Abstract
Background:
Organophosphorus poisoning remains an important cause of morbidity and mortality, but no definite parameters have been identified as predictors of outcome. Prediction of morbidity at presentation might help in decision making in places of limited resources like rural settings in developing countries.
Materials and Methods:
A total of 76 cases were included in this retrospective cohort study. Logged relative risk of requirement of mechanical ventilation and hospital stay >7 days was measured in patients with serum acetylcholinesterase (s. acetylcholinesterase) <1000 versus >1000, presenting in <2 h versus ≥ 2 h after exposure, with Glasgow Coma Scale (GCS) ≤12 versus >12 and in patients with SpO2 <85% versus ≥85% at room air at presentation.
Results:
S. acetylcholinesterase <1000, time elapsed after ingestion to presentation ≥ 2 h and SpO2 (at room air) at presentation <85% were found to have positive association with requirement of ventilation. GCS ≤ 12 had a significant association with both requirement of ventilation and hospital stay >7 days.
Conclusion:
S. acetylcholinesterase, SpO2 at room air, GCS, and duration of exposure at presentation can be used to identify the requirement of special care in acute organophosphorus poisoning. This can aid in decision making regarding admission to intensive care unit and referral in the places with limited resources.
Keywords: Acute organophosphorus poisoning, glasgow coma scale, morbidity, serum acetylcholinesterase, SpO2, time elapsed after exposure
Introduction
Organophosphate (OP) poisoning is an important cause of poisoning all over the world. OPs inhibit the enzyme acetylcholinesterase resulting in excessive acetylcholine accumulation, which affects muscarinic and nicotinic receptors at synapses within the peripheral and central nervous systems.[1] It causes neurotoxic sequelae and has a high mortality rate.[2,3,4] Many patients have respiratory failure and cardiorespiratory arrests after admission.[5] It is estimated to kill around 200,000 people each year, largely in the Asia-Pacific region.[6] The World Health Organization estimated that the incidence of pesticide poisoning in developing countries doubled during 10 years period from 1990.[6] There is 10-20% case fatality rate in developing countries compared with much lower fatality in developed countries.[7] OP poisoning is also of great interest to developed countries vulnerable to terrorist or military attack with nerve agents.[8] A number of systems have been proposed for predicting outcome in OP poisoning. Many are reliant on laboratory tests.[9,10,11,12,13] Others that use clinical parameters have been validated using small numbers of patients.[14]
In this study, we intended to observe if morbidity in terms of requirement of ventilation and hospital stay can be assessed from clinical parameters at presentation. We also tried to identify the levels of these parameters at which they indicate chances of significant morbidity and mortality. This might enable clinicians to identify patients in need of intensive monitoring and treatment. This will be of great help in resource constrained places in decision making regarding admission to intensive care unit (ICU) or keeping under observation or referring to a higher center.
Materials and Methods
This retrospective cohort study was conducted at a city in Western India. This hospital receives patients from surrounding areas of Waghodia as well as from adjoining states; MP and Rajasthan. The area surrounding our center is mostly agricultural therefore, OPs are widely used in fields and thus are readily available. The patients presenting with suspected poisoning are subject to routine investigations as well as serum acetylcholinesterase (s. acetylcholinesterase) levels and are treated according to the standard protocol i.e., gastric lavage, atropine infusion until the signs of atropinization appear, pralidoxime (6-8 g/day in three divided doses for 3 days) and mechanical ventilation when indicated.[15]
The study included patients admitted during May 2009 to October 2012. All patients admitted with a history of ingestion or inhalation of organophosphorus compounds (as indicated by the patient or relatives, the transferring doctor, or the pesticide bottle) and clinical manifestations (constricted pupils, diarrhea and vomiting, crepitations, altered sensorium) were included in the study. The information on initial parameters at presentation such as pulse, blood pressure, total leucocyte count (TLC), SpO2 at room air, s. acetylcholinesterase (reference range 3000-8000 IU/L), time elapsed after ingestion/inhalation to presentation and Glasgow Coma Scale (GCS) was collected from the records. The necessary permission was taken from the institutional ethics committee.
