Abstract
Objective
Regression of atherosclerosis is a vital treatment goal of atherosclerotic vascular disease. Inhibitors of the microsomal triglyceride transfer protein (MTP) have been shown to reduce apolipoprotein B (apoB)-containing lipoproteins in animals and humans effectively. Therefore, the major aim of our study is to evaluate the effect of MTP inhibition on atherosclerotic plaque regression.
Methods
LDL-receptor-deficient (LDLr−/−) mice were fed a Western diet for 16 weeks and then harvested for baseline (n=8), switched to chow diet (n=8) or chow diet containing MTP inhibitor (BMS 212122; n=8) for 2 weeks before harvesting.
Results
Treatment with MTP inhibitor led to rapid reduction in plasma lipid levels, which were accompanied by a significant decrease in lipid content and monocyte-derived (CD68+) cells in atherosclerotic plaques compared to baseline and chow diet control groups. MTP inhibitor-treated mice had increased collagen content, a marker associated with increased stability in human plaques. Furthermore, plaques of these mice showed a significant decrease in tissue factor and pro-inflammatory M1 macrophage marker monocyte chemoattractant protein-1 (MCP-I) and an increase in anti-inflammatory M2 macrophage markers arginase-I and mannose receptor 1.
Conclusion
Reversal of hyperlipidemia in atherosclerotic mice by inhibition of MTP leads to rapid and beneficial changes in the composition and inflammatory state of the plaque.
Keywords: Atherosclerosis, Regression, Hyperlipidemia, Microsomal triglyceride transfer protein
Introduction
Lowering of non-HDL-cholesterol (VLDL-C and LDL-C) is the most common approach to decrease coronary artery disease (CAD) risk. For example, various clinical trials have shown that lowering of LDL-C by statins reduces event rates in all age groups [1]. In the ASTEROID trial, aggressive LDL-C lowering with rosuvastatin therapy resulted in regression of coronary atherosclerosis assessed by intravascular ultrasound (IVUS) [2]. Statins increase the clearance of LDL-C. Another approach of lowering LDL-C is to reduce the production of its precursor, VLDL. Microsomal triglyceride transfer protein (MTP) is expressed in the liver, intestine and the heart and is required for the proper assembly of VLDL and chylomicrons [3]. In the Reversa mouse (Ldlr−/−Apob100/100Mttpfl/flMx1Cre+/+), reversal of hyperlipidemia by conditional inactivation of MTP led to regression of atherosclerosis accompanied by favorable changes in the composition and the inflammatory state of the atherosclerotic plaque [4,5]. MTP inhibitors have been shown to reduce apolipoprotein B (apoB)-containing lipoproteins in animals and humans effectively, and are undergoing clinical testing in select populations; but chronic treatment has been associated with hepatic fat accumulation, though this may lessen over time [3,6].
Long term treatment of apoE−/− mice on a high cholesterol diet with MTP inhibitor, Implitapide, resulted in decreased progression of atherosclerosis [7]. In the present study, we used the MTP inhibitor, BMS 212122, on a short-term basis (2 weeks) to test its ability to induce regression of atherosclerosis in LDLr−/− mice. As will be presented, mice treated with MTP inhibitor had a significant decrease in plaque macrophage and lipid content and an improved inflammatory status of the plaque compared to control mice.
