Abstract
Purpose
The aim of this survey is to critically review the available information on one-electron oxidation reactions of nucleobases in cellular DNA with emphasis on damage induced through the transient generation of purine and pyrimidine radical cations. Since the indirect effect of ionizing radiation mediated by hydroxyl radical is predominant in cells, efforts have been made to selectively ionize bases using suitable one-electron oxidants that consist among others of high intensity UVC laser pulses. Thus, the main oxidation product in cellular DNA was found to be 8-oxo-7,8-dihydroguanine as a result of direct bi-photonic ionization of guanine bases and indirect formation of guanine radical cations through hole transfer reactions from other base radical cations. The formation of 8-oxo-7,8-dihydroguanine and other purine and pyrimidine degradation products was rationalized in terms of the initial generation of related radical cations followed by either hydration or deprotonation reactions in agreement with mechanistic pathways inferred from detailed mechanistic studies. The guanine radical cation has been shown to be implicated in three other nucleophilic additions that give rise to DNA-protein and DNA-DNA cross-links in model systems. Evidence was recently provided for the occurrence of these three reactions in cellular DNA.
Conclusion
There is growing evidence that one-electron oxidation reactions of nucleobases whose mechanisms have been characterized in model studies involving aqueous solutions take place in a similar way in cells. It may also be pointed out that the above cross-linked lesions are only produced from the guanine radical cation and may be considered as diagnostic products of the direct effect of ionizing radiation.
Keywords: Ionizing radiation, oxidatively generated DNA damage, one-electron oxidation, intra and interstrand DNA cross-links, DNA-protein cross-links
Introduction
Major progress has been accomplished in the last two decades on the delineation of the molecular effects of ionizing radiation on cellular DNA in terms of damage induction to the nucleobases and the 2-deoxyribose moiety (von Sonntag 1987a, 2006, Dedon 2008, O’Neill and Wardman 2009, Cadet et al. 2010, Cadet and Wagner 2013, Georgakilas et al. 2013). Evidence is now accumulating that the indirect effect of ionizing radiation, predominantly mediated by the highly reactive hydroxyl radical (•OH), gives rise to several modified purine and pyrimidine bases including 5,6-dihydroxy-5,6-dihydrothymine (TGly), 5-hydroxymethyluracil (5-hmU), 5-formyluracil (5-foU), 8-oxo-7,8-ihydroguanine (8-oxoG), 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG), 8-oxo-7,8-dihydroadenine (8-oxoA) and 4,6-diamino-5-formamidopyrimidine (FapyA) (Pouget et al. 2002, Douki et al. 2006, Cadet et al. 2008). As an important achievement, it was found that the yield of these modifications, which are likely to be composed of clustered lesions or tandem base damage in most cases, varied between 1 and 80 lesions per 109 bases and per Gy on the basis of accurate HPLC-MS/MS measurements. Although these values are 2 to 3 orders of magnitude lower than those generally accepted until the end of the 1990s (Mori and Dizdaroglu 1994, Olinski et al. 1996), they fit better with predictions concerning the levels of base damage (Goodhead 1994). Another important message was that aqueous solution systems used for mechanistic studies are able to reproduce the cellular conditions in terms of reactivity and chemical reactions (Cadet et al. 2009, 2010). This is not the case when DNA and its components are exposed to ionizing radiation either as partially hydrated solids or in frozen aqueous solutions. In addition to the lack of any significant contribution of •OH-mediated reactions, the degradation pathways led to the formation of fragmented, dimeric and/or reduced lesions that have not been detected in cellular DNA. This may be explained at least partly in these systems by the lack of significant reaction of oxygen with radiation induced radicals (Shaw and Cadet 1990, Swarts et al. 1996, Gromova et al. 1998, Milligan et al. 2000, Swarts et al. 2007, Peoples et al. 2012). This contrasts with the efficient O2 addition reactions with transiently generated base and 2-deoxyribose radicals in aqueous solutions (von Sonntag 2006, Dedon 2008, Cadet et al. 2010) that are likely to participate in the enhancement of the biological effectiveness of low LET ionizing radiations (Hill et al. 2002, O’Neill and Wardman 2009). Therefore, several one-electron oxidants that are described in the following section have been considered to partly mimic the direct effect of ionizing radiation in model mechanistic studies involving aqueous solutions. In this respect, the contribution of Clemens von Sonntag to the delineation of one- is of electron oxidation reactions of pyrimidine bases using peroxodisulfate as the source of SO4•− major important importance (von Sonntag 1987b, 1991, Schuchmann et al. 1987, Rashid et al. 1991, Aravindakumar et al. 2003).
This article is aimed at critically reviewing available data on one-electron oxidation reactions of nucleobases in model compounds and cellular DNA both in terms of mechanisms and final decomposition products. This includes several classes of oxidatively-generated DNA damage such as single oxidized bases, tandem lesions and more complex modifications as the result of one initial radical hit. Emphasis is placed on recent developments concerning the formation of DNA protein cross-links, intra and interstrand DNA cross-links as a result of the initial generation of the guanine radical cation followed by nucleophilic addition reactions.
Mechanisms of single base lesion formation upon one-electron oxidation
The most easily oxidizable natural DNA base is guanine. The one-electron oxidation characteristics of the base moiety of 2′-deoxyribonucleosides can be understood in terms of their standard reduction potentials E∘(dN•+/dN). In aqueous solutions, the standard reduction potentials estimated from the values measured in aprotic solvents (acetonitrile, dimethylformamide), after corrections for solvation effects, increase in the following order: 1.47 V (2′-deoxyguanosine) <1.94 (2′-deoxyadenosine) <2.09 (thymidine) ~ 2.12 V (2′-deoxycytidine) vs. NHE (Seidel et al. 1996). These values are reasonably well correlated with the E∘ (dN•+/dN) values of 1.58 V for 2′-deoxyguanosine (dGuo) and 2.03 V for 2′-deoxyadenosine (dAdo) vs. NHE calculated from equilibria with reference radicals of known potentials as determined by pulse radiolysis methods in aqueous solutions (Steenken and Jovanovic 1997). In agreement with these thermodynamic characteristics, the radical cations of all four free natural nucleosides (dAdo, dGuo, dCyd and dThd) can be generated by one-electron oxidation with sulfate radical anions, SO4• − (Steenken 1989, von Sonntag 1991). The latter radicals are strong one-electron oxidants with the standard reduction potential of E∘ = 2.43 V vs. NHE (Huie et al. 1991), and rapidly oxidize free nucleosides with rate constants close to the diff usion-controlled limit (O’Neill and Davies 1987, Candeias and Steenken 1989, 1993, Deeble et al. 1990, Aravindakumar et al. 2003). The inorganic carbonate and dibromine radical anions, CO3•− and Br2• −, with the reduction potentials E∘ (CO3•−/CO32−) = 1.59 V and E∘ (Br2• − /2Br−) = 1.62 V vs. NHE, are weaker oxidants than SO4• − radicals, and they only oxidize guanine out of all four DNA bases (Candeias and Steenken 1989, Shafirovich et al. 2001). The radical cations of purine bases (A and G) can also be generated by single photon ionization with 193 nm laser light pulses (Candeias and Steenken 1992) and two-photon ionization with 248 nm (Candeias and Steenken 1992) and 266 nm (Angelov et al. 1997, Spassky and Angelov 1997, Douki et al. 2001) laser excitation. The spectroscopic absorption characteristics of different nucleobase radical cations generated by reaction with SO4• − radicals in aqueous solutions, as well as their hydration and deprotonation were explored utilizing transient absorption methods in real time (pulse radiolysis and flash photolysis) (Steenken 1989, von Sonntag 1991). Several photosensitizers that predominantly operate through a type I photosensitization mechanism have also been used to generate not only the radical cation of guanine but also that of other bases including adenine, thymine and cytosine. This is the case of riboflavin (Douki and Cadet 1999), benzophenone (Cuquerella et al. 2012) 2-methyl-1,4-naphtoquinone (Decarroz et al. 1986, 1987, Wagner and Cadet 2010) and anthraquinone (Joy et al. 2006). One may also quote three other one-electron agents that have been shown to have biological relevance through oxidation of guanine. Interestingly 6-thioguanine, an immunosuppressor that is able to incorporate DNA has been found to act as an internal DNA oxidant upon UVA excitation (Brem and Karran 2012a,b). The carbonate anion radical (CO3• −) may be generated during inflammation through the decomposition of nitrosoperoxycarbonate, the reaction product of peroxynitrite with bicarbonates (Shafirovich et al. 2001, Medinas et al. 2007). There is also evidence that bromate once metabolized is able to undergo one-electron oxidation of guanine (Kawanishi and Murata 2006).
