Abstract
Previous investigations into whether the APOE-ε4 allele exerts cognitive effects at midlife have been inconclusive. We have advanced a “cognitive phenotype” hypothesis arguing that the ε4 allele of the apolipoprotein E gene (APOE) is associated with lower efficiency of neuronal plasticity thereby resulting in poorer cognitive performance independently of the pathology of Alzheimer’s disease (Greenwood et al., 2005). This hypothesis is best tested at midlife, prior to the neuron loss associated with AD diagnosis. This hypothesis predicts that the ε4 allele would alter cognition regardless of age through plasticity mechanisms, but would not induce longitudinal decline in midlife. The alternative “prodrome” hypothesis predicts that the APOE-ε4 allele would be associated with longitudinal cognitive decline as early as midlife due to prodromal effects of AD. We tested these hypotheses with a working memory task in a large cross-sectional sample of cognitively screened APOE-ε4 carriers and non-carriers and also in a small longitudinal sample over 3 years. The sample was divided into middle-aged (mean age 50, range 40-59) and older (mean age 69, range 60-84) individuals. Cross-sectionally, we observed that older, but not middle-aged, APOE-ε4 carriers had lower accuracy than ε4 non-carriers, mainly under the hardest discrimination condition. Longitudinally, we observed increases in accuracy in middle-aged APOE-ε4 carriers, suggesting a cognitive phenotype that includes ability to benefit from experience. We observed a longitudinal decrease in older APOE-ε4 carriers, suggesting an AD prodrome.
The ε4 allele of the APOE gene is a well-known risk factor for Alzheimer’s disease (AD) (Corder et al., 1993) and has also been associated with poorer cognitive performance in older adults (for reviews, see (Greenwood & Parasuraman, 2003; Parasuraman, Greenwood, & Sunderland, 2002)). Previous work has not resolved whether cognitive decline in APOE ε4 carriers is seen in prior to old age. Two competing hypotheses have been advanced to explain the effects of APOE on cognition. The “prodrome” hypothesis assumes that the poorer cognitive performance in groups of people with the APOE-ε4 allele is due to a larger subpopulation with developing AD compared to non-carriers (Smith et al., 1998). We previously advanced an alternative “cognitive phenotype” hypothesis that assumes the ε4 allele is associated with lower efficiency of neuronal plasticity and myelin formation and repair (Greenwood & Parasuraman, 2003), thereby resulting in poorer cognitive performance independently of AD pathology (Greenwood, Lambert, Sunderland, & Parasuraman, 2005). Effects of the APOE-ε4 allele on cognition in healthy adults have been confirmed by meta-analyses (Small, Rosnick, Fratiglioni, & Backman, 2004; Wisdom, Callahan, & Hawkins, 2011), although the studies included in these meta-analyses involved mainly older participants who may have pre-symptomatic AD. Therefore, the question remains whether there are effects of the ε4 allele that are independent of AD pathology. Longitudinal assessment in midlife provides one way to test this hypothesis. The cognitive phenotype hypothesis predicts that the ε4 allele exerts effects on the brain and cognition early in life that are not associated with the pathognomonic lesions of AD – plaques and tangles. Alternatively, the prodrome hypothesis predicts that the APOE-ε4 allele would be associated with longitudinal cognitive decline by midlife due to prodromal effects of the developing disease. This hypothesis is relevant only in midlife, insofar as late in life the likelihood of developing AD pathology is increased in ε4 carriers (Corder et al., 1993) and the pathology itself would induce cognitive decline.
The cognitive phenotype hypothesis was initially based on evidence that APOE-ε4 carriers show poorer cognitive performance in midlife (Greenwood et al., 2005; Greenwood, Sunderland, Friz, & Parasuraman, 2000; Negash et al., 2009), at a mean age more than a decade younger than the typical age of AD diagnosis of 75 (Corder et al., 1993; Wilson, Leurgans, Boyle, & Bennett, 2011). Specifically, the APOE ε4 allele exerts negative effects on attention and working memory (WM) beginning in the 4th decade of life (Blair et al., 2005; Flory, Manuck, Ferrell, Ryan, & Muldoon, 2000; Negash et al., 2009). There is also brain-based evidence from studies of neonates and children not known to have plaques and tangles. Regional gray matter volume differences as a function of APOE genotype have been observed in neonates (Dean et al., 2014; Knickmeyer et al., 2013) and in children and adolescents (aged 8-20) (Shaw et al., 2007). Regional brain activation differences as a function of APOE genotype have been observed in middle-aged APOE-ε4 carriers with an increased BOLD response in medial temporal lobe and prefrontal and association cortices during encoding (Trachtenberg, Filippini, & Mackay, 2012). Similar results have been seen in older APOE-ε4 carriers (Kukolja, Thiel, Eggermann, Zerres, & Fink, 2010). However, that an increased BOLD response was seen in both ε2 and ε4 carriers compared to an ε3/3 group (Trachtenberg et al., 2012) indicates this finding was not related to prodromal AD, as the ε2 allele has been found to be protective against development of AD {Corder, 1994 #4688; Lippa, 1997 #2790}. In support of that, Trachtenberg also found similarities in resting state networks between middle-aged ε2 and ε4 carriers (Trachtenberg, Filippini, Ebmeier, et al., 2012). In summary, this evidence that APOE genotype influences brain structure in infancy and childhood and influences brain activation and cognition beginning in young adulthood provides a basis for the cognitive phenotype hypothesis given that AD pathology is unknown in those age ranges.
What is the basis for the APOE-ε4 cognitive phenotype? The APOE gene encodes the ApoE lipoprotein, important in lipid transport in the brain. Lipid transport is essential for myelin formation, maintenance, and repair (Poirier, 2005; White, Nicoll, & Horsburgh, 2001) and carriers of the ε4 allele have fewer ApoE molecules with which to transport lipid compared to non-carriers (Utermann, Langenbeck, Beisiegel, & Weber, 1980). Consistent with that evidence, neonatal and infant ε4 carriers showed evidence of slower myelination (Dean et al., 2014). As such, the ApoE lipoprotein appears to have a fundamental role in myelin formation and neuronal repair and plasticity (e.g., (Mauch et al., 2001; Teter et al., 2002; White et al., 2001)). We previously argued from this literature that chronically lower ability to form myelin and repair neuronal damage in APOE-ε4 allele carriers would impair cognition independently of the pathophysiology of AD (Espeseth, Westlye, et al., 2012; Greenwood et al., 2005; Greenwood & Parasuraman, 2003; Negash et al., 2009; Reinvang, Espeseth, & Westlye, 2013).
