Abstract
Background:
Acute hyperglycemia, hypoglycemia and glycemic variability (GV) have been found to be the three principal domains of glycemic control, which can adversely affect patient outcome. GV may be the confounding factor in tight glycemic control trials in surgical and medical patient.
Objective:
This study was conducted to establish if there was any relationship between GV and intensive care unit (ICU) mortality in the Indian context.
Study Design:
A retrospective review of a large cohort of prospectively collected database.
Setting:
Adult Medical/Surgical/Trauma/Neuro ICU of a tertiary care hospital.
Patient Population:
All patients who had four or more blood glucose measured during the ICU stay.
Outcome:
ICU mortality.
Result:
2208 patients with a total of 11,335 blood glucose values were analyzed. GV measured by the standard deviation (SD) of mean blood glucose and glycemic lability index (GLI), both were significantly (P < 0.001) associated with ICU mortality. This relationship was maintained (odds ratio (OR): 2.023, 95% confidence interval (CI): 1.483-2.758) even after excluding patients with hypoglycemia (<60 mg/dl). Patients with blood glucose values in the euglycemic range but highest SD had higher mortality (54%) compared to mortality (24%) in patients above the euglycemic range. Similarly patients with blood sugar values below the average for study cohort and high GLI, another marker of GV had higher mortality (OR: 5.62, CI: 3.865-8.198) than compared to patients in the hyperglycemic range, reflecting the importance of GV as a prognostic marker in patients with blood sugar in the euglycemic range.
Conclusion:
This study demonstrated that high glucose variability is associated with increased ICU mortality in a large heterogeneous cohort of ICU patients. This effect was particularly evident among patients in the euglycemic range.
Keywords: Critically ill, glycemic lability index, glycemic variability, hypoglycemia, intensive care unit mortality
Introduction
Three domains of glycemic control, acute hyperglycemia, hypoglycemia and glycemic variability (GV) are an important marker of prognostication in critically ill patient. GV may be the confounding factor in the various tight glycemic control studies in medical and surgical patients which did not take this domain of prognostication into account and measured mean blood sugar as their target variable.[1,2,3,4,5] It is likely that apart from acute hyperglycemia which is an established adverse prognostic factor in many subsets of critically ill, glycemic excursion during intensive care unit (ICU) stay may also become a major indicator of a bad outcome.[6,7,8,9]
Observational studies have shown that increased GV measured by various indices is detrimental in acutely ill patients. Hence factors other than mean blood glucose (MBG), like glycemic excursion or glucose variability need to be explored in a heterogeneous ICU population.
Materials and Methods
The study was conducted in Medical/Surgical/Neuro/Trauma ICU of a 400 bed tertiary care hospital in Eastern India during the period from January 2009 to November 2009. This was a retrospective review of a large cohort of prospectively collected database. Two full time dedicated nurses collected data prospectively on every patient. All patients with four or more blood glucoses measured during their ICU stay during the study period were included. A total of 2,528 patients were admitted in ICU from January 2009 to November 2009. 2,208 patients were finally included in the analysis. Rest of the patients (n = 320) were excluded because they had less than four capillary blood glucose (CBG) readings during the ICU stay. Diabetic status was not retrieved during data collection.
Blood glucoses were checked as per patient requirement, both by point of care testing, with bedside finger prick capillary glucose measurement and also in central biochemistry laboratory when necessary. ICU nurses had an orientation program which included training in bedside glucose estimation and ICU insulin protocol training during in service as a hospital policy. Interobserver variability was not ascertained during the study. Cross checking with central laboratory was left to physician discretion and this data was not retrieved during analysis. The study was conducted in a semiclosed ICU with mandatory intensivist consult. An ICU insulin protocol was utilized in all patients included in the study thereby limiting heterogeneity of insulin delivery.
A nurse- driven insulin protocol using both subcutaneous and intravenous insulin was followed in the ICU. This protocol has been modified from Yale protocol[10] of insulin infusion. Type of insulin and route of administration was left to the discretion of the treating physician. All patients in shock were treated with intravenous insulin, subcutaneous route was only used in patients who were less sick and in those who has recovered from shock state, prior to transfer to wards.
