Abstract
Clinical trials of nicotine vaccines suggest that they can enhance smoking cessation rates but do not reliably produce the consistently high serum antibody concentrations required. A wide array of next-generation strategies are being evaluated to enhance vaccine efficacy or provide antibody through other mechanisms. Protein conjugate vaccines may be improved by modifications of hapten or linker design or by optimizing hapten density. Conjugating hapten to viruslike particles or disrupted virus may allow exploitation of naturally occurring viral features associated with high immunogenicity. Conjugates that utilize different linker positions on nicotine can function as independent immunogens, so that using them in combination generates higher antibody concentrations than can be produced by a single immunogen. Nanoparticle vaccines, consisting of hapten, T cell help peptides, and adjuvants attached to a liposome or synthetic scaffold, are in the early stages of development. Nanoparticle vaccines offer the possibility of obtaining precise and consistent control of vaccine component stoichiometry and spacing and immunogen size and shape. Passive transfer of nicotine-specific monoclonal antibodies offers a greater control of antibody dose, the ability to give very high doses, and an immediate onset of action but is expensive and has a shorter duration of action than vaccines. Viral vector-mediated transfer of genes for antibody production can elicit high levels of antibody expression in animals and may present an alternative to vaccination or passive immunization if the long-term safety of this approach is confirmed. Next-generation immunotherapies are likely to be substantially more effective than first-generation vaccines.
1. INTRODUCTION
Nicotine vaccines appear quite promising in animals but have been disappointing in initial clinical trials for enhancing smoking cessation rates. There are a number of likely reasons for this lack of translation, most of which should be addressable with improvements in vaccine design or the manner in which vaccines are used. This chapter will focus on understanding the limitations of first-generation nicotine vaccines studied to date and how to overcome them. Readers are referred to other reviews for a more detailed discussion of nicotine vaccine development and the mechanism of action (Bevins, Wilkinson, & Sanderson, 2008; LeSage, Keyler, & Pentel, 2006; Raupach, Hoogsteder, & Onno van Schayck, 2012; Shen, Orson, & Kosten, 2012).
2. STATUS OF FIRST-GENERATION NICOTINE VACCINES
Animal data supporting nicotine vaccine efficacy are robust. A variety of nicotine vaccines have been shown to reduce the distribution to the brain of single, clinically relevant nicotine doses by up to 90% (Cerny et al., 2002; Maurer et al., 2005; Pravetoni et al., 2011; Satoskar et al., 2003). With repeated nicotine dosing simulating 1–2 packs of cigarettes per day, nicotine distribution to the brain is reduced to a lesser extent but each nicotine dose reaches the brain more slowly and is presumably less reinforcing (Hieda, Keyler, Ennifar, Fattom, & Pentel, 2000; Pentel, Dufek, Roiko, Lesage, & Keyler, 2006). Nicotine vaccines readily block or attenuate many nicotine addiction-related behaviors including the acquisition, maintenance, and reinstatement of nicotine self-administration (LeSage, Keyler, Hieda, et al., 2006; Lindblom et al., 2002). These general results have been reproduced in many laboratories with different vaccines, adding confidence to the findings.
Clinical trial results of nicotine vaccines, although some are available only as press releases, have not mirrored the strong preclinical findings (Hartmann-Boyce, Cahill, Hatsukami, & Cornuz, 2012). Except for one phase II clinical trial of 3′-AmNic-rEPA (NicVAX) (Hatsukami et al., 2011), overall efficacy for smoking cessation has not been greater than with placebo vaccine (Fahim, Kessler, & Kalnik, 2013). The follow-up phase III trials of NicVAX failed to confirm the earlier finding of the overall efficacy. However, the phase II trials of both NicQb and NicVAX had a similar efficacy signal in subgroup analyses; one-third of subjects with the highest serum antibody concentrations or titers showed an approximately doubled smoking cessation rate compared to controls (Cornuz et al., 2008; Hatsukami et al., 2011). A phase II trial of another conjugate vaccine, Niccine, reported no such efficacy signal but antibody levels were uniformly low and efficacy would not be expected (Tonstad et al., 2013). These data suggest that improved vaccines that consistently generate higher antibody levels could be effective therapies.
3. LIMITATIONS OF FIRST-GENERATION NICOTINE VACCINES
3.1. Importance of achieving high antibody levels
Vaccines generate antibodies that bind nicotine and alter its access to the brain. Vaccine effects on nicotine pharmacokinetics correlate closely with the amount of antibody generated, as estimated by the serum nicotine-specific antibody concentration or the antibody titer (a functional measure that is sensitive to both antibody concentration and antibody affinity for nicotine) (Keyler et al., 2005; Maurer et al., 2005; Pravetoni et al., 2011). Because the amount of nicotine consumed by smokers can approach or exceed the binding capacity of the available antibody produced by vaccination, it is critical to generate antibody levels that are as high as possible. In rats or mice, serum IgG antibody concentrations of >100 μg/ml are associated with significant effects on nicotine distribution and attenuation of its behavioral effects, and antibody levels of 100–500 μg/ml can be achieved (Keyler, Roiko, Earley, Murtaugh, & Pentel, 2008; Maurer et al., 2005). High antibody levels are more readily achieved in rodents than in humans because it is possible to use strong or experimental adjuvants that are not suitable for clinical use. It is also possible to use much higher mg/kg immunogen doses in rodents (typical 50–100 μg/kg) than in humans (typical 1–5 μg/kg) because larger ml/kg vaccine volumes are acceptable for use in animals. Acute nicotine exposure in rats does not interfere with nicotine vaccine immunogenicity (Hieda et al., 2000), but smokers have reduced antibody responses to some infectious disease vaccines (Crothers et al., 2011; Finklea et al., 1971; Roseman, Truedsson, & Kapetanovic, 2012; Winter, Follett, McIntyre, Stewart, & Symington, 1994). Therefore, difficulty inducing sufficient antibody levels in humans could be anticipated, and this has proven to be the principal challenge presented by the use of nicotine vaccines for smoking cessation.
Because of limited clinical experience with addiction vaccines, it is difficult to predict the nicotine-specific antibody serum concentration required for clinical efficacy, but initial studies provide an estimate. In phase II clinical trials of the 3′-AmNic-rEPA vaccine, a peak geometric mean serum IgG concentration of 45 μg/ml was associated with higher smoking cessation rates (Hatsukami et al., 2011). In a clinical laboratory study of the same vaccine, subjects with serum antibody levels of 40–160 μg/ml had a significant but minimal (mean of 12%) reduction in occupancy of brain nicotinic cholinergic receptors (nAChRs) compared to controls after receiving a single i.v. dose of nicotine (Esterlis et al., 2013). These data suggest that serum nicotine-specific antibody levels of greater than 50–100 μg/ml provide an approximate minimum target concentration. This estimate is consistent with rat and mouse data showing that mean serum antibody levels of 50–100 μg/ml produce a significant but small reduction in the distribution of clinically relevant nicotine doses to the brain and that effects increase at higher serum antibody levels (Keyler et al., 2008; Maurer et al., 2005). As in animals, higher serum antibody concentrations in the 3′-AmNic-rEPA phase II study appeared to be more effective for smoking cessation (Hatsukami et al., 2011).
To put this estimate in perspective, the whole-body content of nicotine-specific IgG after vaccination, and of nicotine after smoking, can be estimated. Using a volume of distribution for IgG of 70 ml/kg, which indicates that about 2/3 of IgG exists outside of serum, a serum antibody concentration of 100 μg/ml would provide about 6 μmole of binding sites for nicotine (two binding sites per IgG) (Migone et al., 2009). Smoking one cigarette provides an absorbed nicotine dose of about 1–1.5 mg or 5–7 μmol (Gelal et al., 2005). Therefore, the proposed minimum effective serum nicotine-specific antibody concentration of 50–100 μg/ml would provide the binding capacity for at most one cigarette. By this measure, it is not surprising that the 50–100 μg/ml level would allow only minimal efficacy and that higher concentrations might be required for optimal results.