Based on the role of APOE in lipid transport, white matter integrity is a putative mechanism underlying a cognitive phenotype of APOE. Animal work shows that myelination is a plastic process, which can be heightened over weeks by neuronal firing (Wake, Lee, & Fields, 2011). Balloons and blisters form in myelin sheaths in aged monkeys, but remyelination also occurs along with an age-related increase in numbers of oligodendrocytes (Peters, 2002). In humans, neuroimaging studies have used diffusion tensor imaging to assess white matter integrity in the context of APOE genotype. There is recent evidence of lower white matter integrity in APOE-ε4 allele carriers across the adult life span, and independent of age (Jochemsen, Muller, van der Graaf, & Geerlings, 2012). Both mean diffusion and radial diffusion in ROIs including genu of corpus callosum and supraorbital white matter were greater in ε4 carriers compared to those with an ε3/3 genotype (Bartzokis et al., 2007). Those findings were confirmed by Espeseth and colleagues in a larger sample using tract-based spatial statistics. They found that both ε4 and ε2 carriers showed relatively increased mean and radial diffusivity regardless of age group, consistent with a trait effect of both alleles (Westlye, Reinvang, Rootwelt, & Espeseth, 2012). Because the ε2 allele is associated with reduced risk of AD (Corder et al., 1994), these effects on white matter do not appear to be related to development of AD.
Neuroimaging studies find mixed effects of the APOE-ε4 allele on regional brain activation. Increased regional BOLD signal during memory encoding has been reported in some studies but decreased BOLD signal was reported in others (reviewed in (Trachtenberg, Filippini, & Mackay, 2012)). However, the only imaging study to date restricted to middle-aged people observed that ε4 (and ε2) carriers showed greater activation in medial temporal lobe and PFC compared to those with an ε3/3 genotype (Trachtenberg, Filippini, Cheeseman, et al., 2012). The significance of increased or decreased regional activation is not clear. Increased activation has been linked to better cognitive performance by some investigators (Gray, Chabris, & Braver, 2003) but linked to worse cognitive performance by others (Rypma et al., 2006). However, if lower activation reflects greater “processing efficiency” as claimed, then the increased regional activation seen in middle-aged ε4 carriers (Trachtenberg, Filippini, Cheeseman, et al., 2012) would be consistent with the decreased white matter integrity seen also in middle-aged ε4 carriers (Westlye et al., 2012).
The above-reviewed evidence that the ε4 allele influences cognition, brain activation, and white matter integrity regardless of age predicts effects on cognition early in life. There is some evidence that the ε4 allele confers benefits in children and young adults. In children, cognitive benefits of the ε4 allele have been reported on IQ (Yu, Lin, Chen, Hong, & Tsai, 2000) and verbal fluency (Alexander et al., 2007; Oria et al., 2005). In young adults cognitive benefits of the ε4 allele have been reported on a range of tasks, including attention (Rusted et al., 2013), episodic memory (Mondadori et al., 2007), and executive function (Chen et al., 2007). Consistent with that, Jochemsen and colleagues found an increase over 4 years in working memory performance in ε4 carriers below age 57 (based on a median split) but a decrease in memory in ε4 carriers above that age (Jochemsen et al., 2012). That sample was symptomatic for atherosclerotic disease but the APOE associations were not dependent on cardiovascular factors. Han and Bondi argued that the pattern of ε4-related benefits early in life but costs later in life is an example of “antagonistic pleiotropy” (Han & Bondi, 2008). However, a recent meta-analysis was unable to confirm effects of APOE on a range of cognitive tasks (whether categorized as high or low in executive demands) in children and young adults (Ihle, Bunce, & Kliegel, 2012). They did not include studies of middle-aged people.
Evidence from twin studies that genetic effects on cognition increase with age (McClearn et al., 1997; McGue & Christensen, 2002) predicts that a cognitive phenotype would be more detectable in middle-aged than in younger people. We found selective deficits in visuospatial attention in middle-aged and young-old ε4 carriers (Negash et al., 2009; Greenwood et al., 2005; Espeseth et al., 2006) and in working memory in middle-aged ε4 carriers (Greenwood et al., 2000) (Greenwood et al., 2005). Consistent with those findings, Caselli et al. concluded from a large sample assessed longitudinally over 5 years that the negative effects of the ε4 allele begin before age 60 (Caselli et al., 2009). In another large longitudinally assessed sample, Kozauer et al. (2008) tested in 4 waves over 22 years and again found negative effects of the ε4 allele on delayed recall, only in those who were younger than 65 at the time of the 4th testing. In contrast, Bunce et al. (2013) found no cognitive effects over 8 years in young or middle-aged as a function of APOE genotype. The failure of some studies to find effects of the APOE-ε4 allele in very old age (after age 80) may be due to a healthy survivor effect (Negash et al., 2009; Kozauer et al., 2008).
Overall, the evidence on cognitive effects of the ε4 allele in midlife is mixed, with previous evidence of positive effects, negative effects, and no effects. However, most of those studies used standardized neuropsychological tests, which may not be sensitive to subtle cognitive change (e.g. Kozauer et al. used the MMSE). We used an information-processing working memory task (manipulating load and discrimination difficulty) that we previously found to be sensitive to APOE genotype in middle-age (Greenwood et al., 2005; Greenwood et al., 2000). Deficits in working memory and semantic memory were the earliest functions to undergo decline during the AD prodrome (Wilson et al., 2011). The cognitive phenotype hypothesis predicts effects of the ε4 allele on working memory accuracy beginning at least in midlife, but without longitudinal decline. The prodrome hypothesis predicts effects of the ε4 allele on working memory accuracy in midlife, with longitudinal decline.
Methods
Participants
The data reported here were collected as part of a large study of cognitive aging conducted jointly by the Norwegian Cognitive NeuroGenetics study (NCNG, Espeseth et al., 2012) and the Cognitive Genetics Group of George Mason University (GMU). All persons gave informed consent and were screened by questionnaire for neurological and psychiatric disease. Demographic characteristics are given in Table 1. The average inter-test interval was 1.4 years between years 1 and 2 and 1.0 years between years 2 and 3. Participants reported whether they had a first degree relative diagnosed with dementia.
Table 1.
Demographics means and standard deviations.
| Genotype Group | n | Age | Education | MMSE1 |
|---|---|---|---|---|
| Full Sample | ||||
| Middle | ||||
| APOE ε4+ | 75 | 50.9±5.5 | 15.6±2.5 | 29.2 ± 1.1 |
| APOE ε4− | 174 | 49.9±5.5 | 15.8±2.7 | 28.8 ±1.4 |
| Older | ||||
| APOE ε4+ | 99 | 68.7±5.5 | 15.6±2.5 | 28.7±1.5 |
| APOE ε4− | 243 | 69.6±6.2 | 15.8±2.7 | 28.6±2.2 |
| Longitudinal sample | ||||
| Middle | ||||
| APOE ε4+ | 31 | 50.3±5.3 | 15.7±2.9 | 29.23 ±1.0 |
| APOE ε4− | 68 | 50.4±5-4 | 15.6±2.9 | 29.22 ±1.2 |
| Older | ||||
| APOE ε4+ | 19 | 65.6±4.8 | 15.4±2.7 | 29.04±0.9 |
| APOE ε4− | 27 | 64.6±3.9 | 15.9±3.3 | 28.90±1.3 |
Mini-Mental State Exam (Folstein et al., 1975)
Neuropsychological Testing
A neuropsychological battery was administered: Wechsler Memory Scale-Revised Logical Memory subtest (Wechsler, 1987), WAIS Letter-number sequencing (Wechsler, 1981), and the Mini-Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975) to screen for dementing illness. All were administered at least twice over the 3 years.