Methods
Animals
All procedures were approved by the NYU Institutional Animal Use and Care Committee. LDL−/− mice (B6.129S7-LDLrtm1Her/J; The Jackson Laboratories, Bar Harbor, USA) were weaned at 4 weeks and placed onto a Western diet (Dyets Inc., Bethlehem, USA, Dyet #101977) for 16 weeks. Mice were then harvested for baseline analysis (baseline group), switched to a chow diet (chow group; this group served as a control for the diet change that was also performed in the MTP inhibitor group) or to a chow diet containing MTP inhibitor (BMS 212122; concentration: 25 mg per kg chow diet) (MTPi group) for 14 days before harvesting (n=8 in each group). To achieve a maximal and rapid reduction in plasma lipids the MTP inhibitor treatment was combined with a diet switch to a normal chow diet in the present study, since in a previous study, feeding hyperlipidemic apoE−/− mice with a western-type diet along with MTP inhibitor treatment maintained high plasma levels of cholesterol (~500 mg/dL) and failed to result in regression of atherosclerosis. In addition, we wished to simulate the clinical scenario, in which lipid lowering drugs are considered to be an adjunct to life style changes, a major one being the adoption of a healthier diet. The mean body weight of the mice was 26.8 ± 1.3 g after 16 weeks of Western diet and did not differ significantly between all three groups.
Lipid and Lipoprotein Analysis
Plasma and hepatic total cholesterol, triglyceride and plasma high-density lipoprotein cholesterol (HDL-C) levels were determined by enzymatic assays (Wako Chemicals, Richmond, USA). Hepatic lipids were normalized to the hepatic protein content, which was measured with the DC Protein Assay Kit (Bio-Rad, Hercules, USA) according to the manufacturer’s instructions.
Hepatic lipid content
Hepatic triglyceride and cholesterol content were determined as previously described [8]. Briefly, frozen livers were homogenized, and lipids were extracted. After evaporation of the final chloroform phase, the lipids were dissolved in isopropanol. Triglyceride and cholesterol concentrations were determined as described above.
Liver transaminases
Levels of liver transaminases (AST and ALT) were measured by ANTECH Diagnostics (Farmingdale, New York, USA).
Histology and Morphometrics
At harvesting, aortic roots were removed, perfused with 10% sucrose/saline and embedded in OCT. Serial sections were cut and stained for CD68 (AbD Serotec, Oxford, UK) as previously described [9]. Additional sections were stained with Oil Red O (Sigma), and for monocyte chemoattractant protein-1 (MCP-I antibody; BioLegend, San Diego, USA), arginase-I (arginase-I antibody; Santa Cruz Biotechnology, Santa Cruz, USA) mannose receptor 1 (CD206 antibody; AbD Serotec, Oxford, UK) and tissue factor. The tissue factor antibody (TF632) was generously provided by Dr. Mark B. Taubman (University of Rochester, USA) [10]. For arginase-I and MCP-I stainings, immunofluorescence microscopy was used. Collagen content of lesions was assessed with Sirius Red–stained sections under polarizing light [4]. Negative controls were performed for all stainings. Intimal lesions and stained areas were quantified by computer-aided morphometric analysis of digitized images (ImagePro Plus 3.0 software, Silver Spring, USA).
Grading of steatohepatitis was performed according to Brunt et al. [11] on paraffin embedded liver sections after hematoxylin and eosin (HE) staining.
Statistical Analysis
Data are expressed as mean ± SEM. Data were analyzed by one-way ANOVA with post hoc multiple comparison Tukey tests (Prism 5.0, GraphPad Software Inc., San Diego, USA). Additional comparisons between just the chow and MTPi groups were performed by t-test (Supplemental Fig. 1F), which did not change the general conclusions. For comparison of plasma total cholesterol and triglyceride levels between the chow and MTPi groups at each time-point the unpaired t-test was used; p<0.05 was considered significant.
Results
The MTP inhibitor BMS 212122 was well tolerated by the mice; none of them developed signs of a severe steatohepatitis (see Supplemental Fig. 1).