Guanine
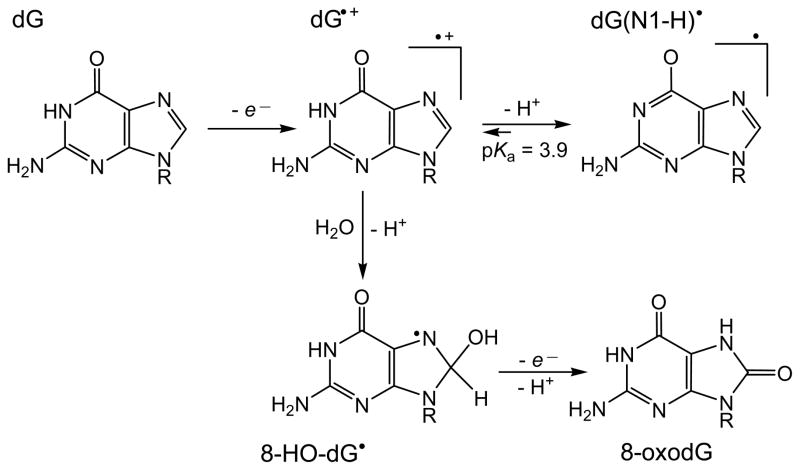
Pulse radiolysis-transient absorption and conductometric detection methods have shown that the guanine radical cation, dGuo•+ produced by one-electron oxidation of 2′-deoxyguanosine (dGuo) with SO4• − radical, is a Brønsted acid with a pKa = 3.9 (Candeias and Steenken 1989) that rapidly deprotonates to form the neutral guanine radical, G(-H)• with a rate constant 1.8×107 s−1 at pH 7.0 (Kobayashi and Tagawa 2003) (Figure 1).
Figure 1.
Guanine radicals produced by one-electron oxidation.
In double-stranded DNA, the deprotonation rates are small (≥ 3×106 s−1) and depend on base sequence context; the transformation of G•+ to G(-H)• is complete within the sub-millisecond time window (Kobayashi and Tagawa 2003, Kobayashi et al. 2008). Electron spin resonance studies in aqueous solutions (Hildenbrand 1990, Bachler and Hildenbrand 1992) and in glass media at low temperatures (Adhikary et al. 2006, 2009, 2010) have shown that the unpaired electron in the G•+ radical cation is mostly localized on the N2, N3, and C8-H but not on N1; however, the deprotonation of the G•+ occurs via dissociation of the H-N1 proton to form the G(N1-H)• radical (Figure 1) in which N1 also shows no evidence of hyperfine coupling in the ESR spectra.
The G•+ radical is a strong electrophile that directly reacts with water to form the 8-hydroxy-7,8-dihydroguanyl radical (8-HO-G•). The latter species is a weakly reducing radical that can undergo oxidation in the presence of oxygen, or other weak oxidants, to yield 8-oxoG or competitive one-electron reduction to give FapyG (Figure 1) (Candeias and Steenken 2000, Cadet et al. 2008). The nucleophilic addition of a water molecule to this radical has been confirmed by studies of guanine oxidation reactions in H218O with the incorporation of 18O in the 8-oxoG product (Kasai et al. 1992). Laser flash photolysis experiments have shown that G•+ radicals produced by one-electron oxidation of 5′-d(TCGCT) sequence with SO4• − radicals in acid solutions (pH 2.5) decay within ~ 3 ms, thus giving an upper limit for the rate constant of hydration of ≤ 330 s−1 (Rokhlenko et al. 2012). In neutral solutions, the deprotonation of G•+ radicals dominates and 8-oxoG is not efficiently formed under these conditions either in the free nucleoside form or in single-stranded DNA (Kasai et al. 1992, Rokhlenko et al. 2012). In double-stranded DNA, guanine radicals retain some cationic character because G-C Watson-Crick base-pairing diminishes the rate of release of the proton from G(-H)• … H+C base pairs (Steenken 1992, 1997, Reynisson and Steenken 2002a, 2002b); thus, 8-oxoG lesions can be formed by the hydration pathway (Kasai et al. 1992). However, this mechanism is not completely understood and requires further investigation.
Adenine
One-electron abstraction from 2′-deoxyadenosine, dAdo, by the SO4• − radical or photoionization with 193 nm laser light generates adenine radical cations, dAdo•+ (O’Neill and Davies 1987, Candeias and Steenken 1992). The dAdo•+ radical is a strong Brønsted acid with pKa ≤ 1 that rapidly deprotonates via proton release from exocyclic amino group to form the neutral dA(N6-H)• radical (Steenken 1989). Hydration is likely to generate 8-hydroxy-7,8-dihydroadenyl radical (8-HO-A•) that upon one-electron oxidation leads to 8-oxoA; in turn, one-electron reduction of this radical initiates fast opening of the imidazole ring at the C8-N9 bond followed by formation of FapyA (Figure 2) (Steenken 1989).
Figure 2.
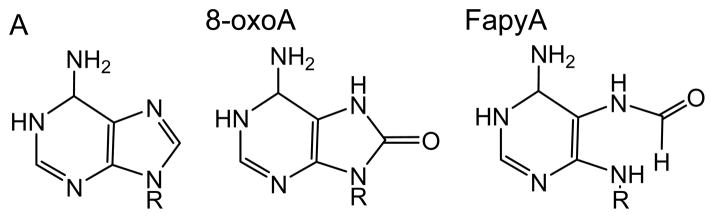
End-products of one-electron oxidation of adenine.
Near-UV photolysis of modified dAdo containing a phenylhydrazone moiety at N6 induces homolytic cleavage of the hydrazine to produce dAdo N6-aminyl radicals and benzylidene iminyl radicals (Kuttappan-Nair et al. 2010). The resulting dAdo N6-aminyl radicals were shown to undergo H-abstraction to give dAdo, deamination to give 2′-deoxyinosine, and addition to dAdo moiety to give dimeric products (Kuttappan-Nair et al. 2010).
Thymine
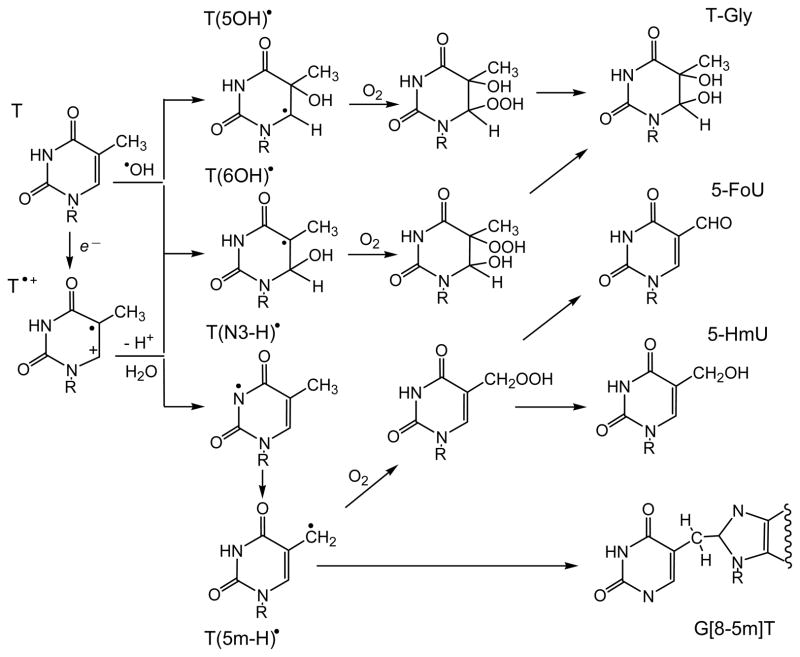
One-electron oxidation of thymidine (Thd) by SO4• − radical generates the thymine radical cation, T•+, which is a typical Brønsted acid with pKa = 3.6 that rapidly deprotonates via proton release from N3 to form the neutral T(N3-H)• radical as shown in Figure 3 (Deeble et al. 1990).
Figure 3.
Free radical reactive intermediates and end-products generated by the one-electron oxidation of thymine.
Deprotonation of the 5-methyl group in T•+ is irreversible and leads to the 5-(uracilyl)methyl radical, T(5m-H)•, which is also formed via H-atom abstraction from the 5-methyl group (von Sonntag and Schuchmann 1986, Deeble et al. 1990). Nucleophilic water addition to T•+, generates mostly the oxidizing 6-hydroxy-5,6-dihydrothymin-5-yl radical, T(6OH)•, whereas the reducing 5-hydroxy-5,6-dihydrothymin-6-yl radical, T(5OH)• forms with a lower yield (Hildenbrandt et al. 1989, Deeble et al. 1990). The T(6OH)• and T(5OH)• radicals are also formed via diffusion-controlled addition of •OH radicals to the C5-C6 double bond of thymine (von Sonntag and Schuchmann 1986, von Sonntag 1987a, Hildenbrandt et al. 1989). Recently, the interconversion of T(6OH)• and T(5OH)• radicals were reported by ESR spectroscopy in basic glassy media at low temperatures (Adhikary et al. 2013).