Materials and Procedures
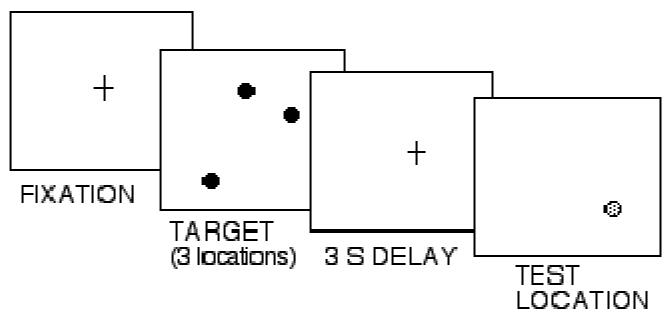
The delayed match-to-sample WM task systematically varied both memory load and spatial distance between target and test stimuli (Figure 1). On match trials, target and test dots appeared in the same location (0° between target and test locations). On non-match trials, target and test dots appeared randomly at different locations (2°, 4° or 8° apart). Participants were seated so their eyes were 60 cm from the computer screen and given task instructions. Each trial began with a 1 sec fixation cross in the center of the display. One, two, or three black target dots (0.67° in size) were displayed at random locations for 500 ms. Immediately following target offset, the centered fixation cross reappeared for 3 sec – the WM maintenance interval. At the end of that interval, a single red test dot (0.67°) appeared alone, either in the same location as one of the target dots (match trial) or at a different location (non-match trial). There were also three levels of memory load (number of dot locations, termed load 1, load 2, load 3) at each distance. Participants made a speeded judgment indicating whether or not the test dot was in the same location as one of the targets by pressing the “same” or “different” button. The response period began with the appearance of the red test dot and lasted for 2 s. In this design the ability to maintain up to 3 items in WM is assessed under conditions (a) when discrimination difficulty was not manipulated (match) and (b) when discrimination difficulty was manipulated (non-match). There were a total of 30 match trials (Distance 0) and 54 non-match trials (18 at each Distance). The speed and accuracy of same/different judgments were measured based on the time of the button press in response to the test stimulus.
Figure 1.
Schematic of task. Working memory load of 3 under a non-match, distance 2 condition is illustrated.
Statistical Analyses
Neuropsychological and working memory data were analyzed in univariate and repeated measures ANOVAs, with age group and genotype group as between-subjects factors and task conditions and year of testing as within subjects factors. The degrees of freedom for all F tests involving repeated measures factors were corrected for violations of the sphericity assumption by using the Greenhouse-Geisser procedure. The alpha level was set at .05. To determine whether APOE genotype was related to the presence of a first degree relative with dementia diagnosis, a univariate ANOVA was conducted to test for age × genotype interactions.
Genotyping
GMU: Genomic DNA was extracted using the BuccalAmp™ DNA Extraction Kit from Epicentre Biotechnologies (Madison, WI USA) according to manufacturers’ instructions. Each individual was genotyped for the rs429358 and rs7412 SNP in the APOE gene with pyrosequencing. PCR was performed to obtain an 218 amplicon (Zivelin et al., 1997) which covered the area of interest (rs429358 and rs7412) with primer 5′ TCCAAGGAGCTGCAGGCGGCGCA 3′ and 5′ biotinylated primer 5′ Biotin-GCCCCGGCCTGGTACACTGCCA 3′. The amplicon was denatured to isolate the single strand DNA on the Streptavidin Sepharose bead and immersed into 0.5uM annealing buffer with sequencing primer (5′ GCGGACATGGAGGAC 3′ for rs429358 and 5′ CCGATGACCTGCAGA 3′ for rs7412). The pyrosequencing was performed on PyroMark Q24 machine (Qiagen, USA) according to manufacture suggested reagents and protocol. The results were analyzed with software PyroMark Q24 version 2.0.6 (Qiagen, USA).
NCNG: DNA was extracted from whole blood using MagNA Pure LC DNA Isolation Kit – Large Volume on the MagNA Pure LC. APOE genotyping was performed by real-time PCR with allele-specific fluorescence energy transfer probes and melting curve analyses on LightCycler 480 (Roche Diagnostics, Mannheim, Germany) with primers and probes as specified by Aslanidis and Schmitz (Aslanidis & Schmitz, 1999): The sense primer (GAAGGCCTACAAATCGGAACTG) was truncated 2 nucleotides in the 5′ end, whereas the antisense primer (GGCTGCCCATCTCCTCCATC) was truncated 2 nucleotides in both ends. The detection probe (LC-red705-ACATGGAGGACGTGCGCG-p) for the ε4 allele was shortened one nucleotide in the 3′end, and LC-red705 was used as fluorophore instead of LC-red640 to allow for one tube duplex PCR. The corresponding anchor probe (AGGCGGCGCAGGCCCGGCTGGGCGC-fluorescein) was truncated 4 nucleotides 5′. The probe pair for ε2 was as originally published. The 20 μL duplex PCR reaction mix consisted of 1 × LightCycler 480 Probes Master (Roche), 0,1 μM of sense and 0,5 μM of antisense primers, 0,07 μM of each probe, 10% DMSO and 5 μL of diluted DNA eluate (10-100 ng). The PCR touchdown protocol consisted of initial denaturation of DNA and activation of the polymerase (95°C, 5 min); 40 cycles of denaturation (95°C, 10 sec), annealing (63°C stepping down 0.4°C/cycle to 59 °C, 10 sec), elongation (72°C, 10 sec); denaturation and polymerase inactivation (99°C, 5 min) and melting curve analysis (38°C (1 min) to 77° (ramp rate 1°C/sec). The ε4 allele (rs429358) was identified by melting temperature (Tm) 63°C vs. 55°C for wild type. The ε2 allele (rs7412) was identified by Tm 55°C (63°C for wild type).
Results
Participants
We used a MMSE cut off score of 27, found to be appropriate for a well-educated population (O’Bryant et al., 2008). Demographic information for participants, including genotype and age, is presented in Table 1. The age groups were middle-aged (40-59, mean age 50.2+/− 5.5) and older (60-83, mean age 69.1+/− 6.0). GMU Participants reported race and ethnicity on a questionnaire using NIH-specified categories. Analysis of family history of AD (self-reported) as a function of APOE genotype and age group showed no significant effects. There was a tendency for more first degree relatives in the ε4 carrier groups, but that was not significant.