3.2. Variability in vaccine immunogenicity
An additional limitation of first-generation nicotine vaccines, in both animals and humans, is large individual variability in the immune response. Serum nicotine-specific antibody titer or concentration ranges of 30-fold or greater have been reported and appear to be the rule rather than the exception with the nicotine vaccines studied to date (Cornuz et al., 2008; Hatsukami et al., 2011; Tonstad et al., 2013). This wide range reflects in part the underlying variability of immune responses in a population and is also found with cocaine or opioid vaccines and many infectious disease vaccines (Kosten et al., 2002). Large variability results in a substantial number of non-responders with very low antibody levels that are unlikely to be effective. The goal of next-generation vaccine design must be both to enhance the mean antibody response and to reduce the number of nonresponders by either reducing variability or enhancing mean efficacy enough to raise the lowest responders into an acceptable range.
While most studies have focused on serum antibody concentrations or titers as a surrogate for concentration, antibody affinity for nicotine also influences efficacy in rats (Keyler et al., 2005). Antibody affinity is measured as the dissociation rate constant Kd, with lower values indicating greater affinity for nicotine. The Kd value corresponds with the nicotine concentration at which antibody binding sites are half saturated with nicotine. When the serum nicotine concentration is above the Kd, the antibody is highly saturated and most of its binding capacity is utilized. When the serum nicotine concentration is below the Kd, saturation is low and less of the binding capacity is being utilized. Venous serum nicotine concentrations in smokers are generally around 5–40 ng/ml (30–240 nM), so that antibodies with a Kd near or below the lower end of this range should be most effective. This estimate corresponds well with vaccine or passive immunization experience in rats or mice, where effective antibodies have had Kd’s in the range of 10–100 nM (as measured by equilibrium dialysis or soluble radioimmuno-assay), and this range provides a target for vaccine or immunotherapy development (Maurer et al., 2005; Moreno et al., 2010; Pentel et al., 2000). The mean affinity of antibodies generated in a phase I study of NicQb was 33 nM, also within the proposed target range (Maurer et al., 2005). The extent of individual variability in antibody affinity was not reported, but we have found a 10-fold range in affinity for nicotine in rats vaccinated with 3′-AmNic-rEPA (unpublished data). Measuring this parameter in clinical trials might add insight into the causes of variability in vaccine efficacy (Orson et al., 2007).
4. LIMITATIONS OF VACCINATION AS A GENERAL STRATEGY FOR TREATING NICOTINE ADDICTION
It is likely that sufficiently immunogenic nicotine vaccines will be effective for enhancing smoking cessation rates. Advances in vaccine design, outlined in Section 6, should substantially enhance vaccine efficacy in the next few years and provide tools for testing this hypothesis. A more general question is how effective even the most immunogenic vaccines can be and which aspects of tobacco addiction they can address.
4.1. Nicotine versus other tobacco components
Nicotine is the principal addictive component of tobacco but other chemicals may contribute to tobacco addiction through their effects on nicotine absorption (e.g., alkali) (Anon, 1988), enhancement of nicotine reinforcement (other tobacco alkaloids and acetaldehyde) (Cao et al., 2007; Clemens, Caille, Stinus, & Cador, 2009), or modulation of mood or cognition (monoamine oxidase inhibitors) (George & Weinberger, 2008). Nicotine vaccines can attenuate only the effects of nicotine and perhaps its active but minor metabolite nornicotine. Whether other components of tobacco or tobacco smoke are sufficient to maintain addiction by themselves or in the presence of the lower concentrations of nicotine that persist even after vaccination is not clear. Addressing the effects of these non-nicotine components may prove helpful or necessary for maximizing the impact of nicotine vaccines.
4.2. Immediate versus long-lasting effects of nicotine exposure
The nicotine-specific antibodies generated by vaccination modify nicotine’s access to brain nAChRs by altering its distribution to tissues and its elimination. These actions can only occur when nicotine is present. The primary target of vaccination is therefore the immediate effects of nicotine. Longer-lasting effects of nicotine such as withdrawal and craving, which occur when nicotine is no longer present, cannot be directly altered by vaccination. It is possible that a persistent reduction of nicotine’s acute effects may indirectly lead to less subsequent withdrawal or craving (Lindblom et al., 2005), but a role for this in humans is speculative. It is more likely that additional measures, such as counseling or other medications, will be needed to complement vaccination in order to achieve optimal efficacy.
4.3. Quantitative limits on antibody effects
Immunotherapies can reduce but not completely prevent nicotine distribution to the brain. Even if there is sufficient antibody on a molar basis to bind all of the nicotine present, binding is reversible and governed by the dissociation rate constant (Kd). For example, viral vector-directed production of extremely high serum antibody concentration in mice (1 mg/ml) reduced the distribution of a single nicotine dose to the brain by only 85% (Hicks et al., 2012). This is important because even low serum concentrations of nicotine may be sufficient to produce addiction-relevant effects. Smoking just one low-nicotine Quest cigarette (5% of the nicotine content of a standard cigarette) produces 26% occupancy of brain nAChRs (Brody et al., 2009). The serum nicotine concentration estimated from these data to produce 50% nAChR occupancy was 0.75 ng/ml, considerably lower than typical serum nicotine concentration in smokers of 10–40 ng/ml. It is possible that immunotherapy will benefit from other measures or medications to address the residual effects of low levels of nicotine.
These considerations do not minimize the encouraging results found to date with vaccines or argue against the use of vaccines for nicotine addiction. Rather, they point to the need to consider vaccination in the larger context of addiction treatment and to take advantage of complementary approaches, in addition to optimizing vaccine immunogenicity.
5. DESIGN OF NICOTINE VACCINES
5.1. Types of nicotine vaccines
First-generation nicotine vaccines consist of protein conjugate immunogens (nicotine linked, or conjugated, to a carrier protein) mixed with adjuvant. Next-generation vaccines comprise a diverse array of approaches employing improvements in protein conjugate design, use of novel vaccine components, or vaccine component display on synthetic nanoparticles. The rationale for these designs and innovations derives from advances in understanding how vaccines engage an immune response and elicit antibody production.
5.2. Vaccine immunology
Generation of an effective humoral immune response (Fig. 14.1) requires linked recognition of the antigenic epitopes bound by B and T cells and coordinated interactions among B cells, T cells, and other antigen-presenting cells (APCs) (dendritic cells and macrophages) and follicular dendritic cells, neutrophils, and NKT cells (see McHeyzer-Williams & McHeyzer-Williams, 2005, for a more detailed review). The key components of a conjugate nicotine vaccine are the following: (1) a B cell epitope, in this case nicotine, to bind and engage those B cells that have the capacity to mature into nicotine-specific antibody-producing cells; (2) a T cell epitope, provided by the carrier protein of a conjugate vaccine or by smaller peptides, to bind and engage the T helper cells that assist B cell activation and maturation; and (3) an adjuvant, such as alum, that enhances vaccine immunogenicity. Sections 5 and 6 that follow are not intended to provide a comprehensive summary of humoral immunity, but rather to focus on those aspects most pertinent to vaccine design and performance.
Figure 14.1.