The addition of oxygen to C-centered T(6OH)• and T(5OH)• radicals generates hydroperoxyl radicals, which further transform, in the case of thymidine (dThd), into eight cis and trans diastereomeric hydroperoxides: 6-hydroxy-5-hydroperoxy-5,6-dihydrothymidine and 5-hydroxy-6-hydroperoxy-5,6-dihydrothymidine (Figure 3) (Wagner et al. 1994). Two-electron reduction of these hydroperoxides leads to the cis and trans diastereomers of 5,6-dihydroxy-5,6-dihydrothymidine (ThdGly).
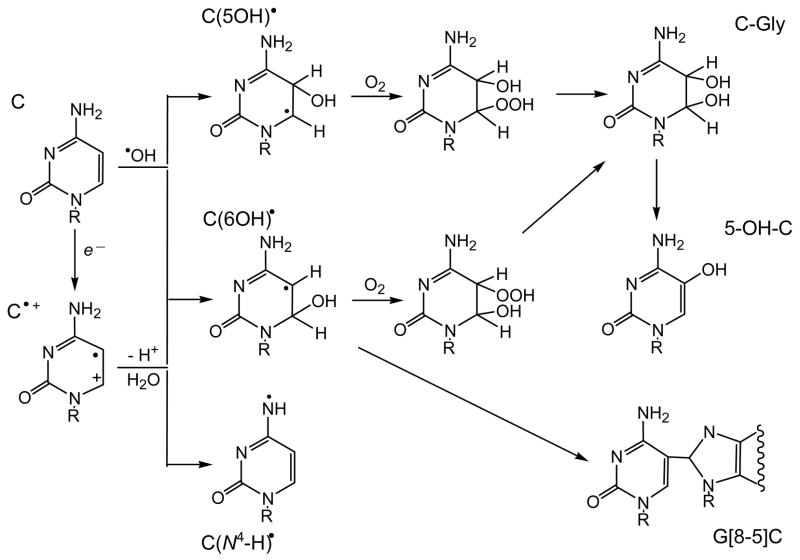
In the presence of oxygen, the 5-(uracilyl)methyl radical forms the corresponding peroxyl radical. The hydroperoxide derived from this radical via H-atom abstraction (or reduction/protonation) decomposes by reduction and dehydration to 5-(hydroxymethyl)-2′-deoxyuridine (5-hmdUrd) and 5-formyl-2′-deoxyuridine (5-fodUrd) as end-products (Wagner et al. 1994). Cytosine and 5-methylcytosine 2′-Deoxycytidine (dCyd) is the most resistant DNA nucleoside to one-electron oxidation that reacts rapidly only with very strong oxidants such as the SO4• − and •OH radicals (von Sonntag and Schuchmann 1986, O’Neill and Davies 1987, von Sonntag 1987a, b, 1991, Hildenbrandt et al. 1989). The radiolytically generated SO4• − radicals produce a mixture of radical intermediates detected by ESR and pulse radiolysis (Hildenbrandt et al. 1989, Catteral et al. 1992, Aravindakumar et al. 2003). The major intermediate, which shows an absorption band at 385 nm by transient absorption spectroscopy does not react with O2, has been identified as the N -centered radical dC(N4 -H)• derived by deprotonation from the exocyclic amino group of cytosine radical cations, C•+ (Decarroz et al. 1987, Aravindakumar et al. 2003) (Figure 4).
Figure 4.
Free radical reactive intermediates and end-products generated by the one-electron oxidation of 2 2-deoxycytidine.
Minor quantities of other intermediates characterized by their strong absorption bands at 340 and 430 nm and their rapid reaction with oxygen, were also detected; these intermediates have been identified as a mixture of 6-hydroxy-5,6-dihydrocytosin-5-yl, C(6OH)• and 5-hydroxy-5,6-dihydrocytosin-6-yl, C(5OH)• radicals derived from the nucleophilic addition of water to C•+ radicals. These radicals are also formed via diffusion-controlled addition of •OH to the C5-C6 double bond of 2′-deoxycytidine Aravindakumar et al. 2003).
Addition of oxygen to the C-centered C(6OH)•, and C(5OH)• radicals generates 5(6)-hydroxy-6(5)hydroperoxyl radicals, which further transform to 6-hydroxy-5-hydroperoxy-5,6-dihydrocytosine and 5-hydroxy-6-hydroperoxy-5,6-dihydrocytosine hydroperoxides (Figure 4). In contrast to the hydroperoxides of thymine, the hydroperoxides of cytosine rapidly decompose to labile products, as the nucleoside or the base in DNA, which include cytosine glycol (C-Gly), 5-hydroxycytosine (5-OHC) and 5-hydroxyuracil (5-OHU) (Tremblay et al. 1999, Wagner et al. 1999, Tremblay and Wagner 2008).
One-electron oxidation base lesions in cellular DNA
Two-quantum photo-ionization of pyrimidine and purine nucleobases has been shown to be an efficient way to generate the corresponding radical cations as free nucleosides and within isolated DNA upon exposure in aqueous solutions to high intensity 266 nm nanosecond laser pulses (Angelov et al. 1997, Douki et al. 2001, 2004). This approach has been successfully used to induce the formation of base radical cations in the DNA of human THP1 monocytes (Douki et al. 2006). Eight oxidized 2′-deoxyribonucleosides including 8-oxodGuo, the four cis and trans diastereomers of ThdGly, 5-hmdUrd and 5-fodUrd were characterized by HPLC-ESI-MS/MS measurement after quantitative enzymatic digestion of extracted DNA (Frelon et al. 2000). The formation of oxidation products was rationalized in terms of the well-established chemical conversion pathways of the guanine and thymine radical cations in model studies. Hydration of the guanine radical cation followed by one electron oxidation of the transiently generated 8-hydroxyl-7,8-dihydroguanyl radical gives rise to 8-oxodGuo. The formation of ThdGly and two methyl oxidation products (5-hmdUrd, 5-foUrd) is explained by initial hydration and deprotonation of thymine radical cations respectively. The accurate quantification of oxidized nucleosides clearly shows a large predominance of 8-oxodGuo over pyrimidine degradation products. This may be accounted for by efficient hole migration of initially produced radical cations of purine and pyrimidine bases with a preferential redistribution of final damage on guanine bases, which thermodynamically are the most likely sinks of positive charge migration, as previously observed in isolated double-stranded oligonucleotides. Similar results were recently reported upon exposure of HeLa cells to high intensity (120 J) nanosecond 266 nm laser pulse excitation (Madugundu et al. 2013). In addition, 5-hydroxy-2′-deoxycytidine (5-OHdCyd) was shown to be formed with a similar yield as the main thymidine oxidation products. The mechanism of 5-OHdCyd formation involves hydration of the cytosine radical cation followed by the addition of O2 to transiently generated 6-hydroxy-5,6-dihydrocytos-5-yl radical and the conversion of intermediate peroxyl or hydroperoxides to 5,6-dihydroxy-5,6-dihydro-2′-deoxycytidine. Dehydration of the dCyd glycol finally gives 5-OHdCyd (Wagner and Cadet 2010, Cadet and Wagner 2013). Further support for implication of the one-electron oxidation reaction of nucleobases was provided by an increase in the yields of oxidized 2′-deoxyribonucleosides with the intensity of laser pulses (Madugundu et al. 2013).
Tandem base damage initiated by one-electron oxidation
Two main classes of tandem base lesions whose formation may be initiated by the reaction of pyrimidine radicals with vicinal purine bases have been identified so far.
Implication of carbon-centered pyrimidyl radicals
In deoxygenated solutions, 5-(uracilyl) methyl radicals add to the C8 position of guanine to form a radical adduct that is readily oxidized by traces of oxygen or other weak oxidants to so-called tandem lesion, G[8-5m]T (Box et al. 1996, 2001, Budzinski et al. 1997, Romieu et al. 2000, Bellon et al. 2002, Zhang and Wang 2005). Such tandem lesions have been detected in calf thymus DNA exposed to Fenton reagents (Cu2+/H2O2/ascorbate) or ionizing radiation (Hong et al. 2006).
The formation of G[8-5]C lesions has also been detected in X-irradiated oxygen-free aqueous solutions of short DNA fragments (Box et al. 2001). The mechanism of formation of G[8-5]C tandem lesions likely involves the reaction of •OH radicals with C to give intermediate 6-hydroxy-5,6-dihydrocytosyl radicals that add to the C8 position of guanine. Subsequently, the resulting radical can undergo one-electron oxidation and deprotonation to regenerate guanine on one side of the molecule and dehydration to regenerate cytosine on the other side (Cao and Wang 2007).