NCNG participants were recruited in the Oslo and Bergen urban areas with the requirement that they were native speakers of Norwegian. DNA from the entire sample was later genotyped on the Illumina Human610-Quad Beadchip. Quality control was performed with the iterative check.marker function in the R package GenABEL (Aulchenko, Ripke, Isaacs, & van Duijn, 2007). Population structure was assessed by multidimensional scaling (MDS) analysis (100K random SNPs), removing outlying samples with possible recent non-Norwegian ancestry (see (Espeseth, Christoforou, et al., 2012) for details).
Participants who did not perform above chance on the working memory task were eliminated from the analysis.
Baseline analysis
After filtering, there were 591 participants who were tested at least one time, 417 ε4 non-carriers and 174 ε4 carriers. People with ε2/ε2 and ε2/ε3 genotype were categorized as non-carriers as we did not have a large enough sample of them to form a separate group. In that sample, 92% of participants were self-categorized as white (NIH categories). In light of the high racial homogeneity in this sample, no adjustments for stratification were conducted.
The baseline sample was analyzed in an omnibus ANOVA. In this design, match and non-match conditions were not crossed. The non-match condition had 3 levels of target-test distance (2, 4, 8 degrees) in addition to 3 levels of load while the match condition (distance 0 degrees) had 3 levels of load only. Therefore, to conduct an omnibus analysis, match trials were treated as the lowest level of distance (0 degrees of target-test distance). In a repeated measures ANOVA, within subjects factors were four distances (0, 2, 4, 8 degrees) and load (1, 2, 3 targets) and between subjects factors were age group (middle-aged, older) and presence of the APOE ε4 allele (ε4−, ε4+). Accuracy was lowest at the shortest non-match target-test distance and highest at the longest non-match target-test distance (F(1.78, 1046.02) = 1134.78, p < .0001, partial eta squared = .66). The middle-aged were more accurate than the older group (F(1,587) = 23.28, p<.0001, partial eta squared = .04), but that effect interacted with target-test distance (Four Distance × Load × Age Group (F(3.73, 2186.34) = 15.01, p<.0001, partial eta squared = .03). Based on those results, separate follow-up analyses were conducted on non-match trials for middle-aged and old groups separately. The middle-aged group showed no interactions with APOE group. In the older group, the non-carriers showed higher accuracy at the shortest target-test distance only (Distance × APOE group (F(1.39, 15.43) = 3.36, p < .05, partial eta squared = .01).
Longitudinal Analyses
A subset of the sample was assessed longitudinally. After filtering for accuracy, there were 143 participants (middle-aged: 67 ε4−, 31 ε4+; older: 26 ε4−, 19 ε4+) with testing on each of 3 sessions about 1 year apart (Table 1). People with ε2/ε2 and ε2/ε3 genotype were categorized as non-carriers as we did not have a large enough sample to form separate groups. In this sample, 97% of participants were self-categorized as white (NIH categories). In light of the high racial homogeneity in this sample, no adjustments for stratification were conducted. Participants were asked by questionnaire to indicate whether they had a first degree relative diagnosed with dementia.
Longitudinal analysis of neuropsychological data
For three standardized tests, data was collected twice over the 3 years – Wechsler Memory Scale-Revised Logical Memory subtest, WAIS Letter-number sequencing, and the Mini-Mental State Exam (MMSE). These were analyzed in a repeated measures ANOVA with age group (middle-aged, older) and presence of the APOE ε4 allele (ε4−, ε4+) as between subjects factors and year as the within subjects factor. There were no significant effects on the MMSE. On the Letter Number Sequencing task, the middle-aged were more accurate (main effect of age group, F(1,134) = 8.01 p = .005, partial eta squared = .06. Age group did not interact with year or APOE group. No other effects were significant. Wechsler Memory Scale was analyzed in a repeated measures ANOVA with age group (middle-aged, older) and presence of the APOE ε4 allele (ε4−, ε4+) as between subjects factors and year and recall (immediate, delayed) as the within subjects factor. Under the immediate recall condition, all but the older ε4 non-carriers declined over time but under the delayed recall condition, both middle-aged and older ε4 carriers declined while the non-carrier groups did not decline (main effect of year, F(1, 181) = 4.49, p = .036, partial eta squared = .03, interaction of Age Group × Recall, F(1, 162) = 6.26, p = .013, partial eta squared = .04, interaction of Year × Age Group × Recall × Genotype Group (F(1, 162) = 15.43, p = .003, partial eta squared = .05).
Omnibus longitudinal analysis of working memory data
As in the baseline analysis described above, in an omnibus repeated measures ANOVA, within subjects factors were four distance (0, 2, 4, 8 degrees) and load (1, 2, 3 targets) and year (1, 2, 3) and between subjects factors were age group (middle-aged, older) and APOE group (presence of at least one APOE ε4 allele, ε4+, ε4−). There were main effects of year (F(1.98, 263.47) = 5.84, p<.003, partial eta squared = .04), load (F(1.91, 253.92) = 429.92, p<.0001, partial eta squared = .76), and distance (F(1.67, 222.58) = 454.97, p <.0001, partial eta = .77). There was a significant interaction of Year × Distance × APOE × Age Group (F(3.23, 471.66) = 3.20, p = .004, partial eta squared = .02). Based on these significant effects involving distance between target and test stimulus, we conducted separate follow-up ANOVAs on match conditions (distance of 0 degree) and non-match conditions (distances of 2, 4, 8 degrees).
Match condition analyses
A repeated measures ANOVA was conducted on match trials with APOE ε4 presence (ε4−, ε4+) and age group (Middle-aged, Older) as between-subjects factors and load (number of target locations to remember) and year (3) as within subjects factors. There were no significant effects involving APOE group. Accuracy increased over time (main effect of Year, F(1.90, 343.04) = 8.47, p<.0001, partial eta squared = .05) and decreased with load (F(1.69, 303.56) = 203.95, p<.0001, partial eta squared = .53). The interaction of Load × Age Group was significant, F() = 3.45, p = .04, partial eta squared = .02). The interaction of Load × Age Group × APOE was marginally significant (p=.08). There were no other interactions.