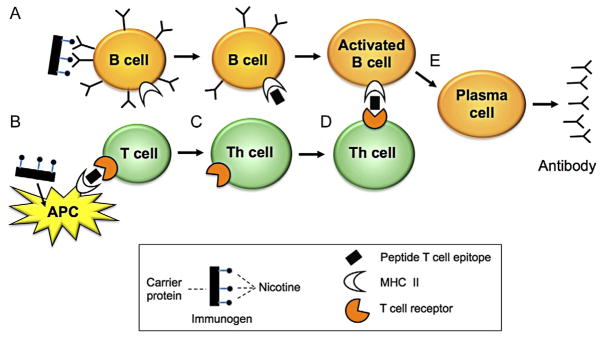
Key elements of conjugate vaccine interaction with immune cells that are relevant to nicotine vaccine design. For a more detailed account of humoral immunity, see McHeyzer-Williams and McHeyzer-Williams (2005). Humans have >108 naive B cells bearing surface antibody (B cell receptor) with different specificities that are capable of binding a wide range of chemical structures, including small molecules such as nicotine. When a nicotine vaccine is administered, (A) the nicotine component (B cell epitope) of the conjugate vaccine binds to those naive B cells that have appropriate surface antibody, and the entire conjugate is internalized. Internalization is enhanced by having multiple nicotine molecules attached to each carrier protein so that many surface antibody interactions take place. The carrier protein is digested within the B cell and peptide fragments (T cell epitopes) are displayed on its surface in association with MHC class II molecules. It is the display of these T cell epitopes that will subsequently allow helper T cells that have encountered the same T cell epitopes to recognize and interact with nicotine-specific B cells. (B) Immunogen is phagocytized by antigen-presenting cells (APCs) such as dendritic cells. This is a nonspecific process (does not involve immunogen receptors) that can be facilitated by the presence of an adjuvant (not shown). Particle uptake is enhanced if it is an appropriate size. APCs digest the carrier protein and display T cell epitope peptides on their surface in association with MHC class II molecules. Only peptides (not nicotine) can serve as T cell epitopes. Some T cells bear surface receptors capable of binding the T cell epitopes presented by APCs. (C) T cell interaction with APCs allows them differentiate into T helper (Th) cells that can provide stimulatory signals to B cells. (D) Those Th cells bearing receptors that are specific for the particular T cell epitope derived from the nicotine immunogen recognize and interact with the correct subset of B cells (those capable of binding nicotine) because those B cells now bear the immunogen’s T cell epitope on their surface. It is this specific recognition of T cell epitopes displayed by nicotine-specific B cells that allows Th cells to interact selectively with these B cells. (E) Activated B cells undergo maturation into antibody-secreting cells and memory cells (not shown) that can be activated by subsequent booster doses of vaccine. Adapted from materials provided by Y. Chang.
5.2.1 B cell epitope
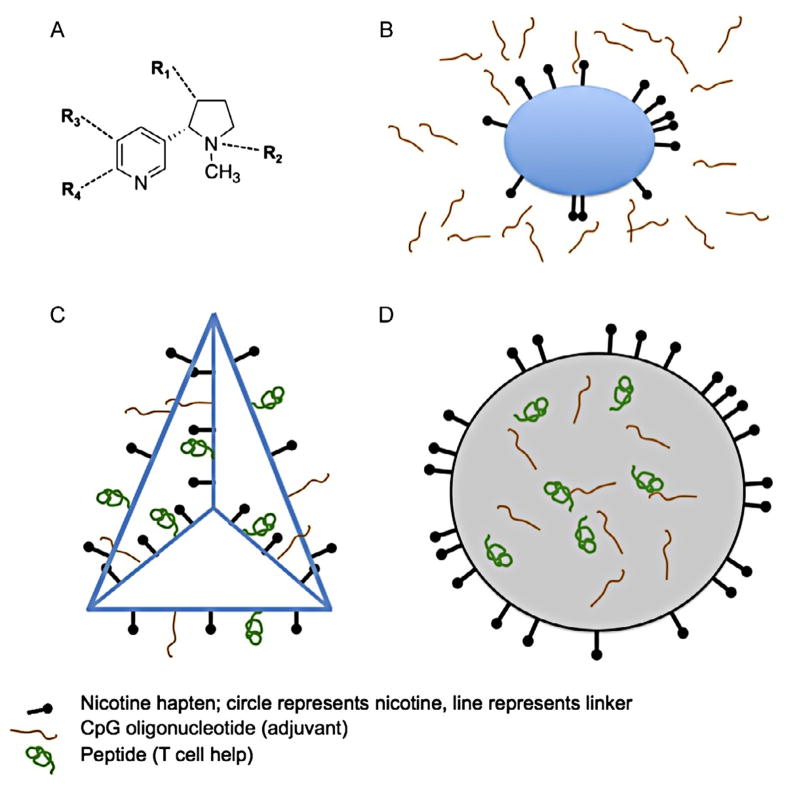
Nicotine serves as the B cell epitope in nicotine vaccines. Nicotine and a source of T cell help (carrier protein or peptide sequences) must be internalized in the same B cell to allow B cell recognition by helper T cells. In protein conjugate vaccines, this is assured by covalently attaching nicotine to a carrier protein through a short linker that allows nicotine to be displayed to the B cell without being buried within the carrier protein surface. To accomplish this, nicotine is modified by adding a chemical “handle” for linker attachment. Linkage of the modified nicotine (hapten) to carrier protein forms the complete immunogen (Fig. 14.2B).
Figure 14.2.
Immunogen structures, not drawn to scale. (A) Nicotine, with positions commonly used for attachment of linkers indicated as R1–4. (B) Conjugate vaccine. Nicotine is covalently attached to a carrier protein through a short linker that allows the nicotine to be accessible for binding to B cells. Some linkers are illustrated in Fig. 14.3A. A high density of nicotine hapten on the carrier protein facilitates its binding and uptake. Covalently linking nicotine to carrier assures that nicotine hapten and carrier protein will be taken into the same B cell. Conjugate immunogens are generally mixed with adjuvant to enhance antibody generation. (C) Nanoparticle vaccine: Synthetic scaffold (illustration is a DNA tetrahedron) (Liu et al., 2012). Nanoparticle scaffolds allow vaccine components to be covalently linked with readily controlled density and stoichiometry. T cell help can be provided by attachment of either whole protein or shorter peptide sequences. Some molecularly defined adjuvants, such as CpG oligonucleotides, can also be covalently linked to the nanoparticle. (D) Nanoparticle vaccine: Liposome or synthetic vesicle. Vaccine components such as hapten that require interactions with cell surface receptors can be attached to or embedded within the vesicle surface, while components intended for delivery to the cell interior can be encapsulated within.
B cells display a transmembrane form of immunoglobulin on their surface (B cell receptor, BCR) and each B cell displays a unique BCR. The naive (nonvaccinated) B cell repertoire for a single individual consists of >108 B cells, each with its own surface antibody and capacity to recognize and bind certain molecules (Taylor, Jenkins, & Pape, 2012). A small fraction of these naive B cells bear antibodies that can recognize and bind nicotine that is linked to a carrier protein (or nanoparticle), initiating internalization of the immunogen (Pape, Taylor, Maul, Gearhart, & Jenkins, 2011).
Once immunogen is internalized, B cells digest the carrier protein to shorter (12–20 amino acid) peptides, which are then displayed on their surface in association with MHC class II molecules (Fig. 14.1A). Therefore, the role of nicotine on the immunogen is to select and engage just those B cells bearing surface antibody that can bind nicotine, while the carrier protein provides peptide sequences (T cell help) that can subsequently recognize and interact with T cells to obtain the signals needed for maturation into antibody-secreting cells. This interaction takes place primarily in the germinal centers of lymph nodes where B and T cells are abundant and in close proximity to each other.
5.2.2 T cell help peptides
B cells require help from T cells, via direct contact and soluble signals, in order to mature into activated B cells capable of producing high-affinity nicotine-specific antibody. The process of engaging T cell help is initiated when immunogen is taken up nonspecifically, a process not dependent upon the presence of hapten, by APCs consisting primarily of dendritic cells and macrophages (Fig. 14.1B). APCs digest the carrier protein to shorter peptides, which are then displayed on their surface by MHC class II molecules. Some T cells bear surface receptors (structurally similar to antibodies) that can recognize the peptide sequences displayed by APCs. This interaction activates those T cells to differentiate into a T helper cell. Because the T helper cell bears surface receptors that recognize peptides derived from the carrier protein, it can now recognize and interact with nicotine-specific B cells displaying this same peptide. This interaction, along with additional surface interactions and release of soluble mediators, activates the nicotine-specific B cell. The B cell then undergoes a complex process of affinity maturation within the germinal center of lymph nodes to select and produce high-affinity nicotine-specific antibodies.
5.2.3 Adjuvant
Adjuvants are a diverse class of distinct chemicals or complex mixtures that can facilitate an immune response (Wilson-Welder et al., 2009). They broadly signal either tissue injury or the presence of something foreign, creating an immune-competent environment at the injection site (Awate, Babiuk, & Mutwiri, 2013). Some adjuvants may also prolong immunogen retention at the injection site or enhance immunogen delivery to regional lymph nodes where the abundance of B and T cells maximizes their opportunity to encounter immunogen and interact. Alum (most often Al(OH)3), the most widely used adjuvant in humans, appears to mimic tissue injury but has other postulated mechanisms of action as well (Spreafico, Ricciardi-Castagnoli, & Mortellaro, 2010). Many newer or experimental adjuvants bind specifically to Toll-like receptors or NOD-like proteins that recognize structural components of bacteria and viruses, for example, CpG oligonucleotides, lipopolysaccharide, and flagellin, or components that are released from injured cells (Klinman, Klaschik, Sato, & Tross, 2009). Some adjuvants have proven more effective than alum for specific immunogens, but it is not clear that any one adjuvant is consistently best. Combining CpG with alum enhances responses to some vaccines, including a nicotine conjugate vaccine (McCluskie et al., 2013), but failed to do so with one other (Bremer & Janda, 2012). Some carrier proteins have intrinsic adjuvant activity but all conjugate addiction vaccines that have advanced to clinical trials to date have used alum as adjuvant.