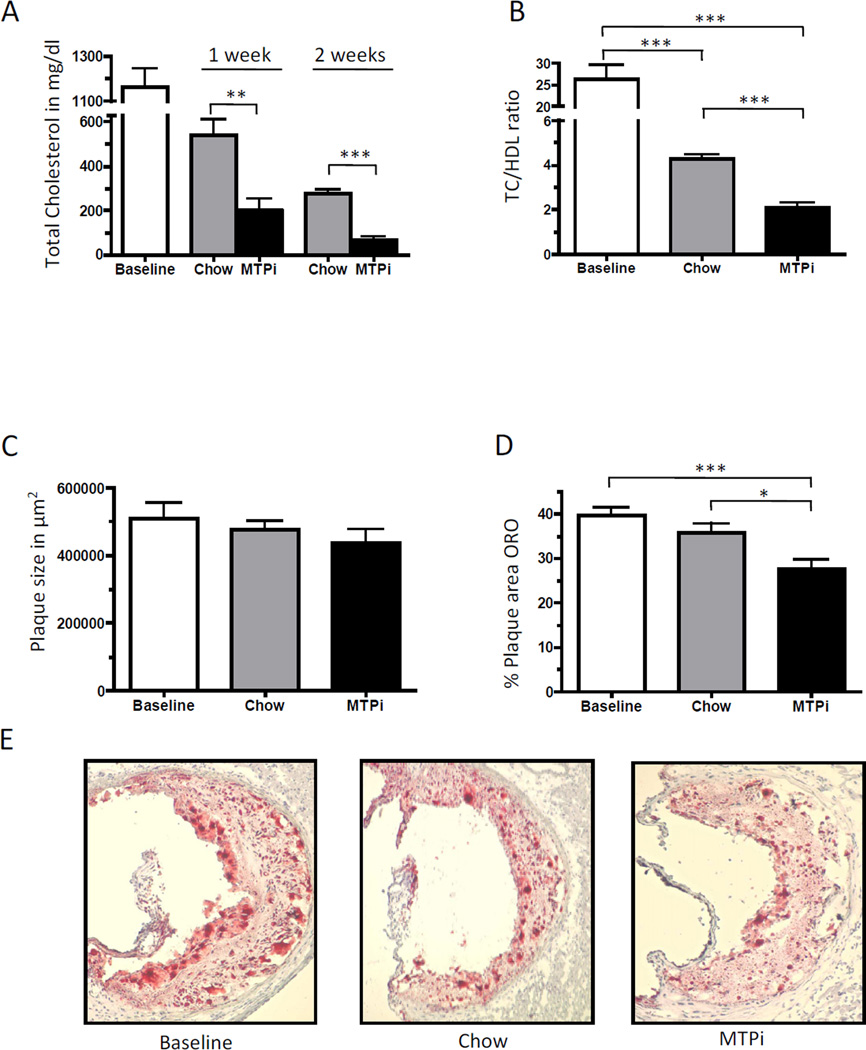
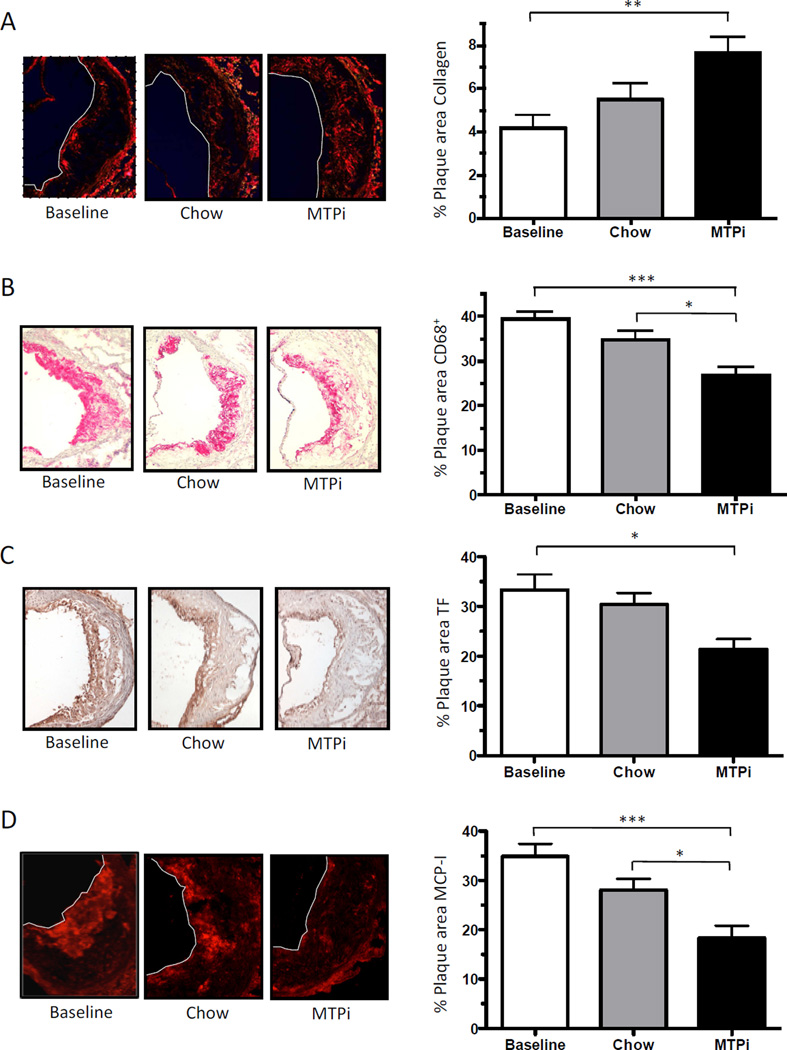
The MTP inhibitor rapidly lowered total cholesterol levels by 83% (201.1 ± 53.8 mg/dl) at 1 week and by 94% (70.0 ± 11.8 mg/dl) at 2 weeks of treatment compared to the baseline group (1160.0 ± 87.0 mg/dl). The chow group, although exhibiting a significant decrease in total cholesterol compared to the baseline group, still had levels >250 mg/dl at 2 weeks, while the MTPi group achieved levels around 70 mg/dl (~ wild-type mouse levels) in the same period (Fig. 1A). TC/HDL ratio decreased in both diet-switched groups compared to baseline (26.3 ± 3.4), while the MTPi group had a ~50% decrease in the TC/HDL ratio compared to the chow group (4.3 ± 0.2 vs. 2.1 ± 0.2, respectively) (Fig. 1B). Plasma triglyceride levels were significantly lower in the MTPi group compared to the baseline and chow group (Supplemental Fig. 1A). There was no significant change in absolute plaque size between all groups (Fig. 1C), but lipid content (i.e., Oil Red O staining) was significantly lower in the MTPi group (27.8 ± 1.9% of plaque area) compared to the