Non-Match condition analyses
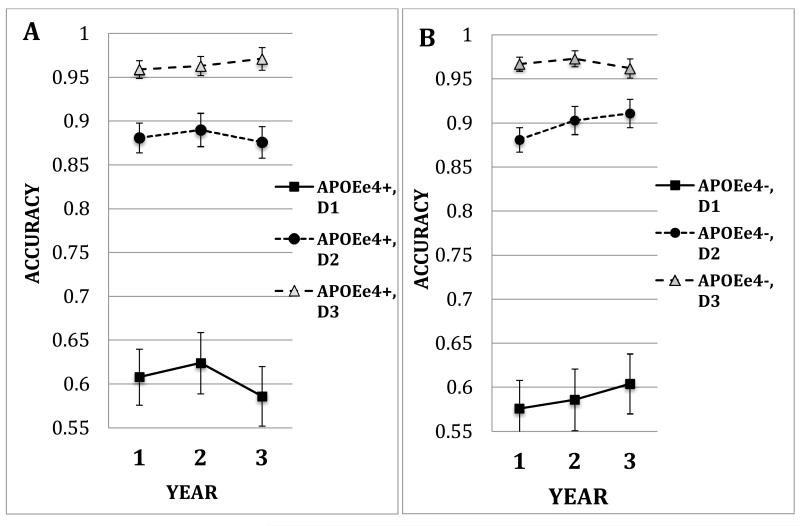
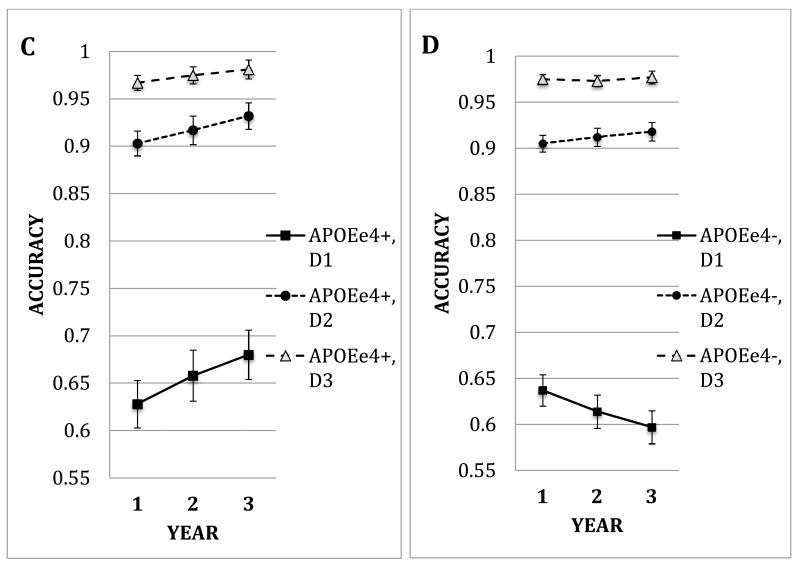
A repeated measures ANOVA was conducted on non-match trials with APOE ε4 presence (ε4−, ε4+) and age group (middle-aged, older) as between-subjects factors and load (number of targets) and target-test distance (2, 4, 8 degrees) as within-subjects factors. The middle-aged group was more accurate overall than the older group (main effect of Age Group (F(1,141) = 4.42, p=.04, partial eta squared = .03). However, the two age groups showed different patterns of change over time as a function of age and APOE genotype (interaction of APOE × Age Group × Year (F(2.00, 281.41) = 3.05, p < .05, partial eta squared = .02). Those effects varied with discrimination difficulty (Figure 2a-d, APOE × Age Group × Year × Distance, F(2.70, 381.06) = 2.91, p = .04, partial eta squared = .02).
Figure 2.
Accuracy under non-match conditions for years 1, 2, 3, plotted as a function of the presence of an APOE-ε4 allele (ε4+, ε4−). Panel A. Older APOE ε4+ Group. Panel B. Older APOE ε4− (non-carrier) Group. Panel C. Middle-aged APOE ε4+ Group. Panel D. Middle-aged APOE ε4− (non-carrier) Group. Error bars are standard errors.
To decompose the 4-way interaction in the non-match analysis, planned follow-up analyses were conducted for each age group separately under the most difficult conditions of each factor.
For the middle-aged groups, analysis of the closest target-test distance (hardest discrimination) with three levels of load, revealed that ε4 carriers increased in accuracy over time while non-carriers decreased in accuracy over years (Figure 2c, d, main effect of Load (F(1.63, 157.96) = 124.85, p < .0001, partial eta squared = .56, interaction of Year × APOE, F(1.96, 157.96) = 5.32, p = .006, partial eta squared = .05). An analysis at the highest level of load, but with three levels of distance, analysis revealed that middle-aged ε4 carriers increased in accuracy over time while non-carriers showed little change. However, that interaction was marginally significantly (Year × APOE, p=.09).
For the old groups, at the closest target-test distance (hardest discrimination) but three levels of load, the analysis revealed no interaction involving APOE genotype, although there was a significant main effect of load (F(1.34, 59.09) = 51.80, p<.0001, partial eta squared = .54). An analysis at the highest level of load, but three levels of distance, analysis revealed the ε4 carriers declined, while the non-carriers increased in accuracy over years (main effect of Distance F(1.59, 76.45) = 365.21, partial eta squared = .88; interaction of Distance × Year × APOE Group (F(2.93, 140.80) = 2.78, p=.045, partial eta squared = .06). Figure 2 a,b shows the greatest decline at the shortest target-test distance.
Discussion
We observed changes over three years in effects of the APOE polymorphism on accuracy on an information processing task of working memory in healthy middle-aged adults. The longitudinal increase in middle-aged APOE-ε4 carriers are consistent with the cognitive phenotype hypothesis, rather than the prodrome hypothesis. The longitudinal decrease in WM and in Wechsler Memory Scale in older APOE-ε4 carriers suggests the presence of an AD prodrome in that group.
Although the APOE genotype has effects on brain structure, it has been harder to show cognitive consequences. Effects of the ε4 allele have been seen in brains of newborns, children, and adolescents, with effects varying with the measurement used and the age of participants. Neonatal ε4 carriers showed greater volume in parietal cortex but lower volume in temporal cortex compared to non-carriers (Knickmeyer et al., 2013), while child ε4 carriers showed thinner left entorhinal cortex compared to non-carriers (Shaw et al., 2008). White matter was slower to myelinate in infant ε4 carriers (Dean et al., 2014). Despite this evidence of effects of the ε4 allele in the brains of young populations, a recent meta-analysis of 20 cross-sectional studies concluded that there are no effects of the ε4 allele on cognitive performance in children or young adults (Ihle et al., 2012).
What about in midlife? There is consensus that the ε4 allele does have cognitive consequences in older adults, (confirmed in a meta-analyses, Wisdom et al., 2011), but our interest was in understanding the development of effects of the APOE-ε4 allele on cognition in midlife before the neuron loss found to occur close in time to AD diagnosis (West, Kawas, Stewart, Rudow, & Troncoso, 2004). Based on evidence that cognitive decline started on average 5-6 years before AD diagnosis at mean age 77.4 in one large study (Wilson et al., 2011), our middle-aged group of mean age 50 is well outside the likely onset of an AD prodrome. That would be the case even if a 10 year prodrome was assumed (Amieva et al., 2008). Only a small subset of the large literature on effects of APOE on cognition has included middle-aged participants. We are aware of 4 longitudinal studies of the effects of APOE on memory in midlife. One study observed a longitudinal decrease in cognition in ε4 carriers including memory in a sample of over 7000 (Blair et al., 2005). Two studies observed no effect of the ε4 allele on longitudinal cognitive change in midlife (Bunce et al., 2012; Caselli et al., 2009), although negative effects were seen in people over age 60 (Caselli et al., 2009). One study observed a longitudinal increase in memory and executive functioning in ε4 carriers in a sample of 375 (Jochemsen et al., 2012), consistent with the present finding on working memory. The only other longitudinal study of APOE with a middle-aged group used MMSE as their sole cognitive measure in midlife (Kozauer, Mielke, Chan, Rebok, & Lyketsos, 2008).