In human HeLa-S3 cells exposed to ionizing radiation, the G[8-5m]T lesions have been detected at a level of 0.05 lesions per 109 nucleosides/Gy (Jiang et al. 2007). The G[8-5] C tandem lesion was detected in cultured human HeLa-S3 cells exposed to ionizing radiation in quantities of 0.037 lesions per 109 ucleosides/Gy (Hong et al. 2007a, Wang 2008). The G[8-5m]T lesions are highly mutagenic (Raychaudhury and Basu 2010, 2011, Colis et al. 2008) and they are more resistant to prokaryotic nucleotide excision repair (NER) in comparison with the pyrimidine (6-4) pyrimidone photoproduct (6-4TT) and the C8 guanine adduct of N -acetyl-2-aminofluorene (AAF) (Yang et al. 2005).
Implication of peroxyl pyrimidine radicals
Evidence has been provided from model studies involving short oligonucleotides and isolated DNA that pyrimidine peroxyl radicals formed upon either deprotonation at the methyl group or hydration of the thymine radical cation are able to react with pyrimidine and purine vicinal bases by addition or one electron abstraction (Douki et al. 2002, Joy et al. 2006, Hong et al. 2007b, Bergeron et al. 2010). However none of the tandem lesions thus formed has been detected so far in cellular DNA (Cadet et al. 2012), probably due to the lack of sensitivity of currently available methods.
Cross-linked lesions involving the guanine radical cation
Following an initial observation that the guanine radical cation is highly susceptible to hydration (Kasai et al. 1992) leading to 8-oxo-7,8-dihydroguanine and 2,6-diamino-4-hydroxy-5-formamidopyrimidine (Cadet et al. 2008), three other addition reactions involving thymine, lysine residues of proteins, and opposite cytosine as nucleophiles have been shown to take place in isolated and cellular DNA.
Intrastrand guanine-thymine adducts
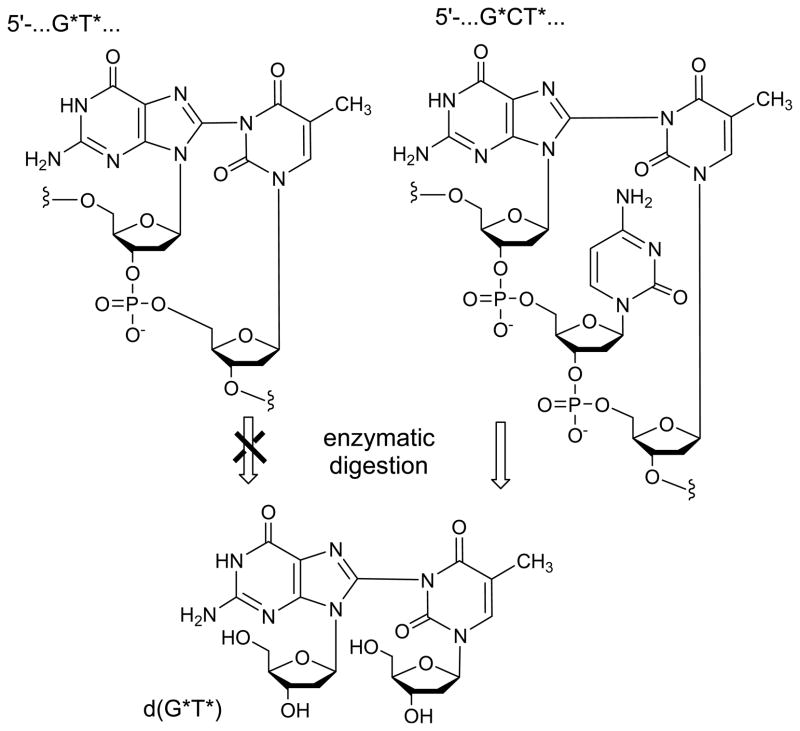
Neutral guanine radicals, G(-H)• do not react with observable rates with weak nucleophiles, such as water molecules (Cadet et al. 2008). However, these radicals retain electrophilic character and can add to thymine bases to form intrastrand G*T* cross-links (Figure 5). The latter modifications were discovered in single-stranded oligonucleotides exposed to one-electron oxidants in air-equilibrated aqueous solutions (pH 7 – 10) (Crean et al. 2008a, 2008b). Of the novel products identified by LC-MS/MS as well as 1D and 2D NMR methods, G and T bases adjacent to one another, or separated by one or two intervening bases (C or A), are linked by a covalent bond between G-C8 and N3-T positions (Figure 5).
Figure 5.
The 5′..G*T* … and 5′…G*CT* … intrastrand cross-links generated by one-electron oxidation of guanine bases.
The efficiency of formation of G* – T* cross-links is base-sequence-dependent such that the greatest efficiency was observed in the case of the 5′-d(GpCpT) sequence (Crean et al. 2008c). In the case of 5′-d(GpT), the efficiency of formation of G* – T* is ~ 3 times lower than that observed for the 5′-d(GpCpT) sequence. When there are two C bridging residues as in 5′-d(GCCT), the yield of cross-linked products was lower than in the case of 5′-d(GpCpT). However, no cross-linked products were detected in the 5′-d(GCCCT) sequence with three bridging C bases. Furthermore, no intrastrand 5′-d(T*pG*) cross-links were detected in the dinucleotide 5′-d(TpG) either, although there was a substantial yield in the case of 5′-d(GpT). This latter result supports the conclusion, further supported by experiments with the sequence isomers 5′-d(GpCpT) and 5′-d(TpCpG), that crosslink formation is more efficient when the T residue is on the 3′-side rather than on the 5′-side of the guanine (Crean et al. 2008c).
Thymine is not the only pyrimidine that can form intrastrand cross-links with guanine radicals derived from one-electron transfer reactions. Similar cross-links can also be generated by the analogous one-electron oxidation of 5′-r(GpCpU) and 5′-d(GpCpU) trinucleotides containing uracil that lack the C5-methyl group of thymine (Crean et al. 2008a). Like thymine, uracil contains an N3-site that is available for cross-link formation with the C8-atom of guanine. Mechanistically, the formation of G[8 – 3]T (or G[8 – 3]U) cross-links requires the loss of two electrons and two protons (Crean et al. 2008a, 2008c). The first step can be initiated by one-electron oxidants that have appropriate reduction potentials for electron abstraction from guanine: CO3• − and SO4• − radicals, and photoexcited riboflavin. The latter is a typical type I photosensitizer that reacts with guanine via an electron transfer mechanism (Ravanat et al. 2003). Insights into the second electron abstraction step were obtained from our observation that the yields of G* – T* (or G* – U*) crosslinked products are negligible in the absence of molecular oxygen (Crean et al. 2008a). We rationalized that the second electron is abstracted by O2, thus forming the superoxide anion O2• −. This second electron can also be removed by other mild oxidants such as 1,4-benzoquinone in deoxygenated aqueous solutions. Benzoquinone has a redox potential E∘ ( BQ/BQ• −) = 0.078 V versus NHE (Wardman 1989), and is a stronger oxidant than O2 with E∘ (O2 / O2• −) = 0.16 V vs. NHE (Stanbury 1989). This mechanism is supported by a strong increase of the yields of the G*T* crosslinked product when the pH is increased from neutral to basic values (pH 10) (Crean et al. 2008b). In strongly basic solutions, T-N3(H) is deprotonated since its pKa = 9.67 (Knobloch et al. 2005), thereby greatly enhancing the nucleophilicity of T-N3. At pH 10, the yield of the G* – T* crosslinked product is ~ 5 times greater than at pH 7.5, which is fully consistent with the nucleophilic addition of T(N3) to the G(C8) radical. Similar enhancements in cross-linked product yields are also observed in the case of 5′-d(GCU) sequences because T-N3(H) and U-N3(H) have the same pKa values (Crean et al. 2008).
Intrastrand G*-T* cross-links can be generated by the chemical mediator of inflammation, nitrosoperoxycarbonate (Yun et al. 2011). The latter, derived from the combination of carbon dioxide and peroxynitrite, rapidly decomposes to reactive species, CO3• − and •NO2 radicals (Pacher et al. 2007) that are known to initiate the selective oxidation and nitration of guanine in DNA (Shafirovich et al. 2009). The reaction of peroxynitrite with native DNA in aqueous solutions (25 mM carbon dioxide/bicarbonate, pH 7.5 – 7.8) generates G*T* cross-links in addition to the well-known nitration/oxidation products of guanine, including 8-nitroguanine (8-nitroG), 5-guanidino-4-nitroimidazole (NIm), 8-oxo-7,8-dihydroguanine (8-oxoG) and spiroiminodihydantoin (Sp) (Yun et al. 2011). The yields of these products, after enzymatic digestion with P1 nuclease and alkaline phosphatase to the nucleotide level, and reversed phase HPLC separation, were compared with those obtained with uniformly, isotopically labeled [15N, 13C]-labeled 2′-deoxyoligoribonucleotides 5′-dGpT and 5′-dGpCpT. The d(G*pT*) and d(G*-T*) crosslinked products derived from di- and tri-oligonucleotides, respectively, were used as standards for identifying the analogous lesions in calf thymus DNA using isotope dilution LC-MS/MS methods in selected reaction-monitoring mode. Thereby, Nim and 8-nitroG were found to be the major products formed ( ~ 0.05% each), followed by 8-oxoG ( ~ 0.02%), and d(G*pT*) and d(G*-T*) enzymatic digestion products ( ~ 0.002% each). It was shown that the formation of d(G*pT*) enzyme digestion product can only arise from intrastrand cross-links, whereas d(G*-T*) can arise from both interstrand and intrastrand cross-linking (Yun et al. 2011).
