/74 (61) 49–71 |
EEG = electroencephalogram.
Table 5.
Comparison of Secondary Outcomes Between the Intervention and the Control Groups
| Outcome | Control Group (n = 76), n (%) 95% CI |
Intervention Group (n = 73), n (%) 95% CI |
p-value‡ |
|---|---|---|---|
| In-hospital mortality | 4 (5.5) 2–13 | 4 (5.3) 2–14 | 1.000 |
| ED LOS in hours, median (IQR)* | 5.5 (3–8) | 5 (4–9) | 0.352 |
| Hospital LOS in days, median (IQR)† | 3 (1–8) | 4 (1–8) | 0.495 |
| Disposition | |||
| Admit (regular ward) | 52 (68) 59–76 | 43 (59) 47–69 | |
| Admit (ICU) | 13 (17) 10–27 | 19 (26) 17–37 | |
| Discharge home | 10 (13) 7–23 | 10 (14) 7–24 | 0.599 |
| Transfer to another facility | 1 (1) 0–8 | 1 (1) 0–8 |
ICU = intensive care unit; IQR = interquartile range; LOS = length of stay.
From triage to disposition order.
From triage to written discharge order (or death).
Fisher’s exact test for categorical and Mann-Whitney U test for continuous variables.
All but one of the 73 EEGs were reviewed by a second epileptologist blinded to the interpretation provided by the first epileptologist, but with access to initial clinical information as described under Methods. Kappa representing agreement of epileptologists (based on a nine-category taxonomy) in blinded interpretation of EEGs was 0.43 (95% CI = 0.23 to 0.64).
The list of final hospital diagnoses for the study cohort is presented in Table 6. Of 76 patients in the control arm, 21 had standard EEG ordered and performed after admission. All but one standard EEG was performed more than 24 hours after the patients left the ED. These EEGs revealed slowing in 16 of 21 (76%, 95% CI = 54% to 90%) and epileptiform discharges in 1 of 21 (5%, 95% CI = 1% to 24%) and were normal in four of 21 (20%, 95% CI = 7% to 41%).
Table 6.
Final Hospital Diagnosis for the Enrolled Subjects*
| Control (n = 76) | microEEG (n = 73) | ||||
|---|---|---|---|---|---|
| Diagnosis | n | % | Diagnosis | n | % |
| Neurologic (seizure only) | 24 | 32 | Neurologic (seizure only) | 26 | 36 |
| Vascular (hypertensive encephalopathy, vasculitis, etc.) | 16 | 21 | Neurologic (nonseizure) | 19 | 26 |
| Stroke | 9 | 12 | |||
| Neurologic (nonseizure) | 16 | 21 | Brain tumor | 3 | 4 |
| Stroke | 7 | 9 | CNS infection | 2 | 3 |
| Brain tumor | 0 | 0 | Other neurological (e.g. dementia) | 5 | 7 |
| CNS infection | 2 | 3 | Metabolic (uremia, hepatic encephalopathy, endocrine, etc.) | 17 | 23 |
| Other neurologic (e.g., dementia) | 7 | 9 | |||
| Metabolic (uremia, hepatic encephalopathy, endocrine, etc.) | 15 | 21 | Vascular (hypertensive encephalopathy, vasculitis, etc.) | 13 | 18 |
| Non-CNS infection (e.g., sepsis) | 10 | 11 | Non-CNS infection (e.g. sepsis) | 8 | 11 |
| Respiratory (e.g. hypoxia) | 9 | 12 | Toxicologic (e.g., ethanol withdrawal/intoxication) | 7 | 10 |
| Toxicologic (e.g., ethanol withdrawal/intoxication) | 8 | 11 | Psychological (acute psychosis, catatonia, etc.) | 5 | 7 |
| Psychological (acute psychosis, catatonia, etc.) | 6 | 8 | Respiratory (e.g., hypoxia) | 3 | 4 |
| Trauma (e.g., traumatic brain injury) | 0 | 0 | Trauma (e.g., traumatic brain injury) | 1 | 1 |
| Undifferentiated AMS/Other | 20 | 26 | Undifferentiated AMS/Other | 14 | 19 |
AMS = altered mental status; CNS = central nervous system; EEG = electroencephalogram.