Demographics of the study population (age, admission diagnosis, acute physiology and chronic health evaluation IV, ICU mortality and ICU length of stay) were retrieved from ICU database which has been collected prospectively. MBG was measured for each patient and GV was calculated as a standard deviation (SD) and glycemic lability index (GLI)[11] of MBG. The SD determines how tightly all the various glucose values are clustered around the mean in a set of data. GLI takes into account frequency of glucose measurement. GLI was measured by the formula GLI = ([mg/dl]2 /h)/week. Each consecutive blood sugar difference was calculated and squared (mg/dl)2 and was divided by the time interval between these two readings. Then, the mean of those values within each week were calculated to compute representative index value for that week. Then, the mean of those representative indices (if stay more than a week) was calculated to obtain the GLI for that patient, frequency of blood glucose estimation was left to the treating physician, but the time of every measurement was recorded in the nursing chart which was retrieved during analysis. Desirable glucose range for the individual patient was left to the discretion of the treating physician.
The entire study cohort was divided into 10 equal deciles (D1-D10) of 200 patients each, in accordance to the value of SD and GLI. For example decile one (D1) had first 200 patients with a SD range in the lowest values (0-8.56) and so forth. This was an unadjusted analysis.
In order to assess the effect of GV across the different range of blood glucose, MBG of the study population was divided into five sub groups of blood glucose in mg/dl (<99, 100-119, 120-139, 140-179, 180-180+). GV measured by SD in each of the five subgroups was divided into quartiles and correlation with mortality was determined. These categories were determined as per Krinsley paper.[12]
For the same purpose, patients were also separated into two groups of below and above the median glucose value for the whole cohort and relationship with GLI and mortality was also observed.
As hypoglycemia (CBG <60 mg/dl), has been identified as an independent predictor of mortality, relationship of GV determined by SD was analyzed for association with mortality excluding hypoglycemia patient.
Blood glucose between 40 and 70 mg/dl and <40 mg/dl were associated with increased mortality.[13] Separate analysis of patient with blood sugar <40 mg/dl was not done.
P < 0.05 was considered to indicate statistical significance. Logistic regression models were also used for SD and GLI to check their associations with mortality rates in ICU.
Institutional review board (ethical committee) of study institution approved the study and waived informed consent requirement due to the observational nature of the study.
Results
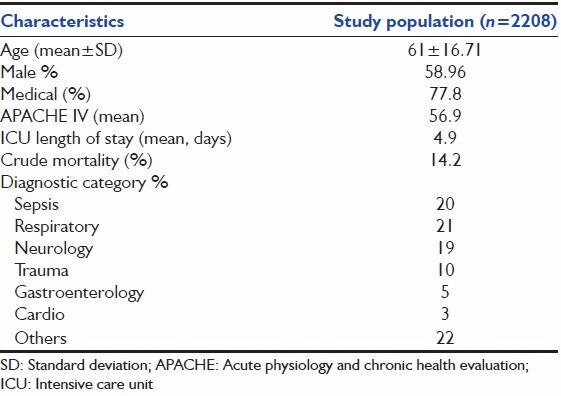
A total of 2,528 patients were admitted in ICU from January 2009 to November 2009. 2,208 patients were finally included in the analysis. Rest of the patients (n = 320) were excluded because they had less than four CBG readings during the ICU stay. Demographics of the study population are given in Table 1. The average age of the study population was 61 years with equal male and female representation. The predominant patient population was medical, with sepsis and respiratory diseases as major admitting diagnoses. The ICU mortality of the study population was 14% with an average length of ICU stay of 5 days.
Table 1.
Demographics of the study population
A total of 11,335 blood glucose records were analyzed from 2,208 patients during the study period. MBG for each patient with SD and GLI was computed. Patients in lowest decile (D1) with an average SD of 5.7 had the lowest mortality (6%) and patients in the highest decile (D10) with an average SD of 94.48 had the highest mortality (23%). Thus, the deciles (D1-D10) of the study population showed increasing ICU mortality as 6, 6, 8, 8, 13, 12, 18, 22, 21 and 23% respectively with increasing SD in each decile [Figure 1]. The deciles of increasing GLI similarly showed increasing ICU mortality as 5, 7, 5, 9, 12, 11, 19, 21, 24 and 24% respectively in each decile with average GLI 6.34 in the lowest decile (D1) and 24.72 in the highest decile (D10) [Figure 2].
Figure 1.
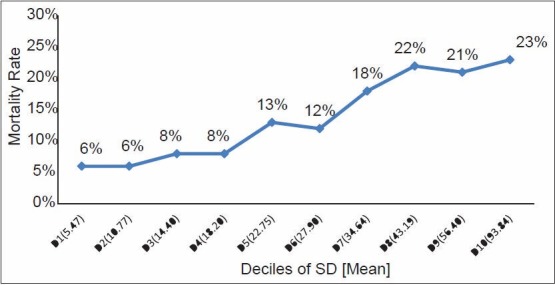
Relation of mortality in relation to deciles of SD
Figure 2.