Recently, we demonstrated that the one-electron oxidation of cellular DNA in cultured human HeLa cells initiated by intense nanosecond 266 nm laser pulse irradiation produces cross-links between guanine and thymine bases (G*-T*), characterized by a covalent bond between C8 guanine (G*) and N3 thymine (T*) atoms (Figure 5) (Madugundu et al. 2013). The DNA lesions were quantified by isotope dilution LC-MS/MS methods in the multiple reaction-monitoring mode using isotopically labeled [15N, 13C]-nucleotides as internal standards. Among several known oxidized pyrimidine bases and 8-oxoG, the G*-T* cross-linked lesions were detected at levels of ~ 0.21 and 1.19 d(G*-T*) lesions per 106 DNA bases at laser intensities of 50 and 280 mJ/cm2 /pulse, respectively (Madugundu et al. 2013). DNA-protein cross-links (DPC) The formation of covalent bonds between DNA and proteins was induced in cells upon exposure to ionizing radiation, in an oxygen independent manner, as the likely result of radical reactions (Barker et al. 2005a, 2005b). Recently, evidence was provided that 6-thioguanine, once metabolized and incorporated into cellular DNA, was able to induce DPC after UVA illumination. This may be rationalized in terms of the initial generation of guanine radical cations since 6-thioguanine has been shown to act as a type I photosensitizer. Further support is gained by previous model studies that have involved several one-electron oxidants (Nguyen et al. 2000, Kurbanyan et al. 2003, Madison et al. 2012) including high intensity UVC laser irradiation (Angelov et al. 2003) as generators of DPC. A detailed mechanistic study describing the covalent attachment of TGT trinucleotide to trilysine peptide (KKK) is now available (Perrier et al. 2006). UVA excited riboflavin-mediated generation of guanine radical cations was followed by an efficient nucleophilic addition of the central lysine amino group of KKK to the C8 position of guanine with subsequent formation of a covalent adduct. This is a relevant model of DNA-protein cross-link that will be further investigated in cellular DNA.
Interstrand DNA cross-links
The formation of interstrand cross-links (ICL) has been observed in 6-thioguanine containing cellular DNA upon UVA irradiation (Brem and Karran 2012a, 2012b). It may also be pointed out that exposure of double-stranded DNA oligodeoxynucleotides to high-intensity UVC nanosecond laser pulses gives rise to ICL involving guanine and likely opposite cytosine (Cadet et al. 2012). It is likely that in both cases nucleophilic addition of the 4-amino group of cytosine to the initially photo-induced guanine radical cation is the key step leading to the generation of ICL (Figure 6).
Figure 6.
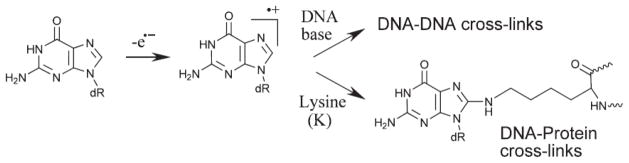
Formation of DNA-protein cross-link and interstrand cross-link involving the guanine radical cation.
Conclusion
The effects of one-electron oxidation of nucleobases in cellular DNA mimic partly the effects of •OH because of similarities in the structure of the final oxidation products. The situation is quite different from a quantitative point of view with the overwhelming formation of 8-oxodGuo under conditions of one-electron oxidation that can be triggered by the direct effect of ionizing radiation and several oxidants including type I photosensitizers, peroxyl pyrimidine radicals and biologically relevant CO3• −. This may be explained by the significant occurrence of hole transfer in cellular DNA that is accompanied by the redistribution of the initially damaged sites along oligonucleotide chains. As a result, the final lesions are preferentially located on guanine bases that exhibit the lowest ionization potential among DNA bases. Increasing evidence is provided for a major role played by addition reactions of various nucleophiles to the guanine radical cation. If the hydration of G•+ gives rise to 8-oxoG and FapyG, lesions that are also produced by •OH, the formation of G*-T* intrastrand cross-links, DNA protein cross-links and interstrand cross-links appear to be specific of one electron oxidants. Further work is however required in order to establish the detailed mechanisms of formation of the latter classes of damage in cellular DNA that involve only one radical hit through ionization of the guanine bases. Information is now available in cellular DNA on final nucleobase degradation products induced by •OH oxidation and ionization reactions. These are expected to be part of radiation-induced oxidatively-generated clustered DNA damage as the result of multiple radical and excitation hits. Identification and measurement of the latter class of DNA lesions which together with DNA double strand breaks constitute the molecular signatures of ionizing radiation (Georgakilas 2011, Steward et al. 2011) remain a highly challenging analytical issue.
Acknowledgments
This work was supported by the National Institute of Environmental Health and Sciences Grant RO1ES011589 (VS), the Natural Science and Engineering Research Council of Canada, and the Canadian Institutes of Health Research of Canada. Components of this work were conducted in the Shared Instrumentation Facility at NYU that was constructed with support from a Research Facilities Improvement Grant (C06 RR-16572) from the National Center for Research Resources, National Institutes of Health. The acquisition of the MALDI-TOF mass spectrometer was supported by the National Science Foundation (CHE-0958457).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adhikary A, Kumar A, Becker D, Sevilla MD. The guanine cation radical: Investigation of deprotonation states by ESR and DFT. J Phys Chem B. 2006;110:24171–24180. doi: 10.1021/jp064361y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikary A, Khandur D, Sevilla MD. Direct observation of the hole protonation state and hole localization site in DNA-oligomers. J Am Chem Soc. 2009;131:8614–8619. doi: 10.1021/ja9014869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikary A, Kumar A, Munafo SA, Khanduri D, Sevilla MD. Prototropic equilibria in DNA containing one-electron oxidized GC: Intra-duplex vs. duplex to solvent deprotonation. Phys Chem Chem Phys. 2010;12:5353–5368. doi: 10.1039/b925496j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikary A, Kumar A, Heizer AN, Palmer BJ, Pottiboyina V, Liang Y, Wnuk SF, Sevilla MD. Hydroxyl ion addition to one-electron oxidized thymine: Unimolecular interconversion of C5 to C6 OH-adducts. J Am Chem Soc. 2013;135:3121–3135. doi: 10.1021/ja310650n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelov D, Charra M, Müller CW, Cadet J, Dimitrov S. Solution study of the NF-kappa B p50-DNA complex by UV laser protein-DNA cross-linking. Photochem Photobiol. 2003;77:592–596. doi: 10.1562/0031-8655(2003)077<0592:ssotnp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Angelov D, Spassky A, Berger M, Cadet J. High-intensity UV laser photolysis of DNA and purine 2′-deoxyribonucleosides: Formation of 8-oxopurine damage and oligonucleotide strand cleavage as revealed by HPLC and gel electrophoresis studies. J Am Chem Soc. 1997;119:11373–11380. [Google Scholar]
- Aravindakumar CT, Schuchmann MN, Rao BSM, von Sonntag J, von Sonntag C. The reactions of cytidine and 2′-deoxycytidine with SO4• − revisited. Pulse radiolysis and product studies. Org Biomolec Chem. 2003;1:401–408. doi: 10.1039/b209626a. [DOI] [PubMed] [Google Scholar]
- Bachler V, Hildenbrand K. EPR-detection of the guanosyl radical cation in aqueous solution. Quantum chemically supported assignment of nitrogen and proton hyperfine couplings. Radiat Phys Chem. 1992;40:59–68. [Google Scholar]
- Barker S, Weinfeld M, Murray D. DNA-protein crosslinks: Their induction, repair and biological consequences. Mutat Res. 2005a;580:111–115. doi: 10.1016/j.mrrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Barker S, Weinfeld M, Zheng J, Li L, Murray D. Identification of mammalian proteins cross-linked to DNA by ionizing radiation. J Biol Chem. 2005b;280:33826–33838. doi: 10.1074/jbc.M502477200. [DOI] [PubMed] [Google Scholar]