Some patients had more than one final diagnosis.
DISCUSSION
The time-sensitive nature of EEG abnormalities, including NCS and NCSE, demands quicker access to EEG for ED patients, especially those at higher risk of having EEG abnormalities (e.g., NCS), such as patients with AMS. The use of microEEG may overcome some of the hurdles associated with using EEG in the ED setting. However, the clinical applicability of EEG findings and its influence on clinical decision-making and patient management has not been studied adequately. Most studies have evaluated the yield of EEG in AMS patients based on the number of cases of NCS or NCSE they discovered.5–8,12–15 However, we employed a different approach. We studied the benefits of using EEG in ED patients with AMS by asking ED physicians how their management was influenced by this test. We also measured the changes in the list of top differential diagnoses over time between the control and intervention arms using a randomized controlled design. The latter approach sheds additional light on how EEG affects the clinical decision-making process while managing AMS patients.
We selected patients with AMS as our target cohort in this study mainly because of the challenges associated with their management in the ED.16,17 In most cases, the clinical evaluation is difficult, especially if a thorough history cannot be obtained and a complete physical examination is not possible.16 Therefore, EPs mostly rely on laboratory and imaging studies for making the diagnosis. Information pertaining to cerebral function, provided by EEG, could be of significant value in such circumstances. However, the utility of EEG is not limited to this entity.18
Praline et al.13 studied the value of emergent EEG (EEG ordered on an emergent basis from anywhere in the hospital) on clinical decision-making in a prospective study. These investigators reported that EEG contributed to the diagnosis in 78% of cases. In the study by Praline et al., EEG also resulted in changes in diagnostic management in 47% of cases and changes in treatment plan in 38% of cases.13 These results are in accord with our findings. In our study, EEG helped establish the diagnosis in 56% of cases, changed diagnostic management in 49% of cases, and changed treatment plans in 42% of cases. As mentioned, the study by Praline et al. was not limited to ED patients.13 In fact, the majority of emergent EEGs were ordered from the ICU (70% of cases). In another study conducted in the ED, Ziai et al.11 assessed the role of EEG in ED patients with AMS in a single-center, prospective cohort study. Patients were enrolled in the study if they had witnessed seizure activity in the ED or had AMS. Patients underwent 20-minute EEGs using a 16-channel portable digital EEG device. In the study by Ziai et al., the EEG helped to establish the diagnosis in 51% of the cases, but changed the ED management only in 4% of the cases.11 The difference between the results of this study and our findings could be explained by the nature of the study sample (not all patients had AMS), sampling method (patients were enrolled 1 day a week only), and the spectrum of the sample (no cases of NCS or NCSE were identified) in the study by Ziai et al. Instead, our enrollment occurred 24 hours a day 7 days a week, and the rate of NCS/NCSE in our cohort was 4%.
We used a unique approach to present the changes in the differential diagnosis between various time points. Presenting the changes using this approach provides the readers with a visual plot of the raw categorical changes. Inspection of the data indicates that the differences in differential diagnosis from T0 to T1 (before EEG in intervention arm and before completion of workup in control arm) were not distinct. However, the changes in probability of diseases were more noticeable from T0 to T3 (initiation of enrollment to completion of disposition) between the two arms and T1 to T2 (before and after the EEG, intervention arm only).
Our randomized trial was not powered to assess clinical outcomes such as ED or hospital length of stay or mortality. However, we included these outcomes as secondary outcomes in our study to estimate the effect sizes that could help us calculate the sample size of a future, larger multicenter trial that would be needed to accurately assess these outcomes.
In our study, we used RAs for recording EEGs instead of certified EEG technicians. These RAs were trained to set up the device, perform troubleshooting, annotate events occurring during EEG recording (e.g., eye opening), and transmit and store the recordings. This indicates that the application of the head cap and EEG acquisition could be taught to a group of ED personnel with a variable degree of sophistication. This could obviate the need for an EEG technician in the ED, at least during the off-hours.