6. STRATEGIES FOR IMPROVING VACCINE EFFICACY
6.1. Vaccine design: Conjugate immunogens
Many promising improvements in nicotine vaccines have been studied. It is difficult to compare the efficacy of nicotine vaccines to each other from the available literature because (1) the density of hapten on carrier protein, a key determinant of immunogenicity, is often not measured or reported; (2) immunogenicity is often reported only as antibody titer, a measure that is method-dependent; and (3) many vaccines and adjuvants are proprietary and not available for direct comparison. Nevertheless, some general principles are apparent.
The basic components of nicotine vaccines are summarized in Fig. 14.2. All nicotine vaccines (and cocaine vaccines as well) in clinical trials reported to date have been conjugate vaccines consisting of nicotine linked, or conjugated, to a carrier protein and then mixed with alum adjuvant. Conjugate vaccine development has focused on choosing the most effective (1) site of attachment of linker to nicotine, (2) linker composition and length, (3) number of nicotine molecules linked to each carrier protein (higher ratios are generally more effective), and (4) carrier protein. There are few rules to guide these choices, and design generally proceeds empirically by systematically comparing alternatives.
6.1.1 Nicotine hapten
A variety of positions for linker attachment to nicotine have been used (Fig. 14.2A) and are effective (de Villiers et al., 2010; Isomura, Wirsching, & Janda, 2001; Pravetoni et al., 2012). No one linker position stands out as superior. Linker attachment away from the site of metabolism to its primary metabolite cotinine, keeping this site exposed, should favor production of antibodies with greater specificity for nicotine over cotinine, but many linker positions appear to accomplish this. Because nicotine can rotate about the bond between its pyridine and pyrrolidine rings, a rigid conformationally constrained hapten was studied and found to be more immunogenic than its nonconstrained counterpart (Moreno, Azar, Koob, & Janda, 2012). Whether it is more effective than other nicotine haptens is not clear. Fluorination of drugs, in which a hydrogen is replaced by the sterically similar but more electronegative fluorine, is a common method of enhancing drug–receptor binding and might also affect the binding of haptens to BCRs. Fluorination of a cocaine hapten did not alter the affinity for cocaine of the antibodies produced by vaccination of mice, but modestly increased the mean cocaine-specific serum antibody level (Cai, Tsuchikama, & Janda, 2013). Because ligand fluorination is a general method, it may be useful for other haptens as well.
6.1.2 Linker
The purposes of a linker are to tether nicotine to its carrier or scaffold, provide a geometry that allows binding of nicotine hapten to B cells, and display a high enough density of nicotine haptens to optimize hapten binding to B cells. The most commonly manipulated variables are linker length and lipophilicity. Published comparisons of linker attributes can be difficult to interpret if the haptenation ratio (number of haptens conjugated to each protein molecule) is not reported. Thus, a linker that forms a conjugate with particularly high immunogenicity might be doing so either because of its ability to effectively present nicotine to B cells or because of the efficiency with which it can be conjugated to protein. Most linkers are 5–15 atoms long and consist of simple unsubstituted chains in order to minimize their own immunogenicity (de Villiers et al., 2010; Pravetoni et al., 2012).
6.1.3 Carrier proteins or peptides
A variety of foreign proteins known to be highly immunogenic have proven useful as carriers for conjugate vaccines. Several proteins, such as keyhole limpet hemocyanin or tetanus toxoid, are generally highly effective and widely used in animal models but it is far from clear that any one protein is best for all haptens or in all species. Carrier proteins used in nicotine conjugate vaccines reaching clinical trials include recombinant P. aeruginosa exoprotein A, recombinant cholera toxin B subunit, and tetanus toxoid (Pentel et al., 2000; Tonstad et al., 2013; Anon, 2004). All were immunogenic in rodents but less so in humans. Some viruses are highly immunogenic because they are readily recognized as foreign, and their large size promotes cellular uptake. One vaccine reaching clinical trials, NicQb, consists of a nicotine hapten conjugated to the outer protein capsid of bacteriophage Qb (Maurer et al., 2005). The capsid therefore serves the same function as the protein in a standard conjugate vaccine. This noninfectious construct proved highly immunogenic in animals but, like the conjugate vaccine NicVAX, showed efficacy in clinical trials only in the minority of subjects who achieved the highest antibody levels (Cornuz et al., 2008). Disrupted adenovirus has also proven quite effective as a carrier for nicotine or other haptens in animals but clinical data are not available. Immunogenicity was preserved even in animals with prior exposure to this common virus (De et al., 2013).
Only certain peptide sequences within carrier proteins provide T cell help. Haptens can be linked directly to these peptides rather than to the whole carrier protein, but it is not clear if there is an advantage to this. Peptides may however prove particularly useful in the construction of nanoparticle vaccines (see succeeding text) because they can be more readily and predictably incorporated into these structures. Nicotine haptens have also been linked directly to an agonist of C5a receptors found on APCs, which provides direct adjuvant activity and, presumably, T cell help (Sanderson et al., 2003). This construct is immunogenic, but no more so than standard protein conjugate vaccines.
6.2. Vaccine design: Nanoparticles
Conjugate vaccines are effective but it can be challenging or impossible to reliably achieve the desired hapten density on the protein or to try new designs such as directly attaching other vaccine components (e.g., adjuvant) to the immunogen or to alter the immunogen’s size, shape, or chemical composition to enhance cellular uptake or engagement of the initial immune response. Nanoparticle scaffolds have been designed to facilitate these kinds of manipulations and allow a wider range of immunization strategies to be studied (Peek, Middaugh, & Berkland, 2008; Zaman, Good, & Toth, 2013).
One approach under development is the use of liposomes or self-assembling synthetic vesicles constructed from nontoxic biodegradable polymers (Kasturi et al., 2011; Lockner et al., 2013). The outer surface of these particles can be chemically modified with handles for component attachment. Lipophilic components such as monophosphoryl lipid A adjuvant can also be embedded in liposomal membranes, or components can be enclosed within (Matyas et al., 2013; Peek et al., 2008). One such nicotine vaccine consisting of a synthetic vesicle with nicotine haptens linked to its surface, and T cell help peptides and adjuvant packaged within, has proven highly immunogenic in rodents and primates and has advanced to early clinical trials (Kishimoto, Altreuter, Johnston, Keller, & Pittet, 2012). Other scaffolds are in early stages of development for nicotine and other types of vaccines and may provide even greater flexibility and control of composition. DNA scaffolds are of particular interest because they can be fashioned into a wide variety of complex, self-assembling sizes and shapes through DNA origami using base pairing algorithms (Han et al., 2011). Precise control of the number and placement of handles for hapten, T cell peptides, and adjuvant linkage is possible (Liu et al., 2012). This assures control of component density and stoichiometry. Self-assembling peptides are being developed that provide both a scaffold for vaccine components and an intrinsic adjuvant activity (Rudra, Tian, Jung, & Collier, 2010). These and other nanoparticle designs provide a means of more systematically studying novel vaccine components and better defining the qualitative and quantitative determinants of vaccine efficacy.