- Bellon S, Ravanat J-L, Gasparutto D, Cadet J. Cross-linked thymine-purine base tandem lesions: Synthesis, characterization, and measurement in gamma-irradiated isolated DNA. Chem Res Toxicol. 2002;15:598–606. doi: 10.1021/tx015594d. [DOI] [PubMed] [Google Scholar]
- Bergeron F, Auvré F, Radicella J-P, Ravanat J-L. HO• radicals induce an unexpected high proportion of tandem base lesions refractory to repair by DNA glycosylases. Proc Natl Acad Sci USA. 2010;107:5528–5533. doi: 10.1073/pnas.1000193107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box HC, Budzinski EE, Dawidzik JD, Wallace JC, Evans MS, Gobey JS. Radiation-induced formation of a crosslink between base moieties of deoxyguanosine and thymidine in deoxygenated solutions of d(CpGpTpA) Radiat Res. 1996;145:641–643. [PubMed] [Google Scholar]
- Box HC, Dawidzik JB, Budzinski EE. Free radical-induced double lesions in DNA. Free Radic Biol Med. 2001;31:856–868. doi: 10.1016/s0891-5849(01)00653-0. [DOI] [PubMed] [Google Scholar]
- Brem R, Karran P. Multiple forms of DNA damage caused by UVA photoactivation of 6-thioguanine. Photochem Photobiol. 2012a;88:5–15. doi: 10.1111/j.1751-1097.2011.01043.x. [DOI] [PubMed] [Google Scholar]
- Brem R, Karran P. Oxidation-mediated DNA cross-linking contributes to the toxicity of 6-thioguanine in human cells. Cancer Res. 2012b;72:4787–4795. doi: 10.1158/0008-5472.CAN-12-1278. [DOI] [PubMed] [Google Scholar]
- Budzinski EE, Dawidzik JB, Rajecki MJ, Wallace JC, Schroder EA, Box HC. Isolation and characterization of the products of anoxic irradiation of d(CpGpTpA) Int J Radiat Biol. 1997;71:327–336. doi: 10.1080/095530097144210. [DOI] [PubMed] [Google Scholar]
- Cadet J, Douki T, Ravanat J-L. Oxidatively generated damage to the guanine moiety of DNA: Mechanistic aspects and formation in cells. Acc Chem Res. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]
- Cadet J, Douki T, Gasparutto D, Ravanat J-L, Wagner JR. Chemical reactions of the radical cations of nucleobases in isolated and cellular DNA. Formation of single-base lesions. In: Greenberg MM, editor. Radical and radical ion reactivity in nucleic acid chemistry. New York: Wiley; 2009. pp. 69–97. [Google Scholar]
- Cadet J, Douki T, Ravanat JL. Oxidatively generated base damage to cellular DNA. Free Radic Biol Med. 2010;49:9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Cadet J, Ravanat J-L, TavernaPorro M, Menoni H, Angelov D. Oxidatively generated complex DNA damage: Tandem and clustered lesions. Cancer Lett. 2012;327:5–15. doi: 10.1016/j.canlet.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harbor Perspect Biol. 2013;5:a012559. doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candeias LP, Steenken S. Structure and acid-base properties of one-electron-oxidized deoxyguanosine, guanosine, and 1-methylguanosine. J Am Chem Soc. 1989;111:1094–1099. [Google Scholar]
- Candeias LP, Steenken S. Ionization of purine nucleosides and nucleotides and their components by 193-nm laser photolysis in aqueous solution: Model studies for oxidative damage of DNA. J Am Chem Soc. 1992;114:699–704. [Google Scholar]
- Candeias LP, Steenken S. Electron transfer in di(deoxy)nucleoside phosphates in aqueous solution: Rapid migration of oxidative damage (via adenine) to guanine. J Am Chem Soc. 1993;115:2437–2440. [Google Scholar]
- Candeias LP, Steenken S. Reaction of HO• with guanine derivatives in aqueous solution: Formation of two different redoxactive OH-adduct radicals and their unimolecular transformation reactions. Properties of G(-H)•. Chem Eur J. 2000;6:475–484. doi: 10.1002/(sici)1521-3765(20000204)6:3<475::aid-chem475>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cao H, Wang Y. Quantification of oxidative single-base and intrastrand cross-link lesions in unmethylated and CpG-methylated DNA induced by Fenton-type reagents. Nucleic Acids Res. 2007;35:4833–4844. doi: 10.1093/nar/gkm497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catteral H, Davies MJ, Gilbert BC. An EPR study of the transfer of radical-induced damage from the base to sugar in nucleic acid components: Relevance to the occurrence of strand-breakage. J Chem Soc Perkin Trans. 1992;2:1379–1385. [Google Scholar]
- Colis LC, Raychaudhury P, Basu AK. Mutational specificity of gamma-radiation-induced guanine-thymine and thymine-guanine intrastrand cross-links in mammalian cells and translesion synthesis past the guanine-thymine lesion by human DNA polymerase eta. Biochemistry. 2008;47:8070–8079. doi: 10.1021/bi800529f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean C, Geacintov NE, Shafirovich V. Intrastrand G-U cross-links generated by the oxidation of guanine in 5′-d(GCU) and 5′-r(GCU) Free Radic Biol Med. 2008a;45:1125–1134. doi: 10.1016/j.freeradbiomed.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean C, Lee YA, Yun BH, Geacintov NE, Shafirovich V. Oxidation of guanine by carbonate radicals derived from photolysis of carbonatotetramminecobalt(III) complexes and the pH dependence of intrastrand DNA cross-links mediated by guanine radical reactions. ChemBioChem. 2008b;9:1985–1991. doi: 10.1002/cbic.200800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean C, Uvaydov Y, Geacintov NE, Shafirovich V. Oxidation of single-stranded oligonucleotides by carbonate radical anions: Generating intrastrand cross-links between guanine and thymine bases separated by cytosines. Nucleic Acids Res. 2008c;36:742–755. doi: 10.1093/nar/gkm1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuquerella MC, Lhiaubet-Vallet V, Cadet J, Miranda MA. Benzophenone photosensitized DNA damage. Acc Chem Res. 2012;45:1558–1570. doi: 10.1021/ar300054e. [DOI] [PubMed] [Google Scholar]
- Decarroz C, Wagner JR, van Lier JE, Murali Krishna M, Riesz P, Cadet J. Sensitized photo-oxidation of thymidine by 2-methyl-1,4-naphthoquinone. Characterization of the stable photoproducts. Int J Radiat Biol. 1986;50:491–505. doi: 10.1080/09553008614550901. [DOI] [PubMed] [Google Scholar]
- Decarroz C, Wagner JR, Cadet J. Specific deprotonation reactions of the pyrimidine radical cation resulting from the menadione mediated photosensitization of 2′-deoxycytidine. Free Rad Res Commun. 1987;2:295–301. doi: 10.3109/10715768709065295. [DOI] [PubMed] [Google Scholar]
- Deeble DJ, Schluchmann MN, Steenken S, von Sonntag C. Direct evidence for the formation of thymine radical cations from the reaction of SO4• − with thymine derivatives: A pulse radiolysis study with optical and conductance detection. J Phys Chem. 1990;94:8186–8192. [Google Scholar]
- Dedon PC. The chemical toxicology of 2-deoxyribose oxidation in DNA. Chem Res Toxicol. 2008;21:206–219. doi: 10.1021/tx700283c. [DOI] [PubMed] [Google Scholar]
- Douki T, Cadet J. Modification of DNA bases by photosensitized one-electron oxidation. Int J Radiat Biol. 1999;75:571–581. doi: 10.1080/095530099140212. [DOI] [PubMed] [Google Scholar]
- Douki T, Angelov A, Cadet J. UV laser photolysis of DNA: Effects of duplex stability on charge transfer efficiency. J Am Chem Soc. 2001;123:11330–11336. doi: 10.1021/ja016426a. [DOI] [PubMed] [Google Scholar]
- Douki T, Ravanat J-L, Angelov D, Wagner JR, Cadet J. Effect of duplex stability on charge-transfer efficiency within DNA. Top Curr Chem. 2004;236:1–25. [Google Scholar]
- Douki T, Rivière J, Cadet J. DNA tandem lesions containing 8-oxo-7,8-dihydroguanine and formamido residues arise from intramolecular addition of thymine peroxyl radicals to guanine. Chem Res Toxicol. 2002;15:445–454. doi: 10.1021/tx0155909. [DOI] [PubMed] [Google Scholar]
- Douki T, Ravanat J-L, Pouget JP, Testard I, Cadet J. Minor contribution of direct ionization to DNA base damage induced by heavy ions. Int J Radiat Biol. 2006;82:119–127. doi: 10.1080/09553000600573788. [DOI] [PubMed] [Google Scholar]