LIMITATIONS
This study was conducted in two urban academic institutions serving a culturally and ethnically diverse population of low socioeconomic status. Therefore, the findings of the study may not be generalizable to other populations.
We relied on ED attending physician surveys to identify change in management or change in differential diagnosis. The changes in management were “expected.” We did not measure the actual change in patients’ management. Additionally, changes in diagnosis and practice may not necessarily yield changes in outcome. We did not control for physician experience or years of practice, which might affect how management is affected by any test.
As previously mentioned, due to logistic issues, our study was not powered to measure the differences in mortality or length-of-stay outcomes in the study cohort. We believed that assessing these outcomes require a much larger sample size. The data collected in our study make estimating the accurate sample size for a larger multicenter trial possible.
Although the enrollment occurred 24/7, the investigators relied on EPs to refer the subjects for enrollment by calling the study hotline. To reduce the risk of missing qualified patients, the study coordinators screened the EDs several times a day to identify potential candidates. Regardless, it is possible that some AMS patients were not referred for enrollment in the study or that physicians preferentially referred patients at higher risk of seizure (e.g., those with history of seizure) for enrollment, subjecting the study to a sampling bias. However, we believe that our sampling strategy reduced this risk to a minimum.
The interrater reliability among EEG interpreters was low. This poor agreement is associated with EEG as a diagnostic test and is not limited to our study. In fact it has been extensively discussed in the literature.19,20 In addition, the epileptologists were not blinded to the clinical information; they might have provided clinical feedback (as neurologists) based on the clinical history and not exclusively based on the EEG findings. However, since the information from EEG interpretations was being used in clinical management, we did not think that blinding the epileptologists to the clinical information would be appropriate. In addition, since blinding of EPs was not possible in our study, a recall bias might have contributed to the higher chance of change of management in the intervention arm.
It should be pointed out that an EEG only records about 30 minutes of brain activity. Seizure or other abnormal activities or patterns might have occurred outside this “snapshot” view of the brain activity. Last, although 93% of the EEGs were interpreted as abnormal, not all abnormal findings could have affected clinical decisions.
CONCLUSIONS
Electroencephalograms obtained by micro-electroencephalogram can affect diagnostic and therapeutic management of altered mental status patients in the ED. These findings, paired with the high incidence of electroencephalographic abnormalities in altered mental status patients, further support the need to incorporate electroencephalogram in the work-up of these patients in the ED.
Acknowledgments
Supported by NIH grant 1RC3NS070658 to Bio-Signal Group Inc. (BSG). SZ, ACG, RS, GC, and JW received salary support through a subcontract to Downstate Medical Center. SGAB and AO are BSG employees. AF is the founder of BSG. ACG serves on the BSG advisory board. All income derived from this position is donated directly from BSG to the Downstate College of 2 Medicine Foundation. Drs. Zehtabchi and Sinert, associate editors for this journal, had no role in the peer review or publication decision processes for this paper.
The authors appreciate the assistance and support of the following individuals without whom conducting this study would not have been possible: Helen Valsamis, Ewa Koziorynska, Douglas Maus, Tresa McSween, Katherine Mortati, Alexandra Reznikov, Roger Cracco, Sage Wiener, Vanessa Arnedo, John Gridley, Krishnakant Nammi, and Shweta Malhotra.
Footnotes
Presented at the Society for Academic Emergency Medicine Annual Meeting, Atlanta, GA, May 2013.