6.3. Multivalent vaccines (Fig. 14.3A)
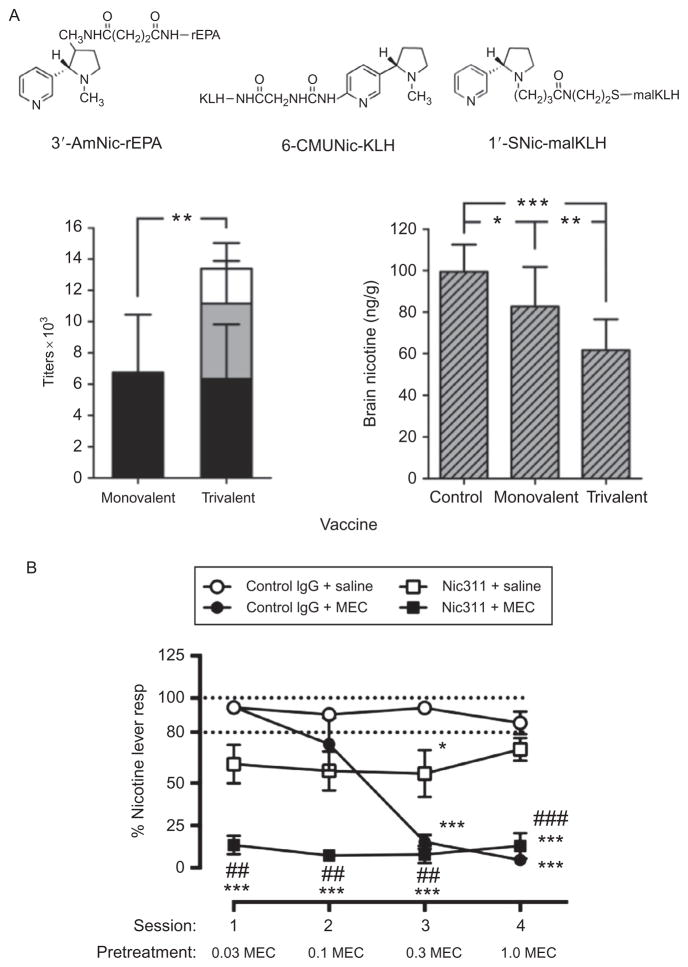
Figure 14.3.
Potential options for combining nicotine vaccines or using them in combination with medications. (A) Trivalent nicotine vaccine (de Villiers et al., 2013). Rats were vaccinated with three structurally distinct nicotine immunogens (linkers attached at different positions, carrier proteins; rEPA, recombinant exoprotein A; KLH, keyhole limpet hemocyanin; malKLH, maleimide activated KLH) or a dose-matched monovalent immunogen s.c. with alum adjuvant. After the last of three vaccine doses, rats received a single i.v. dose of 0.03 mg/kg nicotine. The trivalent immunogen elicited higher nicotine-specific antibody titers and concentrations than the monovalent vaccine (left) and reduced nicotine distribution to the brain to a greater extent (right). *p <0.05. (B) Combined use of the nicotine-specific mAb Nic311 and mec-amylamine to block the subjective effects of nicotine in rats trained in a two-lever nicotine discrimination assay. Each point is the mean (±SEM)% responding on the nicotine lever during consecutive daily test sessions with the 0.4 mg/kg nicotine training dose in each treatment group following administration of control antibody (IgG) and saline (open circles), Nic311 alone (open squares), ascending doses of MEC alone (solid circles), or both (solid squares). Dashed horizontal lines indicate criterion levels of performance for discrimination of the 0.4 mg/kg nicotine training dose. Significantly different from control IgG +saline, *p <0.05, ***p <0.001. Significantly different from Nic311+saline, ##p <0.01, ###p <0.001 (LeSage et al., 2012).
It may be possible to enhance nicotine vaccine efficacy by combining two or more effective immunogens. Multivalent vaccines are widely used for the prevention of infectious diseases. Individual immunogens are combined to provide coverage for related pathogens (e.g., a 23-valent pneumococcal vaccine containing capsular polysaccharides from 23 of the most important serotypes of pneumococcus), or unrelated immunogens can be combined as a convenience so that fewer immunization injections are needed (measles, mumps, and rubella vaccine). This is possible because the immune system has the capacity to respond to multiple simultaneous immune challenges. This capacity can be exploited for nicotine vaccines by immunizing animals with two or three structurally distinct nicotine immunogens, containing haptens with linkers at different positions. If these haptens are sufficiently distinct, they can elicit independent immunologic responses by activating different populations of B cells (Keyler et al., 2008; Pravetoni et al., 2012). Each of the immunogens elicits antibodies against nicotine, but the responses involve different populations of B cells and so are independent and additive. The resulting antibody levels are higher than for the same dose of a monovalent vaccine (Fig. 14.3A). The effects of bi- or trivalent nicotine vaccines on nicotine distribution in rats mirror the titers and are also additive (de Villiers, Cornish, Troska, Pravetoni, Pentel, 2013). An additional benefit seen in this study is that some animals with a low response to one immunogen had a high response to another, reducing the number of low or nonresponders. The concept of multivalent vaccines is quite general and not limited to the specific immunogens studied. As newer and more effective immunogens are developed, it may be possible to combine the best of these to further enhance their efficacy. Experience with this approach is limited and it has not entered clinical trials.
6.4. Combining vaccines with medications (Fig. 14.3B)
Because the mechanism of action of nicotine vaccines is unique among tobacco addiction treatments, it should be possible to combine vaccines with other types of smoking cessation medications. This is of interest because both vaccines alone and existing medications alone are only partially effective, combining them might enhance the efficacy of both (Bevins et al., 2008; Orson et al., 2007; Raupach et al., 2012). Vaccines cannot directly block withdrawal or craving, which occur when nicotine is no longer present. However, bupropion and varenicline have activities for reducing craving and withdrawal. There are limited clinical data addressing this hypothesis. Two clinical trials of nicotine vaccines plus varenicline failed to find enhanced efficacy but each had limitations. A NicVAX+varenicline trial used a vaccine that is at best minimally effective and provided only a limited period of overlap between varenicline and peak antibody levels (Hoogsteder et al., 2012). It was therefore more of a study of sequential varenicline followed by vaccine than their combined use. A second clinical trial used Niccine vaccine plus varenicline but achieved only very low antibody levels that would not have been expected to show efficacy (Tonstad et al., 2013).
One combination study in rats showed a strong synergistic effect of the nicotine-specific mAb Nic311 in combination with the nicotinic receptor antagonist mecamylamine for reducing nicotine discrimination (LeSage, Shelley, Pravetoni, & Pentel, 2012) (Fig. 14.3B). The mAb Nic311 was used as a surrogate for vaccination. Mecamylamine was used because its blocking of nicotine effects at nAChRs was expected to augment the reduction in nicotine reaching the receptors produced by Nic311. Mecamylamine alone can provide a high degree of receptor blockade but the high doses required cause side effects. The combination blocked nicotine discrimination at mAb Nic311 and mecamylamine doses that by themselves had a modest or no effect, respectively. Although preliminary, these data encourage further study of mechanistically complementary vaccine and medication combinations. They also suggest a potential broader role for vaccines in medication development as a platform for development of pharmacotherapies for tobacco addiction. Vaccines could enhance the efficacy of other types of medications by reducing the brain concentrations of nicotine that compete with a medication for binding to nicotinic receptors or for producing other downstream neural effects. To the extent that this lowers the dose of medication required, vaccines could reduce the side effects of that medication. The excellent safety profile, distinct mechanism of action, and potentially low cost of vaccines make them well suited to serving as a medication development platform.
7. ALTERNATIVES TO VACCINATION
7.1. Passive immunization
The principal limitations of vaccination are the magnitude of the mean antibody response and large individual variability. In addition, vaccines require weeks to months to generate an effective level of antibody, requiring careful planning and timing of smoking cessation attempts. Each of these limitations can be circumvented by passive immunization consisting of the administration of preformed antibodies. Because drug-specific antibodies appear to have no important toxicity, arbitrarily high doses can be used and more uniformly high serum antibody levels achieved. Passive immunization with drug-specific monoclonal antibodies (mAbs) can block the effects of very high doses of phencyclidine (Hardin et al., 2002), methamphetamine (Gentry et al., 2006), nicotine (Carrera et al., 2004; Keyler et al., 2005; LeSage et al., 2012), or cocaine (Fox et al., 1996) in rodents, and a methamphetamine mAb has entered clinical trials (ClinicalTrials.gov, 2013).