Statistical analysis
A total of 76 cases were included in the study. The requirement of ventilator and hospital stay were taken as indicators of morbidity. Relative risk (RR) of requirement of mechanical ventilation and hospital stay >7 days was calculated in patients with initial s. acetylcholinesterase <1000 versus >1000, in patients presenting in <2 h versus ≥2 h after exposure, in patients with GCS ≤12 versus >12 and in patients with SpO2 <85% versus ≥ 85% at room air at presentation. As the sample population was small (n = 76), logged RR was also calculated. Pearson's correlation analysis was done to assess for association between different clinical parameters with hospital stay and need of mechanical ventilation.
Results
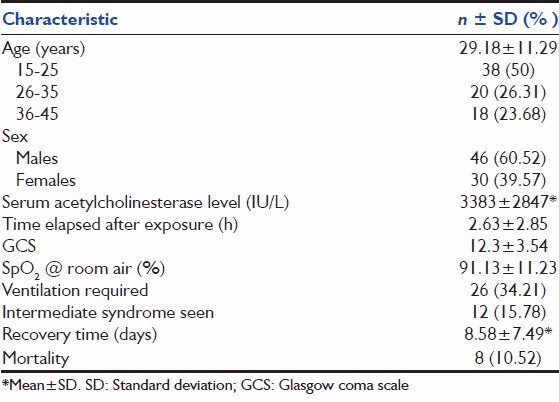
The general characteristics of the 76 study subjects, all of whom had acute OP poisoning at presentation, are summarized in Table 1. The study population included mainly young people, 75% of them coming from 15 to 35 years age group. 60% were males. The mean age was 29.18 ± 11.29 years. The mean hospital stay was 8.5 ± 7.49 days. 26 (34.21%) patients needed mechanical ventilation. 8 (10.25%) of the 76 patients died all of whom were mechanically ventilated.
Table 1.
Basic characteristics of the study population
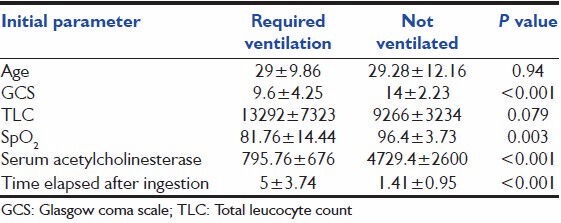
The presentation parameters were compared between the ventilated and nonventilated groups [Table 2]. The GCS score, SpO2 and s. acetyl cholinesterase levels were higher in the nonventilated group and the time elapsed after ingestion and TLC were higher in the ventilated group. Statistically significant difference was observed (between ventilated and nonventilated groups) in the means of GCS, SpO2 , s. acetylcholinesterase and time elapsed after ingestion at presentation [Table 2], but there was no significant difference in the mean of age or TLC between the two groups.
Table 2.
Comparison of the initial parameters in patients with or without requirement of mechanical ventilation
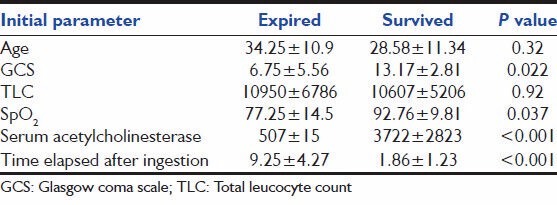
The initial parameters were also compared between patients with hospital stay ≤7 days and >7 days [Table 3]. Statistically significant differences were observed in the means of GCS, SpO2 , s. acetylcholinesterase and time elapsed after ingestion of the two groups. Table 4 shows comparison of initial parameters between expired and survived groups. The means of GCS, SpO2 , s. acetylcholinesterase and time elapsed after ingestion at presentation showed statistically significant difference.
Table 3.