chow group (35.9 ± 1.9% of plaque area, P<0.05) and the baseline group (39.5 ± 2.0% of plaque area, p<0.001) (Fig. 1 D–E). The preservation of plaque size in the MTPi group could be explained by an 80 ± 18% increase in plaque collagen content compared to baseline (p<0.01); there was a 30 ± 5% (p>0.05) increase of plaque collagen in the chow diet group (Fig. 2A). The plaque macrophage (CD68+) content did not differ significantly between the baseline and the chow group (39.4 ± 1.5% versus 34.7 ± 2.0% of plaque area; p>0.05). In contrast, MTPi-treated mice showed a statistically significant 32 ± 5% decrease in plaque macrophages compared to the baseline group (p<0.001) and 23 ± 5% decrease compared to the chow group (p<0.05) (Fig. 2B).
Fig. 1.
A) Total plasma cholesterol levels, B) plasma TC/HDL ratios, and C-E) aortic root plaque sizes and Oil Red O staining (ORO) of plaques from LDLr−/− mice before (Baseline) and after switch to either a chow diet (Chow) or a chow diet containing MTP inhibitor (MTPi) for 2 weeks (magnification: 10×). Values are mean ± SEM; * p<0.05, ** p<0.01, *** p<0.001; n=8 in each group.