- Frelon S, Douki T, Ravanat J-L, Tornabene C, Cadet J. High performance liquid chromatography–mass spectrometry measurement of radiation-induced base damage to isolated and cellular DNA. Chem Res Toxicol. 2000;13:1002–1010. doi: 10.1021/tx000085h. [DOI] [PubMed] [Google Scholar]
- Georgakilas AG. Detection of clustered DNA lesions: Biological and clinical applications. World J Biol Chem. 2011;2:173–176. doi: 10.4331/wjbc.v2.i7.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakilas AG, O‘Neill P, Stewart R. Induction and repair of clustered DNA lesions: What do we know so far? Radiat Res. 2013;180:100–109. doi: 10.1667/RR3041.1. [DOI] [PubMed] [Google Scholar]
- Goodhead DJ. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. Int J Radiat Biol. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- Gromova M, Balanzat E, Gervais B, Nardin R, Cadet J. Direct effect of heavy ions and electrons on thymidine in the solid state. Int J Radiat Biol. 1998;74:81–97. doi: 10.1080/095530098141753. [DOI] [PubMed] [Google Scholar]
- Hildenbrand K. The SO4−• -induced oxidation of 2′-deoxyuridine-5′-phosphate, uridine-5′-phosphate and thymidine-5′-phosphate–an ESR study in aqueous-solution. Z Naturforsch C. 1990;45:47–58. doi: 10.1515/znc-1990-1-210. [DOI] [PubMed] [Google Scholar]
- Hildenbrandt K, Behrens G, Schulte-Frohlinde D, Herak JN. Comparison of the reaction of •OH and of SO4•− tradicals with pyrimidine nucleosides. An electron spin resonance study in aqueous solution. J Chem Soc Perkin Trans. 1989;2:283–289. [Google Scholar]
- Hill MA, Stevens DL, Marsden SJ, Allot R, Turcu IC, Goodhead DT. Is the increased relative biological effectiveness of high LET particles due to spatial or temporal effects? Characterization and OER in V79-4 cells. Phys Med Biol. 2002;47:3543–3555. doi: 10.1088/0031-9155/47/19/308. [DOI] [PubMed] [Google Scholar]
- Hong H, Cao, Wang Y, Wang Y. Identification and quantification of a guanine-thymine intrastrand cross-link lesion induced by Cu(II)/H2O2 /ascorbate. Chem Res Toxicol. 2006;19:614–621. doi: 10.1021/tx060025x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Cao H, Wang Y. Formation and genotoxicity of a guanine-cytosine intrastrand cross-link lesion in vivo. Nucleic Acids Res. 2007a;35:7118–7127. doi: 10.1093/nar/gkm851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong IS, Carter KN, Sato K, Greenberg MM. Characterization and mechanism of formation of tandem lesions in DNA by a nucleobase peroxyl radical. J Am Chem Soc. 2007b;129:4089–4098. doi: 10.1021/ja0692276. [DOI] [PubMed] [Google Scholar]
- Huie RE, Clifton CL, Neta P. Electron transfer reaction rates and equilibria of the carbonate and sulfate radical anions. Radiat Phys Chem. 1991;38:477–481. [Google Scholar]
- Jiang Y, Hong H, Cao H, Wang Y. In vivo formation and in vitro replication of a guanine-thymine intrastrand cross-link lesion. Biochemistry. 2007;46:12757–12763. doi: 10.1021/bi7012195. [DOI] [PubMed] [Google Scholar]
- Joy A, Ghosh AK, Schuster GB. One-electron oxidation of DNA oligomers that lack guanine: Reaction and strand cleavage at remote thymines by long-distance radical cation hopping. J Am Chem Soc. 2006;128:5346–5347. doi: 10.1021/ja058758b. [DOI] [PubMed] [Google Scholar]
- Kasai H, Yamaizumi Z, Berger M, Cadet J. Photosensitized formation of 7,8-dihydro-8-oxo-2 2-deoxyguanosine (8-hydroxy-2 2-deoxyguanosine) in DNA by ribofl avin: A non singlet oxygen mediated reaction. J Am Chem Soc. 1992;114:9692–9694. [Google Scholar]
- Kawanishi S, Murata M. Mechanism of DNA damage induced by bromate differs from general types of oxidative stress. Toxicology. 2006;221:172–178. doi: 10.1016/j.tox.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Knobloch B, Linert W, Sigel H. Metal ion-binding properties of (N3)-deprotonated uridine, thymidine, and related pyrimidine nucleosides in aqueous solution. Proc Natl Acad Sci USA. 2005;102:7459–7464. doi: 10.1073/pnas.0501446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Tagawa S. Direct observation of guanine radical cation deprotonation in duplex DNA using pulse radiolysis. J Am Chem Soc. 2003;125:10213–10218. doi: 10.1021/ja036211w. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yamagami R, Tagawa S. Effect of base sequence and deprotonation of guanine cation radical in DNA. J Phys Chem B. 2008;112:10752–10757. doi: 10.1021/jp804005t. [DOI] [PubMed] [Google Scholar]
- Kurbanyan K, Nguyen KL, To P, Rivas EV, Lueras AMK, Kosinski C, Steryo M, Gonzalez A, Mah DA, Stemp EDA. DNA-protein cross-linking via guanine oxidation. Dependence upon protein and photosensitizer. Biochemistry. 2003;42:10269–10281. doi: 10.1021/bi020713p. [DOI] [PubMed] [Google Scholar]
- Kuttappan-Nair V, Samson-Th ibault F, Wagner JR. Generation of 2′-deoxyadenosine N6-aminyl radicals from the photolysis of phenlhydrazone derivatives. Chem Res Toxicol. 2010;23:48–54. doi: 10.1021/tx900268r. [DOI] [PubMed] [Google Scholar]
- Madison AL, Perez ZA, To P, Maisonet T, Rios EV, Trejo Y, Ochoa-Paniagua C, Reno A, Stemp EDA. Dependence of as studies restriction endonuclease inhibition. Biochemistry. 2012;51:362–369. doi: 10.1021/bi201087q. [DOI] [PubMed] [Google Scholar]
- Madugundu GS, Wagner JR, Cadet J, Kropachev K, Yun BH, Geacintov NE, Shafirovich V. Generation of guanine-thymine cross-links in human cells by one-electron oxidation mechanisms. Chem Res Toxicol. 2013;26:1031–1033. doi: 10.1021/tx400158g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medinas DB, Cerchiaro G, Trindade DF, Augusto O. The carbonate radical and related oxidants derived from bicarbonate buff er. IUBMB Life. 2007;59:255–262. doi: 10.1080/15216540701230511. [DOI] [PubMed] [Google Scholar]
- Milligan JR, Aguilera JA, Paglinawan RA, Ward JF. Mechanism of DNA damage by thiocyanate radicals. Int J Radiat Biol. 2000;76:1305–1314. doi: 10.1080/09553000050151574. [DOI] [PubMed] [Google Scholar]
- Mori T, Dizdaroglu M. Ionizing radiation causes greater DNA base damage in radiation-sensitive mutant M10 cells than in parent mouse lymphoma. Radiat Res. 1994;140:85–90. [PubMed] [Google Scholar]
- Nguyen KL, Steryo M, Kurbanyan K, Nowitzki KM, Butterfield SM, Ward SR, Stemp EAD. DNA protein cross-linking via from oxidation of guanine via the fl ash-quenching technique. J Am Chem Soc. 2000;122:3585–3594. [Google Scholar]
- Olinski R, Zastawny TH, Foksinski M, Windorbska W, Jaruga P, Dizdaroglu M. DNA base damage in lymphocytes of cancer patients undergoing radiation therapy. Cancer Lett. 1996;106:207–215. doi: 10.1016/0304-3835(96)04320-0. [DOI] [PubMed] [Google Scholar]
- O’Neill P, Davies SE. Pulse radiolytic study of the interaction of SO4• − with deoxynucleosides. Possible implications for direct energy deposition. Int J Radiat Biol. 1987;52:577–587. doi: 10.1080/09553008714552071. [DOI] [PubMed] [Google Scholar]
- O’Neill P, Wardman P. Radiation chemistry comes before radiation biology. Int J Radiat Biol. 2009;85:9–25. doi: 10.1080/09553000802640401. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiolog Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples AM, Lee J, Weinfeld M, Milligan JR, Bernhard WA. Yields of damage to C4′ deoxyribose and to pyrimidine in pUC18 by the direct effect of ionizing radiation. Nucleic Acids Res. 2012;40:6060–6069. doi: 10.1093/nar/gks271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier S, Hau J, Gasparutto D, Cadet J, Favier A, Ravanat J-L. Characterization of lysine-guanine cross-links upon one-electron oxidation of a guanine-containing oligonucleotide in the presence of a trilysine peptide. J Am Chem Soc. 2006;128:5703–5710. doi: 10.1021/ja057656i. [DOI] [PubMed] [Google Scholar]