Comparison of the initial parameters between patients with hospital stay≤7 days and>7 days
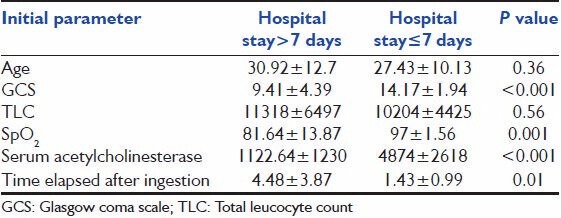
Table 4.
Comparison of the initial parameters between patients who survived and expired
Pearson's correlation analysis was carried out to see for association between different clinical parameters and morbidity. A moderate positive correlation was observed between time elapsed after exposure and hospital stay (r = 0.42, P < 0.001). A moderate negative correlation was seen between s acetylcholinesterase level and hospital stay (r = -0.57, P = <0.05). A moderate negative correlation was seen between GCS and hospital stay (r = -0.51, P = 0.003).
Significant association was observed between mortality and s. acetylcholinesterase <1000 (RR: 5.76; 95% confidence intervals [CI]: 1.25-26.60; logged RR: 1.75), SpO2 <85% (RR: 11.25; 95% CI: 2.50-50.33; logged RR: 2.42), GCS ≤12 (RR: 8.4; 95% CI: 1.84-38.27; logged RR: 2.12) and time elapsed after ingestion to presentation ≥2 h (RR: 12.52; 95% CI: 1.6-98.02; logged RR: 2.52).
Statistically significant association was found between requirement of mechanical ventilation and s. acetylcholinesterase <1000 (RR: 10.57; 95% CI: 4.07-27.46; logged RR: 2.35); time elapsed after ingestion to presentation ≥2 h (RR: 12; 95% CI: 3.04-47.25; logged RR: 2.48); SpO2 (at room air) at presentation <85% (RR: 4.37; 95% CI: 2.55-7.49; logged RR: 1.47) and GCS ≤12 (RR: 4.48; 95% CI: 2.14-6.58; logged RR: 1.49). Hospital stay >7 days was significantly associated with s. acetylcholinesterase <1000 (RR: 5.28; 95% CI: 2.74-10.19; logged RR: 1.66) and GCS ≤ 12 (RR: 4.2; logged RR: 1.43). There was a mild association between hospital stay >7 days and SpO2 (at room air) at presentation <85% (RR: 3.28; 95% CI: 1.71-6.27; logged RR: 1.18) as well as time elapsed after ingestion to presentation ≥2 h (RR: 2.75; 95% CI: 1.40-5.38; logged RR: 1.01).
Discussion
Organophosphate poisoning is a serious clinical entity and causes considerable mortality and morbidity. The estimated mortality from OP ingestion ranges from 10% to 20%.[3,16,17,18] In this study, the mortality was 10.52% (8 deaths in 76 patients), and 26 (34.21%) patients had to be ventilated.
In our study, age, TLC, GCS at presentation, initial s. acetylcholinesterase level, SpO2 at room air at presentation and time elapsed after exposure were analyzed to find out any association with requirement of ventilation or hospital stay. Since severe OP poisoning can cause central respiratory depression and reduced respiratory rate or tachypnea,[5] respiratory rate would not be a reliable guide to severity. History of amount ingested is also often misleading, and inconclusive, most of the information coming up in terms of number of sips or amount left in the bottle. Hence, we did not include respiratory rate or amount ingested into our analysis. Heart rate and pupil size are also unreliable due to frequent use of atropine prior to assessment, so they were not considered for analysis. Using cyanosis[14] or arterial blood gas[9] readings, without taking into account the inspired oxygen concentration are also likely to be misleading. Hence, oxygen saturation at room air at presentation was considered for analysis. Previous studies have shown that outcome varies according to the specific OP compound consumed, but we could not get proper identification of the specific OP compound ingested in many cases so, a compound-based analysis could not be done.