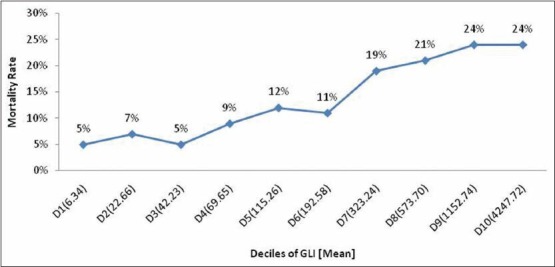
Relation of mortality in relation to deciles of GLI
When separate logistic regression models were fitted to these two indices (SD and GLI) to check their associations with mortality rates in ICU, both the models came out to be significant (P = 0.001). This was an unadjusted risk analysis.
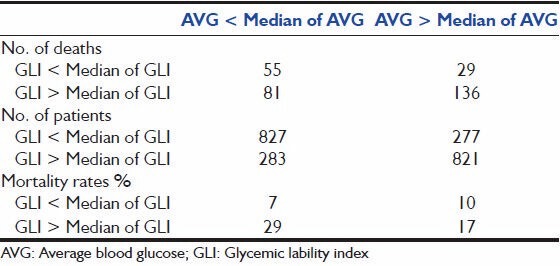
In order to assess the effect of GV across the range of blood glucoses, MBG of the study population was divided into five subgroups in mg/dl (<99, 100-119, 120-139, 140-179 and 180+). Within each subgroup SD was divided into four quartiles and association with mortality was computed. Patients in the highest quartile of SD fared worst when compared to patients in the lower three quartiles of SD across all ranges of blood sugar values. In the patient population with blood sugars in the lowest range (<99 mg/dl) mortality was highest in the fourth quartile signifying GV is more important at lower blood sugar values [Figure 3].[3,4] This observation was further confirmed by correlating MBG above and below the median range with GLI above and below the median range for the whole cohort. Mortality was highest (29%) in the subgroup of patient with GLI above the median and MBG below the median range. (Odds ratio (OR): 5.62: 95% confidence interval (CI): 3.865-8.198). The mortality was lowest (7%) in patient subgroup with MBG < Median and GLI < Median, signifying tight glucose control along with least variability has the best outcome [Table 2].
Figure 3.
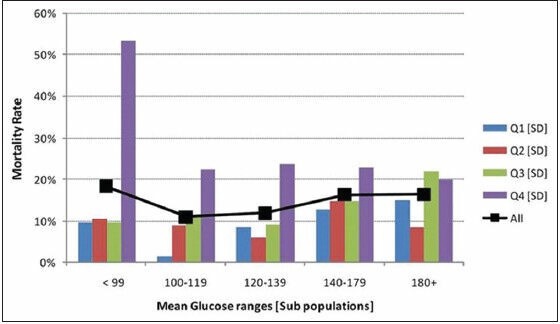
Mortality rates in quartile ranges of SD (Q1-Q4: Quartiles; SD: Standard deviation)
Table 2.
Mortality rates in patients with above and below median GLI
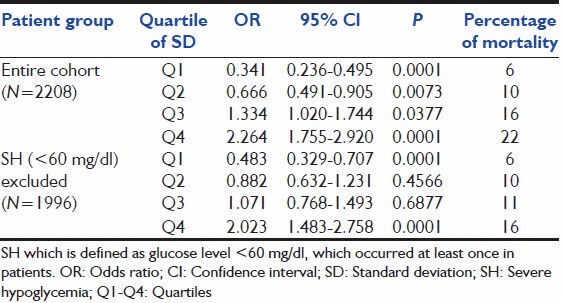
In our study cohort 212 patients (9.6%) had hypoglycemia (CBG <60 mg/dl) of which 84 patients died (39% mortality). As previous studies have shown that hypoglycemia was a strong predictor of mortality we reanalyzed the study cohort after excluding patients with hypoglycemia to assess whether GV was an independent predictor of mortality when hypoglycemia was excluded as a confounding factor. 1,996 patient dataset (excluding those with hypoglycemia) were analyzed for MBG and SD The latter was divided into four quartiles. In this cohort also mortality increased from 6, 10, 11, 16% respectively with rising SD, a result similar to the whole cohort. The relation with mortality reached statistical significance (OR: 2.023, 95% CI: 1.483-2.758) in the highest quartiles of SD [Table 3].
Table 3.