References
- 1.Privitera MD, Strawsburg RH. Electroencephalographic monitoring in the emergency department. Emerg Med Clin North Am. 1994;12:1089–1100. [PubMed] [Google Scholar]
- 2.Omurtag A, Baki SG, Chari G, et al. Technical and clinical analysis of microEEG: a miniature wireless EEG device designed to record high-quality EEG in the emergency department. Int J Emerg Med. 2012;5:35. doi: 10.1186/1865-1380-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zehtabchi S, Grant AC, Abdel Baki SG, et al. Diagnostic accuracy of a novel emergency electroencephalography device (microEEG) in identifying non-convulsive seizures and other EEG abnormalities in the emergency department patients with altered mental status [abstract] Acad Emerg Med. 2012;19(Suppl 1):s378. [Google Scholar]
- 4.Zehtabchi S, Abdel Baki SG, Omurtag A, et al. Prevalence of non-convulsive seizure and other electroencephalographic abnormalities in emergency department patients with altered mental status. Am J Emerg Med. 2013;31:1578–1582. doi: 10.1016/j.ajem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bautista RE, Godwin S, Caro D. Incorporating abbreviated EEGs in the initial workup of patients who present to the emergency room with mental status changes of unknown etiology. J Clin Neurophysiol. 2007;24:16–21. doi: 10.1097/WNP.0b013e318030e8cb. [DOI] [PubMed] [Google Scholar]
- 6.Kapadia FN, Vadi S, Shukla U, Gursahani R. Utility of electroencephalogram in altered states of consciousness in intensive care unit patients. Indian J Crit Care Med. 2005;9:19–21. [Google Scholar]
- 7.Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994;18:155–166. doi: 10.1016/0920-1211(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 8.Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–345. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- 9.Agresti A, Coull BA. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Am Statistician. 1998;52:119–126. [Google Scholar]
- 10.De Maesschalck R, Jouan-Rimbaud D, Massart DL. The Mahalanobis distance. Chemomet Intell Lab Sys. 2000;50:1–18. [Google Scholar]
- 11.Ziai WC, Schlattman D, Llinas R, Venkatesha S, Truesdale M, Schevchenko A. Emergent EEG in the emergency department in patients with altered mental states. Clin Neurophysiol. 2012;123:910–917. doi: 10.1016/j.clinph.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 12.Varelas PN, Spanaki MV, Hacein-Bey L, Hether T, Terranova B. Emergent EEG: indications and diagnostic yield. Neurology. 2003;61:702–704. doi: 10.1212/01.wnl.0000078812.36581.97. [DOI] [PubMed] [Google Scholar]
- 13.Praline J, Grujic J, Corcia P, et al. Emergent EEG in clinical practice. Clin Neurophysiol. 2007;118:2149–2155. doi: 10.1016/j.clinph.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 15.Zehtabchi S, Abdel Baki SG, Malhotra S, Grant AC. Nonconvulsive seizures in patients presenting with altered mental status: an evidence-based review. Epilepsy Behav. 2011;22:139–143. doi: 10.1016/j.yebeh.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanich W, Brady WJ, Huff JS, et al. Altered mental status: evaluation and etiology in the ED. Am J Emerg Med. 2002;20:613–617. doi: 10.1053/ajem.2002.35464. [DOI] [PubMed] [Google Scholar]
- 17.Sporer KA, Solares M, Durant EJ, Wang W, Wu AH, Rodriguez RM. Accuracy of the initial diagnosis among patients with an acutely altered mental status. Emerg Med J. 2013;30:243–246. doi: 10.1136/emermed-2011-200452. [DOI] [PubMed] [Google Scholar]
- 18.Firosh Khan S, Ashalatha R, Thomas SV, Sarma PS. Emergent EEG is helpful in neurology critical care practice. Clin Neurophysiol. 2005;116:2454–2459. doi: 10.1016/j.clinph.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Gerber PA, Chapman KE, Chung SS, et al. Interobserver agreement in the interpretation of EEG patterns in critically ill adults. J Clin Neurophysiol. 2008;25:241–249. doi: 10.1097/WNP.0b013e318182ed67. [DOI] [PubMed] [Google Scholar]
- 20.Azuma H, Hori S, Nakanishi M, Fujimoto S, Ichikawa N, Furukawa TA. An intervention to improve the interrater reliability of clinical EEG interpretations. Psychiatry Clin Neurosci. 2003;57:485–489. doi: 10.1046/j.1440-1819.2003.01152.x. [DOI] [PubMed] [Google Scholar]