Many studies have tried to identify morbidity and mortality predictors in OP poisoning. In a previous study, an Acute Physiology and Chronic Health Evaluation II (APACHE II) score >26 was reported to be a poor prognostic indicator[12] and others reported that both APACHE II score and GCS <13 predicted outcome.[19,20] A study by Goswamy et al. stated that measurement of the s. acetylcholinesterase level is useful in predicting the prognosis in OP poisoning.[21] However, Aygun et al. have reported that low levels of s. acetylcholinesterase support the diagnosis of acute OP poisoning, but were not related to clinical severity.[22] An Indian study in 2008 reported that the Peradenya organophosphorus poisoning scale and serum cholinesterase at presentation may be useful to assess the severity of and prolonged duration of hospital stay.[23] Another Indian study reported that clinical indices such as GCS, APACHE II, predicted mortality rate (PMR) can be applied in predicting mortality in OP poisoning.[24] In 2011, it was reported that serum creatinine phosphokinase, erythrocyte cholinesterase level, blood pH and total atropine dose were strongly correlated with clinical severity.[25]
Most of the studies have reported association of low s. acetylcholinesterase levels with severity, but other parameters used by these studies like APACHE II and PMR are lengthy. One study observed that values between 870-1200 on day 1 were associated with prolonged ventilation and higher mortality. Our study confirms this finding. Other parameters used in our study were simple, can be estimated bedside and need almost no time.
Although a relation between delayed treatment, hypoxia, and low GCS as shown in our study are not surprising, this study identifies the specific levels around which the morbidity and mortality differ significantly and thus can be used to identify the cases in need of greater attention.
Conclusion
We conclude that s. acetylcholinesterase <1000, SpO2 <85% at room air, GCS ≤12 and time elapsed after exposure before treatment ≥2 h are the initial parameters which have a significant association with morbidity and can be used to develop a severity scoring system in acute OP poisoning cases. This will help in identifying those with greater morbidity and aid in decision making regarding admission to ICU, referral or intensive observation in resource constrained settings like rural or remote areas and developing countries with limited resources and to improve outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Goldfrank LR. Goldfrank's Toxicologic Emergencies. 7th ed. New York: McGraw Hill; 2002. pp. 1346–60. [Google Scholar]
- 2.Bardin PG, van Eeden SF, Joubert JR. Intensive care management of acute organophosphate poisoning. A 7-year experience in the Western Cape. S Afr Med J. 1987;72:593–7. [PubMed] [Google Scholar]
- 3.Munidasa UA, Gawarammana IB, Kularatne SA, Kumarasiri PV, Goonasekera CD. Survival pattern in patients with acute organophosphate poisoning receiving intensive care. J Toxicol Clin Toxicol. 2004;42:343–7. doi: 10.1081/clt-120039539. [DOI] [PubMed] [Google Scholar]
- 4.Rajapakse VP, Wijesekera S. Outcome of mechanical ventilation in Sri Lanka. Ann R Coll Surg Engl. 1989;71:344–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Eddleston M, Mohamed F, Davies JO, Eyer P, Worek F, Sheriff MH, et al. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM. 2006;99:513–22. doi: 10.1093/qjmed/hcl065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2000-2001. Geneva: World Health Organization; 2001. WHO/PCS/01.4. [Google Scholar]
- 7.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley NA, Roberts D, Eddleston M. Overcoming apathy in research on organophosphate poisoning. BMJ. 2004;329:1231–3. doi: 10.1136/bmj.329.7476.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardin PG, van Eeden SF, Moolman JA, Foden AP, Joubert JR. Organophosphate and carbamate poisoning. Arch Intern Med. 1994;154:1433–41. [PubMed] [Google Scholar]