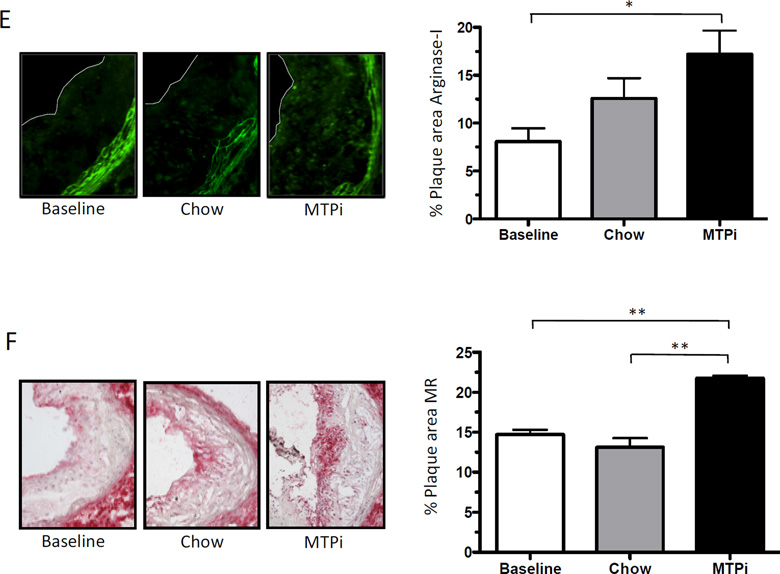
Fig. 2.
Plaques of aortic root sections stained for A) collagen (Sirius Red), B) macrophages (CD68+), C) tissue factor (TF), D) monocyte chemoattractant protein-1 (MCP-I), E) arginase-I and F) mannose receptor 1 (MR) from LDLr−/− mice before (Baseline) and after switch to either a chow diet (Chow) or a chow diet containing MTP inhibitor (MTPi) for 2 weeks (magnification: 10×; arginase-I: 20×). Values are mean ± SEM; * p<0.05, ** p<0.01, *** p<0.001; n=7–8 mice in each group.
In addition to the changes in macrophage content, we were interested in evaluating the inflammatory state of the plaque. We evaluated the expression of tissue factor, which is induced by inflammatory factors [12], in the plaques. Expression of tissue factor was significantly lower in plaques of MTPi-treated mice (21.4 ± 2.1% of plaque area) compared to the baseline group (33.3 ± 3.2% of plaque area; p<0,05), while there was no significant difference between the chow (30.4 ± 2.3% of plaque area) and baseline group (p>0.05) (Fig. 2C).
A simple classification system of tissue macrophages is M1 (pro-inflammatory) and M2 (anti-inflammatory) [13]. We stained for MCP-I (M1 marker), arginase-I (M2 marker) and mannose receptor 1 (M2 marker). We found a significant decrease in MCP-I protein in plaques of the MTPi group (18.4 ± 2.5% of plaque area) compared to the baseline (34.9 ± 2.6% of plaque area; p<0.001) and the chow (28.0 ± 2.3% of plaque area; p<0.05) groups, but the chow data were not statistically different from baseline (p>0.05) (Fig. 2D). Arginase-I was significantly increased in the plaques of the MTPi group (17.2 ± 2.5% of plaque area) compared to the baseline group (8.1 ± 1.4% of plaque area; p<0.05). As with the MCP-I data, the increase in arginase-I in the chow group (12.6 ± 2.1% of plaque area) did not reach statistical significance (p>0.05) (Fig. 2E). The expression of mannose receptor 1 was significantly higher in the MTPi group (20.4 ± 1.0% of plaque area) compared to the baseline (14.6 ± 0.5% of plaque area; p<0.01) and chow group (13.6 ± 1.7% of plaque area; p<0.01) (Fig. 2F).