- Pouget J-P, Frelon S, Ravanat J-L, Testard I, Odin F, Cadet J. Formation of modified DNA bases in cells exposed either to gamma radiation or to high-LET particles. Radiat Res. 2002;157:589–595. doi: 10.1667/0033-7587(2002)157[0589:fomdbi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Raychaudhury P, Basu AK. Replication past the gammaradiation-induced guanine-thymine cross-link G[8,5-Me]T by human and yeast DNA polymerase eta. J Nucleic Acids. 2010;2010:101495. doi: 10.4061/2010/101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhury P, Basu AK. Genetic requirement for mutagenesis of the G[8,5-Me]T cross-link in Escherichia coli: DNA polymerases IV and V compete for error-prone bypass. Biochemistry. 2011;50:2330–2338. doi: 10.1021/bi102064z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid R, Mark F, Schuchmannn HP, von Sonntag C. SO4• − -induced oxidation of 1,3,6-trimethyluracil and 1,3,5-trimethyluracil (1,3-dimethylthymine) by potassium peroxodisulphate in aqueous solution: An interesting contrast. Int J Radiat Biol. 1991;59:1081–1100. doi: 10.1080/09553009114551001. [DOI] [PubMed] [Google Scholar]
- Ravanat J-L, Saint-Pierre C, Cadet J. One-electron oxidation of the guanine moiety of 2′-deoxyguanosine: Influence of 8-oxo-7,8-dihydro-2′-deoxyguanosine. J Am Chem Soc. 2003;125:2030–2031. doi: 10.1021/ja028608q. [DOI] [PubMed] [Google Scholar]
- Reynisson J, Steenken S. DFT calculations on the electrophilic reaction with water of the guanine and adenine radical cations. A model for the situation in DNA. Phys Chem Chem Phys. 2002a;4:527–532. [Google Scholar]
- Reynisson J, Steenken S. DNA-base radicals. Their base pairing abilities as calculated by DFT. Phys Chem Chem Phys. 2002b;4:5346–5352. [Google Scholar]
- Rokhlenko Y, Geacintov NE, Shafirovich V. Lifetimes and reaction pathways of guanine radical cations and neutral guanine radicals in an oligonucleotide in aqueous solutions. J Am Chem Soc. 2012;134:4955–4962. doi: 10.1021/ja212186w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu A, Bellon S, Gasparutto D, Cadet J. Synthesis and UV photolysis of oligodeoxynucleotides that contain 5-(phenylthiomethyl)-2′-deoxyuridine: A specific photolabile precursor of 5-(2′-deoxyuridilyl)methyl radical. Org Lett. 2000;2:1085–1088. doi: 10.1021/ol005643y. [DOI] [PubMed] [Google Scholar]
- Schuchmann HP, Deeble DJ, Olbrich G, von Sonntag C. The SO4• − -induced chain reaction of 1,3-dimethyluracil with peroxodisulphate. Int J Radiat Biol. 1987;51:441–453. doi: 10.1080/09553008714550931. [DOI] [PubMed] [Google Scholar]
- Seidel CAM, Schulz A, Sauer MHM. Nucleobase-specific quenching of fluorescent dyes. 1. Nucleobase one-electron redox potentials and their correlation with static and dynamic quenching efficiencies. J Phys Chem. 1996;100:5541–5553. [Google Scholar]
- Shafirovich V, Crean C, Geacintov NE. Reactions of reactive nitrogen species and carbonate radical anions with DNA. In: Greenberg MM, editor. Radical and radical ion reactivity in nucleic acid chemistry. Hoboken, NJ: John Willey & Sons, Inc; 2009. pp. 325–355. [Google Scholar]
- Shafirovich V, Dourandin A, Huang V, Geacintov NE. Carbonate radical is a site-selective oxidizing agent of guanines in double-stranded oligonucleotides. J Biol Chem. 2001;276:24621–24626. doi: 10.1074/jbc.M101131200. [DOI] [PubMed] [Google Scholar]
- Shaw AA, Cadet J. Radical combination processes under the direct effects of gamma radiation on thymidine. J Chem Soc Perkin Trans II. 1990:2063–2070. [Google Scholar]
- Spassky A, Angelov D. Influence of the local helical conformation on the guanine modifications generated from one-electron DNA oxidation. Biochemistry. 1997;36:6571–6576. doi: 10.1021/bi962761d. [DOI] [PubMed] [Google Scholar]
- Stanbury DM. Reduction potentials involving inorganic free radicals in aqueous solution. Adv Inorg Chem. 1989;33:69–138. [Google Scholar]
- Steenken S. Purine bases, nucleosides, and nucleotides: Aqueous solution redox chemistry and transformation reactions of their radical cations and e- and OH adducts. Chem Rev. 1989;89:503–520. [Google Scholar]
- Steenken S. Electron-transfer-induced acidity/basicity and reactivity changes of purine and pyrimidine bases. Consequences of redox processes for DNA base pairs. Free Rad Res Commun. 1992;16:349–379. doi: 10.3109/10715769209049187. [DOI] [PubMed] [Google Scholar]
- Steenken S. Electron transfer in DNA? Competition by ultra-fast proton transfer? Biol Chem. 1997;378:1293–1297. [PubMed] [Google Scholar]
- Steenken S, Jovanovic SV. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J Am Chem Soc. 1997;119:617–618. [Google Scholar]
- Steward RD, Yu VK, Georgakilas AG, Koumenis C, Park JH, Carlson DJ. Effects of radiation quality and oxygen on clustered DNA lesions and cell death. Radiat Res. 2011;176:587–602. doi: 10.1667/rr2663.1. [DOI] [PubMed] [Google Scholar]
- Swarts SG, Becker D, Sevilla M, Wheeler KT. Radiation-induced DNA damage as a function of hydration. II. Base damage from electron-loss centers. Radiat Res. 1996;145:304–314. [PubMed] [Google Scholar]
- Swarts SG, Gilbert DC, Sharma KK, Razskazovskiy Y, Purkayastha S, Naumenko KA, Bernhard WA. Mechanisms of direct radiation damage in DNA, based on a study of the yields of base damage, deoxyribose damage, and trapped radicals in d(GCACGCGTGC)2. Radiat Res. 2007;168:367–381. doi: 10.1667/RR1058.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Douki T, Cadet J, Wagner JR. 2′-Deoxycytidine glycols: A missing link in the free radical-mediated oxidation of DNA. J Biol Chem. 1999;274:20833–20838. doi: 10.1074/jbc.274.30.20833. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Wagner JR. Dehydration, deamination and enzymatic repair of cytosine glycols from oxidized poly(dG-dC) and poly(dI-dC) Nucleic Acids Res. 2008;36:284–293. doi: 10.1093/nar/gkm1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Sonntag C, Schuchmann H-P. The radiolysis of pyrimidines in aqueous solutions: An updating review. Int J Radiat Biol. 1986;49:1–34. doi: 10.1080/09553008514552201. [DOI] [PubMed] [Google Scholar]
- von Sonntag C. The chemical basis of radiation biology. Philadelphia: Taylor and Francis; 1987a. [Google Scholar]
- von Sonntag C. New aspects in the free-radical chemistry of pyrimidine nucleobases. Free Rad Res Commun. 1987b;2:217–224. doi: 10.3109/10715768709065286. [DOI] [PubMed] [Google Scholar]
- von Sonntag C. The chemistry of free-radical-mediated DNA damage. Basic Life Sci. 1991;58:287–317. doi: 10.1007/978-1-4684-7627-9_10. [DOI] [PubMed] [Google Scholar]
- von Sonntag C. Free radical induced DNA damage and its repair–a chemical perspective. New York: Springer; 2006. [Google Scholar]
- Wagner JR, van Lier JE, Berger M, Cadet J. Thymidine hydroperoxides: Structural assignment, conformational features, and thermal decomposition in water. J Am Chem Soc. 1994;116:2235–2242. [Google Scholar]
- Wagner JR, Decarroz C, Berger M, Cadet J. Hydroxyl-radical induced decomposition of 2′-deoxycytidine in aerated aqueous solutions. J Am Chem Soc. 1999;121:4101–4110. [Google Scholar]
- Wagner JR, Cadet J. Oxidation reactions of cytosine DNA components by hydroxyl radical and one-electron oxidants in aerated aqueous solutions. Acc Chem Res. 2010;43:564–571. doi: 10.1021/ar9002637. [DOI] [PubMed] [Google Scholar]
- Wang Y. Bulky DNA lesions induced by reactive oxygen species. Chem Res Toxicol. 2008;21:276–281. doi: 10.1021/tx700411g. [DOI] [PubMed] [Google Scholar]
- Wardman P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J Phys Chem Ref Data. 1989;18:1637–16755. [Google Scholar]
- Yang Z, Colis LC, Basu AK, Zou Y. Recognition and incision of γ-radiation-induced cross-linked guanine-thymine tandem lesion G[8,5-Me]T by UvrABC nuclease. Chem Res Toxicol. 2005;18:1339–1346. doi: 10.1021/tx050147+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BH, Geacintov NE, Shafirovich V. Generation of guaninethymidine cross-links in DNA by peroxynitrite/carbon dioxide. Chem Res Toxicol. 2011;24:1144–152. doi: 10.1021/tx200139c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang Y. Generation of 5-(2′-deoxycytidyl)methyl radical and the formation of interstrand cross-link lesions in oligodeoxyribonucleotides. Nucleic Acids Res. 2005;33:1593–1603. doi: 10.1093/nar/gki301. [DOI] [PMC free article] [PubMed] [Google Scholar]