Analysis of mortality excluding cohort with hypoglycemia
Discussion
Studies on glucose control in ICU have historically used control of mean or morning blood sugar as their therapeutic target. Discordant results from various glucose control studies in ICU population, has shifted attention towards GV as a potential confounding factor in these studies GV has been found to be detrimental in chronic ambulatory diabetic patient population[14] but its adverse effect in an acute situation has not been well appreciated. It has been shown that rapid fluctuations of blood glucoses increase oxidative stress and is more detrimental than sustained hyperglycemia.[15] Apart from oxidative stress GV can also induce neuronal damage, mitochondrial damage and increase coagulation activity, which are detrimental to acutely ill patient.[16]
Previous studies of GV in acutely ill have been conducted in various patient populations and have used different indices of GV.[17] Meyfroidt et al.[18] in a retrospective study, analyzed Leuven database and noted SD of blood glucose in medical/surgical ICU patient to be significantly associated with hospital mortality irrespective of blood glucose level. Hermanides et al.[19] studied SD and mean absolute glucose in another retrospective study and found these to be significantly associated with ICU and Hospital mortality which was more evident in patients with high MBG. Krinsley[12] in a retrospective chart review in a large heterogeneous ICU population inferred that GV measured as SD was a strong independent predictor of ICU mortality and was more evident in the euglycemic range. They showed a five-fold mortality difference comparing patients from the lowest and highest quartiles of GV within the “best” glycemic range (MBG 70 mg/dl to 99 mg/dl), an observation similar to our study. Ali et al.[20] in the retrospective study looked at GV in a septic cohort, with measures of SD, GLI and mean amplitude of glycemic excursion and came at a similar conclusion. Egi et al.[21] also studied SD as a measure of GV in a large cohort of 7,049 surgical patients and found it to be associated independently with hospital mortality.
The most important observation in our study is that GV is independently associated with increased ICU mortality. This effect was independent of hypoglycemia. Moreover, highest GV in the euglycemic range was associated with the highest mortality similar to observations of Krinsley and Ali et al. Variability could be related to patient factors such as increased insulin resistance or treatment of low blood sugar values with intravenous dextrose resulting in fluctuations of glucose values. In our study, it is apparent that there were less risk associated when both hyperglycemia and hypervariability occur together and lowest risk with tight blood sugar control and least GV. However, it is less clear what was responsible for increased mortality with greater GV in patients with euglycemia.
In our cohort, the occurrence of hypoglycemia (<60 mg/dl) was 9.6%. The incidence higher as we have taken the cut-off of the blood glucose value of <60 mg/dl, compared to that of Egi et al. (7%) and of Van den Berghe et al. who found that 5.1% of patients who received strict glucose control and experienced at least one glucose value <40 mg/dl. In an observational study[13] the incidence of in moderately hypoglycemic group in the conventionally treated arm (blood sugar 40-70 mg/dl) was 15.8% and in severely hypoglycemic group (blood sugar <40 mg/dl) was 0.5%.
Advantages of our study is the large sample size of a heterogeneous critically ill patient, the prospective nature of the data collection, availability of all glucose values of the study cohort and protocolized insulin delivery. The study was conducted in a semiclosed ICU with mandatory intensivist consult. An ICU insulin protocol was utilized in all patients included in the study. Formal assessment of compliance was not collected during the study, which is a limitation of observational nature of the study.
The cohort included only patients with four or more venous glucose samples, so it excluded short stay who may either be less ill or very sick and died within a day, which are the patient subset unlikely to benefit from glucose control. Standardized indices of GV like SD and GLI were analyzed and analysis of the study population after excluding hypoglycemic cohort in order to avoid a major confounder in previous studies. Hypoglycemia was the only confounder analyzed during the study similar to Krinsley paper.[12]
Limitations of our study is of a single center study, absence of protocolized frequency of testing of blood glucose which will influence interpatient and intrapatient variability, predominant medical patient population and exclusion of cardiac and cardiovascular surgery patients. Glycemic ranges have been customarily divided into quartile.[12] GV matrices like SD and GLI are being divided into deciles and correlated with mortality. Each decile was tabulated to include approximately same number of patients. In a non-interventional study uniform frequency of blood glucose measurement could not be enforced. Another limitation of our study was that the diabetic patients were not separately analyzed as GV may have a different effect in this patient population. The findings can be extrapolated to all critically ill patients irrespective of diabetic status as observed by Krinsley. It is conceivable a separate subset analysis of diabetics may yield different results.[12]
Our study and others have firmly established the association of GV with ICU mortality though it is not clear yet whether this is an epiphenomenon or there is a causal relationship. In this study, single database was analyzed to provide three separate information. Firstly increasing GV was significantly associated with increasing mortality across the ranges of blood sugar (expressed in quartiles), secondly different matrices of GV (i.e. SD and GLI are comparable and thirdly GV has worst outcome in patients with sugar in the euglycemic range. These information were previously analyzed in different databases in separate papers. This study also adds to the growing literature of GV in ICU and is the first study of its kind in Indian population. The mechanisms of GV are also not clear and the exact way in which it is detrimental to this patient population is yet to be explained. Pending further research in these fundamental aspects of glycemic control there is enough evidence now to support a policy of measuring some indices of GV in ICUs and apart from keeping MBG in a desirable range, insulin delivery should be protocolized to minimize the fluctuation of blood glucose and should become a quality control measure in ICUs. In fact, there is an urgent need to device an insulin delivery protocol which not only keeps the blood glucose in the desirable range but also minimizes variability. GV indices should be reanalyzed in the database of the randomized studies of tight glucose control conducted so far to elucidate whether GV could have been a confounding variable in these studies. Further studies incorporating continuous glucose monitoring technology should be carried out to capture GV more accurately and finally a randomized, multicenter, study with adequate sample size of heterogenous critically ill patient and mortality as the outcome measure should be conducted with a goal of similar average glucose in both arms but an insulin protocol to favor decreased GV in one arm and present standard of care in the other. This type of study will shed more light on the causative nature of GV as an independent risk factor for mortality.