Some light is shed on this issue by findings that ε4 carriers show selectively enhanced functional connectivity between the “default-mode” network and certain cortical regions in young adulthood (Filippini et al, 2009; (Dennis et al., 2010) and in middle-age (Goveas et al., 2013). In the middle-aged, the increased functional connectivity between the default mode network and frontal, parietal, and temporal cortical regions was associated with better episodic memory performance. A potential role for the default mode network in compensation is interesting in light of evidence that functional connectivity within the default mode network decreases with age (Andrews-Hanna et al., 2007). Increased connectivity involving the default-mode network in ε4 carriers suggests that the ε4 allele is associated with increased “self-referential” thoughts associated with that network (reviewed in Buckner et al., 2008). There is increasing evidence of spontaneous interactions during task processing in the form of dynamic up- and down-regulation between the default mode network and the central executive network (Nygard et al., 2012; Sridharan, Levitin, & Menon, 2008). The increased functional connectivity seen in ε4 carriers between the default mode network and prefrontal regions may be due to greater reliance of ε4 carriers on the executive network as compensation.
Given the strength of the evidence that negative effects of the e4 allele are exerted very early in life (Dean et al., 2014) (Knickmeyer et al., 2013; Westlye et al., 2012), the weakness of the evidence for cognitive consequences in young and middle-aged adults (meta-analysis of Ihle et al., 2012), and the strength of the evidence for cognitive consequences late in life (meta-analysis of Wisdom et al., 2011), it is likely that some form of compensation is active in youth that falters in old age. Although the concept of compensation is not usually applied to the young and middle-aged, we can speculate in the present study that the middle-aged ε4 carriers in our sample show greater practice effects from year to year for the same reason – they put forth greater cognitive effort – perhaps reflected in the observed increased functional connectivity between the default mode network and other cortical regions -- to compensate for lifelong effects of the allele on brain function and structure. The ε4-allele carriers may be characterized by less efficient neuronal plasticity mechanisms, yet retain cognitive plasticity mechanisms.
It is harder to explain why middle-aged ε4 non-carriers showed longitudinal decreases in accuracy, although that was seen only under the hardest discrimination condition. The variability was small, making the finding more convincing. When considered together with the performance of the middle-aged ε4 carriers, this finding is consistent with a benefit of the ε4 allele in midlife.
Regarding the older participants, what is the basis for the longitudinal decrease in accuracy observed in APOE-ε4 carriers? The older ε4 carriers showed longitudinally declining accuracy most strongly under the same conditions in which the older non-carriers showed a longitudinal increase – the hardest discrimination condition. That condition had a target-test distance of about 2 degrees, which requires a difficult judgment about whether the test stimulus appeared at the same location where the target had appeared 3 s previously. These two older genotype groups were very similar in age and MMSE score yet the APOE-ε4 non-carriers benefited from repeated administration of the task by increasing accuracy while the APOE-ε4 carriers did not similarly benefit, especially under the more difficult discrimination conditions. That the strongest genotype effect on accuracy occurred under the hardest discrimination condition (shortest target-test distance) is consistent with previous observations showing the strongest effects of other genes on working memory were seen under demanding discrimination conditions (Greenwood, Lin, Sundararajan, Fryxell, & Parasuraman, in press; Greenwood et al., 2009). It should be noted that these longitudinal results are consistent with the findings in our larger cross-sectional sample which showed that older, but not middle-aged, APOE-ε4 carriers had lower accuracy than ε4 non-carriers at the shortest target-test distance.
It was not unexpected that cognitive change occurred as rapidly as within 3 years. The normally aging brain -- even in people at very low risk of developing Alzheimer’s disease -- has been found to shrink significantly even in one year. That shrinkage has been found to be strongest in structures associated with the default-mode network, although shrinkage in hippocampus predicted memory decline (Fjell et al., 2013). APOE genotype appears to modulate this atrophy. A faster rate of shrinkage over 3 years was seen in ε4 homozygotes compared to non-carriers (both groups were mean age 59 (Chen et al., 2007)). Likewise, ε4 carriers of mean age 61 showed greater thinning in the subiculum and entorhinal cortex compared to non-carriers (Donix et al., 2010). Consistent with these findings, in the present study older ε4 carriers failed to show practice effects observed in the older ε4 non-carriers.
The pattern seen in our older group of decreasing accuracy over time may be at least partly attributable to effects of AD pathology, consistent with the concept of a prodrome. The average age of both older groups in the present study (69 years) approaches the average age of AD diagnosis of APOE heterozygotes of 75 years (Corder et al., 1993). Brain amyloid beta plaque deposition – thought by many investigators to be critical in the pathogenesis of AD (reviewed in Hardy, 2006) – increases with APOE-ε4 allele gene dose (Reiman et al., 2009). According to the amyloid cascade hypothesis of AD, amyloid beta deposition is the earliest event in AD pathology, beginning some 20 years before diagnosis. Evidence from amyloid imaging studies shows that ε4 carriers are more likely to show amyloid deposition (e.g. Morris et al., 2010) and to have associated cognitive decline (Kantarci et al., 2012). Because significant amyloid deposition does not appear before age 50 (Kok et al., 2009), the association of the ε4 allele with increased accuracy in our middle-aged group, is unlikely to be attributable to this AD pathology.
Although presymptomatic AD pathology cannot be ruled out, particularly in the older APOE-ε4 carriers, the ε4 allele also exerts effects on the brain that appear to be independent of AD even in old age. Healthy older APOE-ε4 carriers who were found to be free of amyloid-beta plaques by the imaging tracer Pittsburgh Compound B, nevertheless showed altered patterns of functional connectivity involving the default mode network (Sheline et al., 2010). Such evidence indicates that altered connectivity is a manifestation of the APOE phenotype – perhaps due to effects on white matter integrity (Westlye et al., 2012) induced by a dysfunctional distribution of lipid (Poirier, 2005; Rebeck et al., 1998; White et al., 2001), reviewed in (Greenwood & Parasuraman, 2003). Based on this as well as on the evidence that the ε4 allele increases vulnerability to neuronal injury (reviewed in (Mahley & Huang, 2009)), the results we observed in older APOE-ε4 carriers could be a combination of the APOE phenotype and developing AD.
Limitations
It could be argued that annual assessments are subject to random variation and are therefore not reliable. However, all groups showed orderly effects and the middle-aged groups in particular showed low variability. Our longitudinal findings are consistent with other sources of evidence supporting the emergence of a brain and cognitive APOE-ε4 phenotype in midlife or younger. Moreover, our finding was on an information processing task of working memory with effects seen only at higher levels of difficulty. Nevertheless, due to the relatively small sample sizes in the longitudinal analyses, our findings must be considered as exploratory. The meta-analysis of Wisdom et al. (2011) concluded that effect sizes are generally small for effects of the ε4 allele on cognition. That we found stronger effects with our longitudinal comparisons than with our cross-sectional comparisons suggests that the ε4 allele may particularly affect ability to learn from experience and benefit from practice.