- 10.Goel A, Joseph S, Dutta TK. Organophosphate poisoning: Predicting the need for ventilatory support. J Assoc Physicians India. 1998;46:786–90. [PubMed] [Google Scholar]
- 11.Grmec S, Mally S, Klemen P. Glasgow Coma Scale score and QTc interval in the prognosis of organophosphate poisoning. Acad Emerg Med. 2004;11:925–30. doi: 10.1197/j.aem.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Lee P, Tai DY. Clinical features of patients with acute organophosphate poisoning requiring intensive care. Intensive Care Med. 2001;27:694–9. doi: 10.1007/s001340100895. [DOI] [PubMed] [Google Scholar]
- 13.Matsumiya N, Tanaka M, Iwai M, Kondo T, Takahashi S, Sato S. Elevated amylase is related to the development of respiratory failure in organophosphate poisoning. Hum Exp Toxicol. 1996;15:250–3. doi: 10.1177/096032719601500311. [DOI] [PubMed] [Google Scholar]
- 14.Senanayake N, de Silva HJ, Karalliedde L. A scale to assess severity in organophosphorus intoxication: POP scale. Hum Exp Toxicol. 1993;12:297–9. doi: 10.1177/096032719301200407. [DOI] [PubMed] [Google Scholar]
- 15.Ponte J. Assisted ventilation. 2. Indications for mechanical ventilation. Thora×. 1990;45:885–90. doi: 10.1136/thx.45.11.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka S, Yoshida M, Yamamura Y, Nishimura M, Takaesu Y. A study on acute organophosphorus poisoning - Changes in the activity and isoenzyme patterns of serum cholinesterase in human poisoning. Nihon Eiseigaku Zasshi. 1993;48:955–65. doi: 10.1265/jjh.48.955. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita M, Yamashita M, Tanaka J, Ando Y. Human mortality in organophosphate poisonings. Vet Hum Toxicol. 1997;39:84–5. [PubMed] [Google Scholar]
- 18.Wyckoff DW, Davies JE, Barquet A, Davis JH. Diagnostic and therapeutic problems of parathion poisonings. Ann Intern Med. 1968;68:875–82. doi: 10.7326/0003-4819-68-4-875. [DOI] [PubMed] [Google Scholar]
- 19.Eizadi-Mood N, Saghaei M, Jabalameli M. Predicting outcomes in organophosphate poisoning based on APACHE II and modified APACHE II scores. Hum Exp Toxicol. 2007;26:573–8. doi: 10.1177/09603271060080076. [DOI] [PubMed] [Google Scholar]
- 20.Davies JO, Eddleston M, Buckley NA. Predicting outcome in acute organophosphorus poisoning with a poison severity score or the Glasgow coma scale. QJM. 2008;101:371–9. doi: 10.1093/qjmed/hcn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goswamy R, Chaudhuri A, Mahashur AA. Study of respiratory failure in organophosphate and carbamate poisoning. Heart Lung. 1994;23:466–72. [PubMed] [Google Scholar]
- 22.Aygun D, Doganay Z, Altintop L, Guven H, Onar M, Deniz T, et al. Serum acetylcholinesterase and prognosis of acute organophosphate poisoning. J Toxicol Clin Toxicol. 2002;40:903–10. doi: 10.1081/clt-120016962. [DOI] [PubMed] [Google Scholar]
- 23.Rehiman S, Lohani SP, Bhattarai MC. Correlation of serum cholinesterase level, clinical score at presentation and severity of organophosphorous poisoning. JNMA J Nepal Med Assoc. 2008;47:47–52. [PubMed] [Google Scholar]
- 24.Sam KG, Kondabolu K, Pati D, Kamath A, Pradeep Kumar G, Rao PG. Poisoning severity score, APACHE II and GCS: Effective clinical indices for estimating severity and predicting outcome of acute organophosphorus and carbamate poisoning. J Forensic Leg Med. 2009;16:239–47. doi: 10.1016/j.jflm.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya K, Phaujdar S, Sarkar R, Mullick OS. Serum creatine phosphokinase: A probable marker of severity in organophosphorus poisoning. Toxicol Int. 2011;18:117–23. doi: 10.4103/0971-6580.84263. [DOI] [PMC free article] [PubMed] [Google Scholar]