Conclusion
In summary, this study demonstrated that GV is associated with increased ICU mortality in a large heterogeneous cohort of ICU patients. This effect was particularly evident among patients in the euglycemic range. No prospective RCT to support it. No guideline has proposed it as a gold standard. There is growing evidence but one cannot stretch it too far !.
Acknowledgment
We would like to express to our gratitude to Dr. Sujoy Ghosh, Consultant Endocrinologist, Dr. Subhankar Chowdhry, Consultant Endocrinologist and Dr. Arghya Majumadar, Consultant Nephrologist for their valuable opinion in drafting the manuscript. We also express our thanks to Mr. Tapas Kayal our Librarian for his secretarial assistance and Statlead Corp for statistical assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 3.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 4.Devos P, Preiser JC, Melot C. Impact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycaemia: Final results of the glucontrol study. Intensive Care Med. 2007;33(Suppl 2):S189. [Google Scholar]
- 5.Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 6.Chi A, Lissauer ME, Kirchoffner J, Scalea TM, Johnson SB. Effect of glycemic state on hospital mortality in critically ill surgical patients. Am Surg. 2011;77:1483–9. doi: 10.1177/000313481107701138. [DOI] [PubMed] [Google Scholar]
- 7.Foo K, Cooper J, Deaner A, Knight C, Suliman A, Ranjadayalan K, et al. A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Heart. 2003;89:512–6. doi: 10.1136/heart.89.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RL, Lin JC, Adelson PD, Kochanek PM, Fink EL, Wisniewski SR, et al. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2012;13:85–91. doi: 10.1097/PCC.0b013e3182192c30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–8. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care. 2004;27:461–7. doi: 10.2337/diacare.27.2.461. [DOI] [PubMed] [Google Scholar]
- 11.Zuo YY, Kang Y, Yin WH, Wang B, Chen Y. The association of mean glucose level and glucose variability with intensive care unit mortality in patients with severe acute pancreatitis. J Crit Care. 2012;27:146–52. doi: 10.1016/j.jcrc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Krinsley JS. Glycemic variability: A strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–13. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 13.Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, et al. NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–18. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 14.Di Flaviani A, Picconi F, Di Stefano P, Giordani I, Malandrucco I, Maggio P, et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care. 2011;34:1605–9. doi: 10.2337/dc11-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CM, Hsieh CJ, Huang JC, Huang IC. Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus. Acta Diabetol. 2012;49(Suppl 1):S171–7. doi: 10.1007/s00592-012-0398-x. [DOI] [PubMed] [Google Scholar]
- 16.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 17.Eslami S, Taherzadeh Z, Schultz MJ, Abu-Hanna A. Glucose variability measures and their effect on mortality: A systematic review. Intensive Care Med. 2011;37:583–93. doi: 10.1007/s00134-010-2129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: Effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38:1021–9. doi: 10.1097/CCM.0b013e3181cf710e. [DOI] [PubMed] [Google Scholar]
- 19.Hermanides J, Bosman RJ, Vriesendorp TM, Dotsch R, Rosendaal FR, Zandstra DF, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med. 2010;38:1430–4. doi: 10.1097/CCM.0b013e3181de562c. [DOI] [PubMed] [Google Scholar]
- 20.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244–52. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Ali NA, O'Brien JM, Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–21. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]