The principal drawback of passive immunization is the very high cost of the required doses of mAb. mAbs are widely used to treat cancer or immune disorders, but the doses likely needed to treat tobacco addiction (40–160 mg/kg per dose in rats) are 10–100 times higher (Carrera et al., 2004; Keyler et al., 2005; LeSage et al., 2012). Passively administered antibody also has a somewhat shorter elimination half-life (IgG half-life 3 weeks in humans) (Waldmann & Strober, 1969) than the decline in antibody titer after vaccination (approximate half-life 2 months after nicotine or cocaine vaccines in humans) (Hatsukami et al., 2005), and even fully human antibody can elicit the formation of anti-mAb antibodies that reduce its efficacy or half-life (Presta, 2006). The required dose of drug-specific mAb can perhaps be reduced by combining it with vaccination, administering mAb to only those subjects who have insufficient responses to vaccine alone, but even with this strategy, high mAb doses may still be needed (Cornish et al., 2011; Roiko et al., 2008). Passive immunization is highly effective for blocking nicotine effects in animals but has not been studied in humans.
7.2. Gene transfer
An emerging approach that bypasses the need for either active or passive immunization is the administration of viral vectors containing genes for the expression of drug-specific antibody (Brimijoin, Shen, Orson, & Kosten, 2013; Mingozzi & High, 2011). Adeno-associated virus (AAV) vectors with such sequences can be administered parenterally and become incorporated into the cytoplasm of host cells as plasmids that produce fully formed and functional mAb. Expression of very high levels (up to 1 mg/ml) of nicotine-specific antibody has been demonstrated in mice and nonhuman primates that results in a marked decrease in drug distribution and effects (Hicks et al., 2012). The AAV vectors used are nonreplicating, have low rates of insertion into host DNA, and have shown no important toxicity in initial clinical trials of their use for the transfer of other types of genes (Mingozzi & High, 2011). However, in view of its novelty and the unanticipated toxicities associated with some other types of gene transfer therapies, a high degree of confidence in long-term safety will be needed to allow the use of viral vectors for tobacco addiction treatment. Those addictions that have no other medication therapies available, such as cocaine or meth-amphetamine, may be more attractive initial candidates (Brimijoin et al., 2013).
8. TRANSLATIONAL CONSIDERATIONS
8.1. Adequacy of animal models
Animal models of nicotine vaccines generally involve the administration of nicotine i.v. or s.c. rather than by inhalation and by itself rather than in combination with the thousands of other chemicals present in tobacco and tobacco smoke. One study showed that a nicotine vaccine was equally effective for blocking nicotine distribution to the brain after a single dose of nicotine administered either i.v. or via inhalation of cigarette smoke (Pravetoni et al., 2011). This validates the pharmacokinetic aspects of the i.v. model but does not address the possible behavioral effects of other compounds in tobacco smoke. As such, it is possible that these animal models overestimate the efficacy of vaccines for smoking cessation.
Cigarette smoking occurs in a complex environment of sensory stimuli from smoke inhalation (taste, smell, and “impact” on the respiratory tract), conditioned cues, social influences, and economic and regulatory factors, all of which are important mediators of smoking behavior. Animal models provide only the most basic aspects of some of these, such as a cue light or tone during self-administration procedures. However, all currently approved medications for smoking cessation show activity in animal models of nicotine addiction (Damaj et al., 2010; Le Foll et al., 2012; LeSage, Keyler, Collins, & Pentel, 2003).
There are few data directly addressing whether one or more species of experimental animal is the best predictor of addiction vaccine immunogenicity in humans. Rats and mice are well studied with regard to vaccine immunogenicity and convenient to use for initial vaccine evaluation. It is unclear if, in general, primates offer additional predictive information. Primates are sometimes preferred for infectious disease vaccine studies because the target infections are better modeled in primates than rodents, but nicotine distribution to the brain can be adequately modeled in rats. However, some adjuvants such as certain CpG oligonucleotides are species-specific, and vaccine formulations containing these adjuvants may require testing in primates to better anticipate their immunogenicity in humans (Klinman et al., 2009).
8.2. Design of clinical trials
Phase I clinical trials of nicotine vaccines have focused on establishing an immunogen dose, number of booster doses, and dosing interval. Until the factors controlling vaccine immunogenicity are more fully understood, phase I clinical trials may benefit from examining additional variables such as choice of adjuvant or route of administration. For example, intradermal vaccination is often more immunogenic than intramuscular (La Montagne & Fauci, 2004), and this has now been shown for one nicotine immunogen administered with CpG adjuvant in mice (Chen, Pravetoni, Bhayana, Pentel, & Wu, 2012). Adaptive designs that allow sequential exploration of several of these parameters may prove helpful for optimizing vaccine formulation. Knowing that next-generation vaccines must produce higher serum antibody concentrations than the first-generation vaccines already tested, it is important to identify early in clinical development those immunogens and vaccine formulations with the greatest likelihood of clinical success.
8.3. Individual variability
Strategies for addressing individual variability in vaccine response are needed. With this information, individuals predicted to have a poor response to a particular vaccine could be directed to a different vaccine or to a non-vaccine therapy. No validated predictive biomarkers of nicotine vaccine response are currently available. Cocaine vaccine studies have identified possible effects of HLA type or the presence of low levels of preexisting spontaneous anticocaine antibodies (Orson et al., 2013) as factors in vaccine response. The ability of naive (prevaccination) B cells to bind an opioid hapten in vitro correlates with the magnitude of opioid-specific antibody response to vaccination in mice (M. Pravetoni, personal communication). However, these approaches have not been studied for nicotine vaccines.
8.4. Safety
Nicotine conjugate vaccines have been associated with few serious adverse effects in clinical trials. One subject with a history of penicillin allergy had an anaphylactic reaction after initial vaccination with NicVAX (Hatsukami et al., 2011). Reactogenicity (transient adverse effects associated with vaccine administration) was mild with Niccine (conjugated to tetanus toxoid) or NicVAX (conjugated to recombinant P. aeruginosa exoprotein A) but more marked with NicQb (conjugated to a viruslike particle) (Cornuz et al., 2008; Hatsukami et al., 2011, 2005; Maurer et al., 2005; Tonstad et al., 2013). Side effects from each of these vaccines were seen in control groups as well, so they were likely due to carrier protein and/or adjuvant rather than immunogen. Because all vaccine side effects have been transient, the nicotine-specific antibodies generated by vaccination, which persist in the blood for months, appear to have no adverse effects. The substantially higher levels of nicotine-specific antibody generated in animals by vaccination (Keyler et al., 2008), passive immunization (Keyler et al., 2005), or gene transfer (Hicks et al., 2012) are also well tolerated. This supports the critical premise that it will be safe to generate the higher serum antibody levels needed for nicotine vaccine efficacy. All vaccines studied in clinical trials used alum as adjuvant. While these data are reassuring, new vaccine platforms, nanoparticles, novel adjuvants, and gene transfer strategies will need to be carefully evaluated to establish their safety.
Vaccination does not precipitate nicotine withdrawal. In rats, neither vaccination (Lindblom et al., 2005) nor a very high bolus dose of nicotine-specific mAb (Roiko, Harris, LeSage, Keyler, & Pentel, 2009) elicited withdrawal signs or elevations in the brain reward threshold. No symptoms of withdrawal have been reported in clinical trials of nicotine vaccines (Hartmann-Boyce et al., 2012).
9. CONCLUSION
Abundant opportunities exist for enhancing nicotine vaccine efficacy through optimization of conjugate vaccine components, the use of nanoparticle scaffolds, combining immunogens in a multivalent vaccine, or combining vaccines with other types of medications. Passive immunization or antibody gene transfer offers potential alternatives to vaccination if concerns about cost and long-term safety can be addressed. It is likely that one or more next-generation vaccines or immunotherapies will reliably provide high enough serum nicotine-specific antibody levels to adequately test their efficacy for smoking cessation.
Acknowledgments
We thank Yung Chang for helpful discussions of humoral immune response mechanisms. This work is supported by NIDA grants DA10714 and T32-DA07097.