Acknowledgments
This work was supported in part by NIH grant AG019653 to R.P., by a grant from the Research Council of Norway (177458/V50) to T.E., and by Virginia Center on Aging Grant Alzheimer’s and Related Diseases Research Award Fund 06-2 to P.M.G.
Contributor Information
P.M. Greenwood, Department of Psychology, George Mason University, Fairfax, VA, USA
T. Espeseth, Department of Psychology, University of Oslo, Oslo, Norway
M.-K. Lin, Department of Psychology, George Mason University, Fairfax, VA, USA
I. Reinvang, Department of Psychology, University of Oslo, Oslo, Norway
R. Parasuraman, Department of Psychology, George Mason University, Fairfax, VA, USA
References
- Alexander DM, Williams LM, Gatt JM, Dobson-Stone C, Kuan SA, Todd EG, Gordon E. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol Psychol. 2007;75(3):229–238. doi: 10.1016/j.biopsycho.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, Dartigues JF. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64(5):492–498. doi: 10.1002/ana.21509. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanidis C, Schmitz G. High-speed apolipoprotein E genotyping and apolipoprotein B3500 mutation detection using real-time fluorescence PCR and melting curves. Clin Chem. 1999;45(7):1094–1097. [PubMed] [Google Scholar]
- Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23(10):1294–1296. doi: 10.1093/bioinformatics/btm108. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Geschwind DH, Tingus K, Huang D, Mendez MF, Mintz J. Apolipoprotein E affects both myelin breakdown and cognition: implications for age-related trajectories of decline into dementia. Biol Psychiatry. 2007;62(12):1380–1387. doi: 10.1016/j.biopsych.2007.03.024. doi: 10.1016/j.biopsych.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E, Atherosclerosis Risk in Communities Study, Investigators APOE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005;64(2):268–276. doi: 10.1212/01.WNL.0000149643.91367.8A. doi: 10.1212/01.WNL.0000149643.91367.8A. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Reiman EM, Alexander GE, Caselli RJ, Gerkin R, Bandy D, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am J Psychiatry. 2007;164(6):916–921. doi: 10.1176/ajp.2007.164.6.916. doi: 10.1176/appi.ajp.164.6.916. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Pericak-Vance MA. Gene dose of Apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dean DC, 3rd, Jerskey BA, Chen K, Protas H, Thiyyagura P, Roontiva A, Reiman EM. Brain Differences in Infants at Differential Genetic Risk for Late-Onset Alzheimer Disease: A Cross-sectional Imaging Study. JAMA Neurol. 2014;71(1):11–22. doi: 10.1001/jamaneurol.2013.4544. doi: 10.1001/jamaneurol.2013.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 2010;6(4):303–311. doi: 10.1016/j.jalz.2009.07.003. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, Bookheimer SY. Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. Neuroimage. 2010;53(1):37–43. doi: 10.1016/j.neuroimage.2010.06.009. doi: 10.1016/j.neuroimage.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeseth T, Christoforou A, Lundervold AJ, Steen VM, Le Hellard S, Reinvang I. Imaging and cognitive genetics: the Norwegian Cognitive NeuroGenetics sample. Twin Res Hum Genet. 2012;15(3):442–452. doi: 10.1017/thg.2012.8. doi: 10.1017/thg.2012.8. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Westlye LT, Walhovd KB, Fjell AM, Endestad T, Rootwelt H, Reinvang I. Apolipoprotein E epsilon4-related thickening of the cerebral cortex modulates selective attention. Neurobiol Aging. 2012;33(2):304–322 e301. doi: 10.1016/j.neurobiolaging.2009.12.027. doi: 10.1016/j.neurobiolaging.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Alzheimer’s Disease Neuroimaging, Initiative Brain changes in older adults at very low risk for Alzheimer’s disease. J Neurosci. 2013;33(19):8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory JD, Manuck SB, Ferrell RE, Ryan CM, Muldoon MF. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. American Journal of Medical Genetics. 2000;96(6):707–711. doi: 10.1002/1096-8628(20001204)96:6<707::aid-ajmg1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”-- a practical method for grading the cognitive state of patients for the clinician. Journal of Current Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goveas JS, Xie C, Chen G, Li W, Ward BD, Franczak MB, Li SJ. Functional network endophenotypes unravel the effects of apolipoprotein E epsilon 4 in middle-aged adults. PLoS One. 2013;8(2):e55902. doi: 10.1371/journal.pone.0055902. doi: 10.1371/journal.pone.0055902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6(3):316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19(2):199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Lin MK, Sundararajan R, Fryxell KJ, Parasuraman R. Healthy aging increases the cognitive effects of two genes that influence extracellular dopamine. Psychol Aging. doi: 10.1037/a0036109. in press. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Normal genetic variation, cognition, and aging. Behavioral and Cognitive Neuroscience Reviews. 2003;2(4):278–306. doi: 10.1177/1534582303260641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sundararajan R, Lin MK, Kumar R, Fryxell KJ, Parasuraman R. Both a Nicotinic Single Nucleotide Polymorphism (SNP) and a Noradrenergic SNP Modulate Working Memory Performance when Attention is Manipulated. J Cogn Neurosci. 2009;21(11):2139–2153. doi: 10.1162/jocn.2008.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the varepsilon 4 allele of the apolipoprotein E gene. Proc Natl Acad Sci U S A. 2000;97(21):11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Bondi MW. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimers Dement. 2008;4(4):251–254. doi: 10.1016/j.jalz.2008.02.006. doi: 10.1016/j.jalz.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Hardy J. Has the amyloid cascade hypothesis for Alzheimer’s disease been proved? Curr Alzheimer Res. 2006;3(1):71–73. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- Ihle A, Bunce D, Kliegel M. APOE epsilon4 and cognitive function in early life: a meta-analysis. Neuropsychology. 2012;26(3):267–277. doi: 10.1037/a0026769. doi: 10.1037/a0026769. [DOI] [PubMed] [Google Scholar]