Discussion
Inhibition of MTP has previously been shown to effectively lower the level of apoB-containing lipids in the plasma of animals and humans [3]. The MTP inhibitor (BMS 212122) used in the present study was previously evaluated in Golden Syrian hamsters and cynomolgus monkeys, where it led to a dose-dependent reduction of non-HDL-C plasma levels by over 80% although atherosclerosis was not evaluated [14]. To date MTP inhibition has only been shown to decrease progression of atherosclerosis with long-term treatment in apoE−/− mice [7].
In the present study we show for the first time that reversal of hyperlipidemia by treatment with a MTP inhibitor leads to the regression of atherosclerosis as judged by lipid and macrophage contents of the plaques. This was undoubtedly related to the severe reduction in non-HDL-C plasma levels (~38 mg/dl; wild-type mouse level); In contrast, the non-HDL-C levels in the chow-fed group were ~211 mg/dl, explaining the more mild changes we observed in these mice. Two other notable findings of the regression process induced by MTP inhibitor treatment were changes in plaque composition (more collagen, which in human plaques is considered to be stabilizing) and in the inflammatory state, with evidence that the phenotype of the remaining macrophages resembled that of the M2 state. These results are in accordance with our previous studies in which the plasma lipoprotein profile was modified in a similar way in Reversa mice (i.e., non-HDL-C reduction) [4] and in LDLr−/− mice treated with the LDL receptor by adenovirus [15], or in a different way (selective raising of HDL-C or lowering of non-HDL-C and raising of HDL-C) [16], arguing that the M2 state may be a general characteristic of regressing plaques.
The reduction in plaque size did not follow the decreases in lipid and macrophage contents, but there was a significant increase in collagen [4,17]. We hypothesize that the increase of extracellular matrix counterbalances the decrease in plaque lipids and macrophages. This would be consistent with previous studies, including those in Reversa mice, that have shown an increase in plaque collagen after correction of hyperlipidemia [4,15]. The net content of collagen depends on the balance of its synthesis and degradation. We have previously shown in Reversa mice that after reversal of hyperlipidemia, macrophages become capable of producing collagen, a known property of the M2 phenotypic state [17], thought to be the result of the increase in arginase-I activity, which provides metabolic substrates for collagen synthesis [18]. Similarly, we observed in the present study that in MTP inhibitor treated mice, there was enrichment in plaque macrophage arginase-I. In addition, we have previously reported that the expression of matrix metalloproteinases-2 and -9 mRNA is reduced in laser captured plaque CD68+ cells (macrophages) of Reversa mice after reversal of hyperlipidemia, indicating a decreased degradation of collagen [4,17]. Thus, the increase in collagen content we observed may represent either increased synthesis, decreased degradation, or a combination of both after plasma lipid levels were lowered by inhibiting MTP. It is unlikely to reflect the direct effects of inhibiting MTP in the cells in the plaque, given that we have previously shown that the MTP expressing status of plaque cells did not affect the regression process after lipid lowering [4].
Lowering of non-HDL-C plays a central role in the prevention of cardiovascular disease. Several studies using intravascular ultrasound have demonstrated a strong linear relationship between the degree of LDL-C lowering and the change in atheroma volume [19]. Therefore, the current consensus is that aggressive LDL-C lowering will promote a limited amount of clinical regression if the decrease is chronically maintained below 60 mg/dl [2]. This rationale is supported by our results that demonstrated that an aggressive non-HDL-C lowering is followed by tremendously beneficial changes in the plaque composition. What is particularly attractive about the model we have presented is the ease of simulating aggressive statin trials in man - except for starting with a readily available mouse model: the LDLr−/− mouse - no other genetic or technically challenging manipulations are needed to achieve significant quantitative and qualitative changes in plaques over a relatively short time.
Supplementary Material
Supplemental Fig. 1: A) Plasma triglyceride levels, B) grading of steatohepatitis according to Brunt et al. [11], C) liver transaminases (AST and ALT), D) intrahepatic triglycerides (TG) and E) intrahepatic total cholesterol (TC) of LDLr−/− mice before (Baseline) and after switch to either a chow diet (Chow) or a chow diet containing MTP inhibitor (MTPi) for 2 weeks. Values are mean ± SEM; * p<0.05, ** p<0.01, *** p<0.001; n=8 mice in each group (if not otherwise indicated). F) Direct comparison between the chow and MTPi groups by t-test.