ABBREVIATIONS
- 3′-AmNic
3-aminonicotine
- AAV
adeno-associated virus
- KLH
keyhole limpet hemocyanin
- nAChR
nicotinic cholinergic receptor
- rEPA
recombinant P. aeruginosa exoprotein A
- Th
T helper
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
References
- Anonymous. The health consequences of smoking: Nicotine addiction, a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Office on Smoking and Health; 1988. [Google Scholar]
- Anonymous. Trial watch: Xenova’s TA-NIC vaccine shows promise. Expert Review of Vaccines. 2004;3:386. doi: 10.1586/14760584.3.4.386. [DOI] [PubMed] [Google Scholar]
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Frontiers in Immunology. 2013;4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Wilkinson JL, Sanderson SD. Vaccines to combat smoking. Expert Opinion on Biological Therapy. 2008;8:379–383. doi: 10.1517/14712598.8.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer PT, Janda KD. Investigating the effects of a hydrolytically stable hapten and a Th1 adjuvant on heroin vaccine performance. Journal of Medicinal Chemistry. 2012;55:10776–10780. doi: 10.1021/jm301262z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Shen X, Orson F, Kosten T. Prospects, promise and problems on the road to effective vaccines and related therapies for substance abuse. Expert Review of Vaccines. 2013;12:323–332. doi: 10.1586/erv.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, et al. Brain nicotinic acetylcholine receptor occupancy: Effect of smoking a den-icotinized cigarette. The International Journal of Neuropsychopharmacology. 2009;12:305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Tsuchikama K, Janda KD. Modulating cocaine vaccine potency through hapten fluorination. Journal of the American Chemical Society. 2013;135:2971–2974. doi: 10.1021/ja400356g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Keyler DE, Pentel PR, Leslie FM. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007;32:2025–2035. doi: 10.1038/sj.npp.1301327. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Hoffman TZ, Isomura S, Wirsching P, Koob GF, et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorganic and Medicinal Chemistry. 2004;12:563–570. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Cerny EH, Levy R, Mauel J, Mpandi M, Mutter M, Henzelin-Nkubana C, et al. Preclinical development of a vaccine ‘against smoking’. Onkologie. 2002;25:406–411. doi: 10.1159/000067433. [DOI] [PubMed] [Google Scholar]
- Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine. 2012;31:159–164. doi: 10.1016/j.vaccine.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. The International Journal of Neuropsychopharmacology. 2009;12:1355–1366. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. Safety study of Ch-mAb7F9 for methamphetamine abuse. 2013 http://clinicaltrialsgov/ct2/show/NCT01603147?term=methamphetamine+antibody&rank=4.
- Cornish KE, Harris AC, LeSage MG, Keyler DE, Burroughs D, Earley C, et al. Combined active and passive immunization against nicotine: Minimizing monoclonal antibody requirements using a target antibody concentration strategy. International Immunopharmacology. 2011;11:1809–1815. doi: 10.1016/j.intimp.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, et al. A vaccine against nicotine for smoking cessation: A randomized controlled trial. PLoS One. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers K, Daly KR, Rimland D, Goetz MB, Gibert CL, Butt AA, et al. Decreased serum antibody responses to recombinant pneumocystis antigens in HIV-infected and uninfected current smokers. Clinical and Vaccine Immunology. 2011;18:380–386. doi: 10.1128/CVI.00421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Grabus SD, Navarro HA, Vann RE, Warner JA, King LS, et al. Effects of hydroxymetabolites of bupropion on nicotine dependence behavior in mice. Journal of Pharmacology and Experimental Therapeutics. 2010;334:1087–1095. doi: 10.1124/jpet.110.166850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De BP, Pagovich OE, Hicks MJ, Rosenberg JB, Moreno AY, Janda KD, et al. Disrupted adenovirus-based vaccines against small addictive molecules circumvent anti-adenovirus immunity. Human Gene Therapy. 2013;24:58–66. doi: 10.1089/hum.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers SH, Cornish KE, Troska AJ, Pravetoni M, Pentel PR. Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine. 2013;31:6185–6193. doi: 10.1016/j.vaccine.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers SH, Lindblom N, Kalayanov G, Gordon S, Baraznenok I, Malmerfelt A, et al. Nicotine hapten structure, antibody selectivity and effect relationships: Results from a nicotine vaccine screening procedure. Vaccine. 2010;28:2161–2168. doi: 10.1016/j.vaccine.2009.12.051. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Hannestad JO, Perkins E, Bois F, D’Souza DC, Tyndale RF, et al. Effect of a nicotine vaccine on nicotine binding to beta2*-nicotinic acetylcholine receptors in vivo in human tobacco smokers. The American Journal of Psychiatry. 2013;170:399–407. doi: 10.1176/appi.ajp.2012.12060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim RE, Kessler PD, Kalnik MW. Therapeutic vaccines against tobacco addiction. Expert Review of Vaccines. 2013;12:333–342. doi: 10.1586/erv.13.13. [DOI] [PubMed] [Google Scholar]
- Finklea JF, Hasselblad V, Riggan WB, Nelson WC, Hammer DI, Newill VA. Cigarette smoking and hemagglutination inhibition response to influenza after natural disease and immunization. American Review of Respiratory Disease. 1971;104:368–376. doi: 10.1164/arrd.1971.104.3.368. [DOI] [PubMed] [Google Scholar]
- Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nature Medicine. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- Gelal A, Balkan D, Ozzeybek D, Kaplan YC, Gurler S, Guven H, et al. Effect of menthol on the pharmacokinetics and pharmacodynamics of felodipine in healthy subjects. European Journal of Clinical Pharmacology. 2005;60:785–790. doi: 10.1007/s00228-004-0847-8. [DOI] [PubMed] [Google Scholar]
- Gentry WB, Laurenzana EM, Williams DK, West JR, Berg RJ, Terlea T, et al. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. International Immunopharmacology. 2006;6:968–977. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- George TP, Weinberger AH. Monoamine oxidase inhibition for tobacco pharmacotherapy. Clinical Pharmacology and Therapeutics. 2008;83:619–621. doi: 10.1038/sj.clpt.6100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Pal S, Nangreave J, Deng Z, Liu Y, Yan H. DNA origami with complex curvatures in three-dimensional space. Science. 2011;332:342–346. doi: 10.1126/science.1202998. [DOI] [PubMed] [Google Scholar]
- Hardin JS, Wessinger WD, Wenger GR, Proksch JW, Laurenzana EM, Owens SM. A single dose of monoclonal anti-phencyclidine IgG offers long-term reductions in phencyclidine behavioral effects in rats. Journal of Pharmacology and Experimental Therapeutics. 2002;302:119–126. doi: 10.1124/jpet.302.1.119. [DOI] [PubMed] [Google Scholar]
- Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J. Nicotine vaccines for smoking cessation. Cochrane Database of Systematic Reviews. 2012;8:CD007072. doi: 10.1002/14651858.CD007072.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clinical Pharmacology and Therapeutics. 2011;89:392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical Pharmacology and Therapeutics. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hicks MJ, Rosenberg JB, De BP, Pagovich OE, Young CN, Qiu JP, et al. AAV-directed persistent expression of a gene encoding anti-nicotine antibody for smoking cessation. Science Translational Medicine. 2012;4:140ra87. doi: 10.1126/scitranslmed.3003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, Ennifar S, Fattom A, Pentel PR. Vaccination against nicotine during continued nicotine administration in rats: Immunogenicity of the vaccine and effects on nicotine distribution to brain. International Journal of Immunopharmacology. 2000;22:809–819. doi: 10.1016/s0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Hoogsteder PH, Kotz D, van Spiegel PI, Viechtbauer W, Brauer R, Kessler PD, et al. The efficacy and safety of a nicotine conjugate vaccine (NicVAX(R)) or placebo co-administered with varenicline (Champix(R)) for smoking cessation: Study protocol of a phase IIb, double blind, randomized, placebo controlled trial. BMC Public Health. 2012;12:1052. doi: 10.1186/1471-2458-12-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura S, Wirsching P, Janda KD. An immunotherapeutic program for the treatment of nicotine addiction: Hapten design and synthesis. Journal of Organic Chemistry. 2001;66:4115–4121. doi: 10.1021/jo001442w. [DOI] [PubMed] [Google Scholar]
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Benlhabib E, LeSage MG, St Peter JV, Stewart S, et al. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: Dose- and affinity-response relationships. Drug Metabolism and Disposition. 2005;33:1056–1061. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. Enhanced immunogenicity of a bivalent nicotine vaccine. International Immunopharmacology. 2008;8:1589–1594. doi: 10.1016/j.intimp.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Altreuter D, Johnston L, Keller P, Pittet L. SEL-068 A fully synthetic nanoparticle vaccine for smoking cessation and relapse prevention. SRNT 2012 Annual Meeting; Houston. 2012. [Google Scholar]
- Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Advanced Drug Delivery Reviews. 2009;61:248–255. doi: 10.1016/j.addr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Gonsai K, St Clair Roberts J, Jack L, Bond J, Mitchell E, et al. Phase II human study of cocaine vaccine TA-CD. CPDD Annual Meeting; Quebec City. 2002. [Google Scholar]
- La Montagne JR, Fauci AS. Intradermal influenza vaccination—Can less be more? New England Journal of Medicine. 2004;351:2330–2332. doi: 10.1056/NEJMe048314. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pre-treatment time is used. The International Journal of Neuropsychopharmacology. 2012;15:1265–1274. doi: 10.1017/S1461145711001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: Relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology. 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology. 2006;184:409–416. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: Vaccines and nicotine-specific antibodies. The AAPS Journal. 2006;8:E65–E75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Pravetoni M, Pentel PR. Enhanced attenuation of nicotine discrimination in rats by combining nicotine-specific antibodies with a nicotinic receptor antagonist. Pharmacology, Biochemistry, and Behavior. 2012;102:157–162. doi: 10.1016/j.pbb.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom N, de Villiers SH, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active immunization against nicotine prevents reinstatement of nicotine-seeking behavior in rats. Respiration; International Review of Thoracic Diseases. 2002;69:254–260. doi: 10.1159/000063629. [DOI] [PubMed] [Google Scholar]
- Lindblom N, de Villiers SH, Semenova S, Kalayanov G, Gordon S, Schilstrom B, et al. Active immunisation against nicotine blocks the reward facilitating effects of nicotine and partially prevents nicotine withdrawal in the rat as measured by dopamine output in the nucleus accumbens, brain reward thresholds and somatic signs. Archives of Pharmacology. 2005;372:182–194. doi: 10.1007/s00210-005-0019-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Xu Y, Yu T, Clifford C, Liu Y, Yan H, et al. A DNA nanostructure platform for directed assembly of synthetic vaccines. Nano Letters. 2012;12:4254–4259. doi: 10.1021/nl301877k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockner JW, Ho SO, McCague KC, Chiang SM, Do TQ, Fujii G, et al. Enhancing nicotine vaccine immunogenicity with liposomes. Bioorganic & Medicinal Chemistry Letters. 2013;23:975–978. doi: 10.1016/j.bmcl.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas GR, Mayorov AV, Rice KC, Jacobson AE, Cheng K, Iyer MR, et al. Liposomes containing monophosphoryl lipid A: A potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine. 2013;31:2804–2810. doi: 10.1016/j.vaccine.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, et al. A therapeutic vaccine for nicotine dependence: Preclinical efficacy, and Phase I safety and immunogenicity. European Journal of Immunology. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- McCluskie MJ, Pryde DC, Gervais DP, Stead DR, Zhang N, Benoit M, et al. Enhancing immunogenicity of a 3′ aminomethylnicotine-DT-conjugate anti-nicotine vaccine with CpG adjuvant in mice and non-human primates. International Immunopharmacology. 2013;16:50–56. doi: 10.1016/j.intimp.2013.03.021. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annual Review of Immunology. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, et al. Raxibacumab for the treatment of inhalational anthrax. The New England Journal of Medicine. 2009;361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nature Reviews Genetics. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Azar MR, Koob GF, Janda KD. Probing the protective effects of a conformationally constrained nicotine vaccine. Vaccine. 2012;30:6665–6670. doi: 10.1016/j.vaccine.2012.08.064. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Molecular Pharmaceutics. 2010;7:431–441. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- Orson FM, Kinsey BM, Singh RA, Wu Y, Gardner T, Kosten TR. The future of vaccines in the management of addictive disorders. Current Psychiatry Reports. 2007;9:381–387. doi: 10.1007/s11920-007-0049-z. [DOI] [PubMed] [Google Scholar]
- Orson FM, Rossen RD, Shen X, Lopez AY, Wu Y, Kosten TR. Spontaneous development of IgM anti-cocaine antibodies in habitual cocaine users: Effect on IgG antibody responses to a cocaine cholera toxin B conjugate vaccine. The American Journal on Addictions. 2013;22:169–174. doi: 10.1111/j.1521-0391.2013.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Advanced Drug Delivery Reviews. 2008;60:915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentel PR, Dufek MB, Roiko SA, Lesage MG, Keyler DE. Differential effects of passive immunization with nicotine-specific antibodies on the acute and chronic distribution of nicotine to brain in rats. The Journal of Pharmacology and Experimental Therapeutics. 2006;317:660–666. doi: 10.1124/jpet.105.097873. [DOI] [PubMed] [Google Scholar]
- Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, Lake JR, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacology, Biochemistry, and Behavior. 2000;65:191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Pravetoni M, Keyler DE, Pidaparthi RR, Carroll FI, Runyon SP, Murtaugh MP, et al. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochemical Pharmacology. 2012;83:543–550. doi: 10.1016/j.bcp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Keyler DE, Raleigh MD, Harris AC, Lesage MG, Mattson CK, et al. Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochemical Pharmacology. 2011;81:1164–1170. doi: 10.1016/j.bcp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Advanced Drug Delivery Reviews. 2006;58:640–656. doi: 10.1016/j.addr.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Raupach T, Hoogsteder PH, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: Current status of research. Drugs. 2012;72:e1–e16. doi: 10.2165/11599900-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, Keyler DE, Lesage MG, Zhang Y, Pentel PR. Combined active and passive immunization enhances the efficacy of immunotherapy against nicotine in rats. Journal of Pharmacology and Experimental Therapeutics. 2008;325:985–993. doi: 10.1124/jpet.107.135111. [DOI] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, LeSage MG, Keyler DE, Pentel PR. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacology, Biochemistry, and Behavior. 2009;93:105–111. doi: 10.1016/j.pbb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman C, Truedsson L, Kapetanovic MC. The effect of smoking and alcohol consumption on markers of systemic inflammation, immunoglobulin levels and immune response following pneumococcal vaccination in patients with arthritis. Arthritis Research and Therapy. 2012;14:R170. doi: 10.1186/ar3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson SD, Cheruku SR, Padmanilayam MP, Vennerstrom JL, Thiele GM, Palmatier MI, et al. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. International Immunopharmacology. 2003;3:137–146. doi: 10.1016/s1567-5769(02)00260-6. [DOI] [PubMed] [Google Scholar]
- Satoskar SD, Keyler DE, LeSage MG, Raphael DE, Ross CA, Pentel PR. Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. International Immunopharmacology. 2003;3:957–970. doi: 10.1016/S1567-5769(03)00094-8. [DOI] [PubMed] [Google Scholar]
- Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clinical Pharmacology and Therapeutics. 2012;91:60–70. doi: 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreafico R, Ricciardi-Castagnoli P, Mortellaro A. The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. European Journal of Immunology. 2010;40:638–642. doi: 10.1002/eji.200940039. [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Jenkins MK, Pape KA. Heterogeneity in the differentiation and function of memory B cells. Trends in Immunology. 2012;33:590–597. doi: 10.1016/j.it.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonstad S, Heggen E, Giljam H, Lagerback PA, Tonnesen P, Wikingsson LD, et al. Niccine(R), a nicotine vaccine, for relapse prevention: A Phase II, randomized, placebo-controlled, multicenter clinical trial. Nicotine & Tobacco Research. 2013;15(9):1492–1501. doi: 10.1093/ntr/ntt003. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Strober W. Metabolism of immunoglobulins. Progress in Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: Current challenges and future approaches. Journal of Pharmaceutical Sciences. 2009;98:1278–1316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter AP, Follett EA, McIntyre J, Stewart J, Symington IS. Influence of smoking on immunological responses to hepatitis B vaccine. Vaccine. 1994;12:771–772. doi: 10.1016/0264-410x(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Zaman M, Good MF, Toth I. Nanovaccines and their mode of action. Methods. 2013;60:226–231. doi: 10.1016/j.ymeth.2013.04.014. [DOI] [PubMed] [Google Scholar]