- Jochemsen HM, Muller M, van der Graaf Y, Geerlings MI. APOE epsilon4 differentially influences change in memory performance depending on age. The SMART-MR study. Neurobiol Aging. 2012;33(4):832 e815–822. doi: 10.1016/j.neurobiolaging.2011.07.016. doi: 10.1016/j.neurobiolaging.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Lowe V, Przybelski SA, Weigand SD, Senjem ML, Ivnik RJ, Jack CR., Jr. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012;78(4):232–240. doi: 10.1212/WNL.0b013e31824365ab. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Wang J, Zhu H, Geng X, Woolson S, Hamer RM, Gilmore JH. Common Variants in Psychiatric Risk Genes Predict Brain Structure at Birth. Cereb Cortex. 2013 doi: 10.1093/cercor/bhs401. doi: 10.1093/cercor/bhs401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65(6):650–657. doi: 10.1002/ana.21696. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- Kozauer NA, Mielke MM, Chan GK, Rebok GW, Lyketsos CG. Apolipoprotein E genotype and lifetime cognitive decline. Int Psychogeriatr. 2008;20(1):109–123. doi: 10.1017/S104161020700587X. doi: 10.1017/S104161020700587X. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Eggermann T, Zerres K, Fink GR. Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE epsilon4 carriers. Neuroscience. 2010;168(2):487–497. doi: 10.1016/j.neuroscience.2010.03.044. doi: 10.1016/j.neuroscience.2010.03.044. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Smith TW, Saunders AM, Hulette C, Pulaski-Salo D, Roses AD. Apolipoprotein E-epsilon 2 and Alzheimer’s disease: genotype influences pathologic phenotype. Neurology. 1997;48(2):515–519. doi: 10.1212/wnl.48.2.515. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Alzheimer disease: multiple causes, multiple effects of apolipoprotein E4, and multiple therapeutic approaches. Ann Neurol. 2009;65(6):623–625. doi: 10.1002/ana.21736. doi: 10.1002/ana.21736. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294(5545):1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276(5318):1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. The heritability of level and rate-of-change in cognitive functioning in Danish twins aged 70 years and older. Exp Aging Res. 2002;28(4):435–451. doi: 10.1080/03610730290080416. [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Henke K. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17(8):1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash S, Greenwood PM, Sunderland T, Parasuraman R, Geda YE, Knopman DS, Smith GE. The influence of apolipoprotein E genotype on visuospatial attention dissipates after age 80. Neuropsychology. 2009;23(1):81–89. doi: 10.1037/a0014014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard M, Eichele T, Loberg EM, Jorgensen HA, Johnsen E, Kroken RA, Hugdahl K. Patients with Schizophrenia Fail to Up-Regulate Task-Positive and Down-Regulate Task-Negative Brain Networks: An fMRI Study Using an ICA Analysis Approach. Front Hum Neurosci. 2012;6:149. doi: 10.3389/fnhum.2012.00149. doi: 10.3389/fnhum.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, Lucas JA. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65(7):963–967. doi: 10.1001/archneur.65.7.963. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oria RB, Patrick PD, Zhang H, Lorntz B, de Castro Costa CM, Brito GA, Guerrant RL. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005;57(2):310–316. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Sunderland T. The apolipoprotein E gene, attention, and brain function. Neuropsychology. 2002;16(2):254–274. doi: 10.1037//0894-4105.16.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31(8-9):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer’s disease. Neurobiol Aging. 2005;26(3):355–361. doi: 10.1016/j.neurobiolaging.2004.09.003. doi: 10.1016/j.neurobiolaging.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Alonzo NC, Berezovska O, Harr SD, Knowles RB, Growdon JH, Mendez AJ. Structure and functions of human cerebrospinal fluid lipoproteins from individuals of different APOE genotypes. Experimental Neurology. 1998;149(1):175–182. doi: 10.1006/exnr.1997.6710. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Caselli RJ. Fibrillar amyloid-{beta} burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinvang I, Espeseth T, Westlye LT. APOE-related biomarker profiles in non-pathological aging and early phases of Alzheimer’s disease. Neurosci Biobehav Rev. 2013;37(8):1322–1335. doi: 10.1016/j.neubiorev.2013.05.006. doi: 10.1016/j.neubiorev.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Evans SL, King SL, Dowell N, Tabet N, Tofts PS. APOE e4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. Neuroimage. 2013;65:364–373. doi: 10.1016/j.neuroimage.2012.10.010. doi: 10.1016/j.neuroimage.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D’Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33(3):969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6(6):494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Mintun MA. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, Petersen RC. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer’s disease but not in healthy control subjects. Neurology. 1998;50(2):355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter B, Xu PT, Gilbert JR, Roses AD, Galasko D, Cole GM. Defective neuronal sprouting by human apolipoprotein E4 is a gain-of-negative function. J Neurosci Res. 2002;68(3):331–336. doi: 10.1002/jnr.10221. [DOI] [PubMed] [Google Scholar]
- Trachtenberg AJ, Filippini N, Cheeseman J, Duff EP, Neville MJ, Ebmeier KP, Mackay CE. The effects of APOE on brain activity do not simply reflect the risk of Alzheimer’s disease. Neurobiol Aging. 2012;33(3):618 e611–618 e613. doi: 10.1016/j.neurobiolaging.2010.11.011. doi: 10.1016/j.neurobiolaging.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Trachtenberg AJ, Filippini N, Ebmeier KP, Smith SM, Karpe F, Mackay CE. The effects of APOE on the functional architecture of the resting brain. Neuroimage. 2012;59(1):565–572. doi: 10.1016/j.neuroimage.2011.07.059. doi: 10.1016/j.neuroimage.2011.07.059. [DOI] [PubMed] [Google Scholar]
- Trachtenberg AJ, Filippini N, Mackay CE. The effects of APOE-epsilon4 on the BOLD response. Neurobiol Aging. 2012;33(2):323–334. doi: 10.1016/j.neurobiolaging.2010.03.009. doi: 10.1016/j.neurobiolaging.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Utermann G, Langenbeck U, Beisiegel U, Weber W. Genetics of the apolipoprotein E system in man. American Journal of Human Genetics. 1980;32(3):339–347. [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of Local Protein Synthesis and Initial Events in Myelination by Action Potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised: Manual. Psychological Corporation; New York: 1981. [Google Scholar]
- West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25(9):1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Reinvang I, Rootwelt H, Espeseth T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79(19):1961–1969. doi: 10.1212/WNL.0b013e3182735c9c. doi: 10.1212/WNL.0b013e3182735c9c. [DOI] [PubMed] [Google Scholar]
- White F, Nicoll JA, Horsburgh K. Alterations in ApoE and ApoJ in relation to degeneration and regeneration in a mouse model of entorhinal cortex lesion. Exp Neurol. 2001;169(2):307–318. doi: 10.1006/exnr.2001.7655. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68(3):351–356. doi: 10.1001/archneurol.2011.31. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging. 2011;, 32(1):63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Yu YW, Lin CH, Chen SP, Hong CJ, Tsai SJ. Intelligence and event-related potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers. Neurosci Lett. 2000;294(3):179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]
- Zivelin Ariella, Rosenberg Nurit, Peretz Hava, Amit Yonit, Kornbrot Nurit, Seligsohn Uri. Improved Method for Genotyping Apolipoprotein E Polymorphisms by a PCR-Based Assay Simultaneously Utilizing Two Distinct Restriction Enzymes. Clinical Chemistry. 1997;43(9):1657–1659. [PubMed] [Google Scholar]