Acknowledgments
We thank Dr. David Gordon from Bristol-Myers Squibb Co (Pennington, NJ, USA) for providing the MTP inhibitor (BMS 212122). We are grateful for the excellent technical assistance of Ms. Wing Ki Chung, Ms. Camille McNally, Ms. Yaritzy Astudillo, Dr. Anna Rokhlina, and Ms. Christine Miller.
Funding Source
Dr. med. Bernd Hewing was supported by a fellowship from the German Research Foundation (DFG: HE 6092/1-1). Additional support was from NIH grant HL084312.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel iii guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 2.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The asteroid trial. Jama. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 3.Brautbar A, Ballantyne CM. Pharmacological strategies for lowering ldl cholesterol: Statins and beyond. Nat Rev Cardiol. 2011;8:253–265. doi: 10.1038/nrcardio.2011.2. [DOI] [PubMed] [Google Scholar]
- 4.Feig JE, Parathath S, Rong JX, et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieu HD, Withycombe SK, Walker Q, et al. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 2003;107:1315–1321. doi: 10.1161/01.cir.0000054781.50889.0c. [DOI] [PubMed] [Google Scholar]
- 6.Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 7.Ueshima K, Akihisa-Umeno H, Nagayoshi A, et al. Implitapide, a microsomal triglyceride transfer protein inhibitor, reduces progression of atherosclerosis in apolipoprotein e knockout mice fed a western-type diet: Involvement of the inhibition of postprandial triglyceride elevation. Biol Pharm Bull. 2005;28:247–252. doi: 10.1248/bpb.28.247. [DOI] [PubMed] [Google Scholar]
- 8.Frick F, Bohlooly YM, Linden D, et al. Long-term growth hormone excess induces marked alterations in lipoprotein metabolism in mice. American journal of physiology Endocrinology and metabolism. 2001;281:E1230–E1239. doi: 10.1152/ajpendo.2001.281.6.E1230. [DOI] [PubMed] [Google Scholar]
- 9.Trogan E, Feig JE, Dogan S, et al. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Miller C, Swarthout RF, et al. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–713. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. The American journal of gastroenterology. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 12.Packard RR, Libby P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clinical chemistry. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 13.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 14.Robl JA, Sulsky R, Sun CQ, et al. A novel series of highly potent benzimidazole-based microsomal triglyceride transfer protein inhibitors. J Med Chem. 2001;44:851–856. doi: 10.1021/jm000494a. [DOI] [PubMed] [Google Scholar]
- 15.Van Craeyveld E, Gordts SC, Nefyodova E, et al. Regression and stabilization of advanced murine atherosclerotic lesions: A comparison of ldl lowering and hdl raising gene transfer strategies. J Mol Med. 2011;89:555–567. doi: 10.1007/s00109-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feig JE, Rong JX, Shamir R, et al. Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocytederived cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parathath S, Grauer L, Huang LS, et al. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durante W, Johnson FK, Johnson RA. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clinical and experimental pharmacology & physiology. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. Jama. 2007;297:499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1: A) Plasma triglyceride levels, B) grading of steatohepatitis according to Brunt et al. [11], C) liver transaminases (AST and ALT), D) intrahepatic triglycerides (TG) and E) intrahepatic total cholesterol (TC) of LDLr−/− mice before (Baseline) and after switch to either a chow diet (Chow) or a chow diet containing MTP inhibitor (MTPi) for 2 weeks. Values are mean ± SEM; * p<0.05, ** p<0.01, *** p<0.001; n=8 mice in each group (if not otherwise indicated). F) Direct comparison between the chow and MTPi groups by t-test.