Abstract
Widespread emergence of multidrug resistant (MDR) bacterial pathogens is a problem of global dimension. MDR infections are difficult to treat and frequently associated with high mortality. More than one antibiotic is commonly used to treat such infections, but scientific evidence does not favor use of combination therapy in most cases. However, there are certain subgroups where combination therapy may be beneficial, e.g. sepsis due to carbapenem-resistant Enterobacteriaceae (CRE), bacteremic pneumococcal pneumonia, and patients with multiple organ failure. Well-designed prospective studies are needed to clearly define the role of combination therapy in these subgroups.
Keywords: Carbapenem-resistant enterobacteriaceae, combination antibiotic therapy, Klebsiella pneumoniae carbapenemase, sepsis
Introduction
Combination antibiotic therapy is widely practised in the Indian subcontinent. However, combination therapy has its own disadvantages and irrational use can worsen the already alarming scenario of antibiotic resistance. Antibiotics are most commonly overused in subgroup of less severely ill patients. Identifying the subgroup of patients who are likely to benefit from combination therapy and restricting its use only for those specific indications can be helpful in controlling excessive use of antibiotics.
Combination antibiotic therapy is used in critically ill patients due to widespread emergence of multidrug resistance organisms (MDR). Multidrug resistance is defined as lack of susceptibility to at least one agent in three or more antibiotic categories.[1] Over the past few years, carbapenem-resistant Enterobacteriaceae (CRE) has emerged as one of the most notorious groups due to dissemination of Klebsiella pneumoniae carbapenemase (KPC) and other carbapenemase subtypes like New Delhi metallo-β-lactamase (NDM1), via mobile genetic elements.[2] Using dual coverage for organisms producing these enzymes is intuitively thought to be better by many physicians. Majority of the published literature has shown no mortality benefit with combination therapy when compared with monotherapy in sepsis patients.[3,4] In a recently published exhaustive review on combination therapy for Gram-negative bacteria, Tamma et al., have summarized 10 meta-analyses, out of which one meta-analysis showed that combination therapy improves survival in high-risk life-threatening infections but may be detrimental to low-risk patients, whereas nine showed no mortality benefit with combination therapy as compared with monotherapy.[5] The authors concluded that combination therapy is appropriate in empirical regimens when the organism is unknown, whereas definitive therapies should include single appropriate antibiotic only. However, certain subgroups need to be studied separately, e.g. patients infected with CRE and multiple organ failure patients.
Reasons for using combination therapy
Combination therapy is mostly practiced because of one or more of the following reasons:
Broadening antibacterial spectrum. Use of more than one agent broadens the antibacterial spectrum of the empirical therapy and thus ensures that at least one agent will cover the infecting organism. It has been shown by clinical studies that initial appropriate antibiotic choice is one of the most important determinants of mortality in critically ill patients.[6,7,8] Patients infected with resistant organisms are more likely to get delayed appropriate antibiotic and subsequently mortality increases[9,10]
Polymicrobial infections. Intra-abdominal infections with breach in continuity of gut wall are often polymicrobial and require more than one antibiotic to cover all bacterial pathogens
Synergy. Antibiotic combinations are also used for their synergistic action. Synergy is defined as combined effect of two agents together being greater than the sum of their individual activities, e.g. certain beta-lactams and aminoglycoside combinations. Various synergistic combinations have been tested for CRE organisms also. Pournaras et al., reported in vitro synergy between tigecycline and colistin but not between tigecycline and meropenem in KPC producing K. pneumoniae strain via time kill assay.[11] Synergistic combinations have been proved to be advantageous in animal models but clinical studies are still lacking
Emergence of resistance. Chances of emergence of resistance against two drugs are lower as compared with a single drug. Polymyxin and tigecycline are the two most commonly used antibiotics for CRE. As these antibiotics are last resort for resistant infections, emergence of resistance against these drugs needs to be prevented. There are reports of emergence of resistance against tigecycline and polymyxin, whereas the patient is still on treatment when these agents are used as monotherapy. In a study of 12 patients being treated for KPC infection with polymyxin B monotherapy, three (25%) developed reduced susceptibility to polymyxin B during the therapy. The authors recommended use of combination therapy to prevent emergence of resistance.[12] However, these findings have not been validated by well-designed randomized control trial (RCT).
Combination therapy can be helpful in following conditions.
Targeted therapy for patients with life-threatening CRE infections
Carbapenem monotherapy is frequently used to treat MDR Gram-negative infections, but a new class of enzymes capable of inactivating carbapenems has emerged. These are called carbapenemases. Various types of carbapenemases are KPC, Verona integron-encoded metallo beta lactamases (VIMs), active on imipenem metallo beta lactamases (IMPs), New Delhi metallo betalactamases (NDMs) and so forth.
KPC enzyme is a type of carbapenemase, which was first reported in K. pneumoniae, but over a period of few years it has spread to other bacteria of Enterobacteriaceae family, e.g. Escherichia spp, Proteus spp, Acinetobacter spp, and Pseudomonas spp. There are nine KPC variants reported in literature out of which KPC2 and 3 are most common. KPC infections are health care-associated infections with estimated mortality of 30 to 50%.[13] KPC-producing organisms are frequently resistant to many other classes of antibiotics, including fluoroquinolones and aminoglycosides.[14] There is a paucity of literature regarding appropriate antibiotic choice for KPC infections [Table 1].
Table 1.
Summary of studies on combination therapy for Carbapenem-resistant Enterobacteriaceae

Lee et al., reviewed 38 articles on KPC infection, which included case reports, case series, and retrospective cohort studies.[16] Of 105 patients studied 49 (47%) patients received monotherapy, whereas 56 (53%) cases received combination therapy. Blood was the most common site of infection followed by respiratory tract and urine. The study reported significantly more treatment failure rates with monotherapy as compared with combination therapy (49% versus 25%; P = 0.01). Authors recommended combination therapy for KPC infection. Similarly, Qureshi et al., reported superiority of combination therapy over monotherapy in bacteremia due to KPC-producing K. pneumoniae in a retrospective study conducted over 41 patients.[18] Multivariate analysis showed that combination therapy was independently associated with improved survival. Twenty-eight day mortality was 13.3% in combination group as compared with 57.8% in monotherapy group. These results were seen despite in vitro susceptibility in monotherapy group. Colistin or polymyxin B or tigecycline combined with carbapenem were the most commonly used combinations. The authors concluded that combination regimens should be used for definitive therapy for KPC-K. pneumoniae. Hirsch and Tam reviewed 15 studies/reports on patients infected with bacteria producing KPCs.[15] They reported that polymyxin monotherapy was associated with poor response rate as compared with combination therapy (14% versus 73%) in patients infected with KPC. In a multicentric study conducted in Italy, 125 cases with bloodstream infections caused by KPC-producing K. pneumoniae were studied. Mortality rate was significantly higher in monotherapy group (54% versus 34% in combination therapy group). Multivariate analysis showed that a combination of tigecycline, colistin, and meropenem was associated with decreased risk of death.[19]
It has been shown in various studies that despite being carbapenem resistant, KPC infections can be treated with carbapenems if following aspects are kept in mind, i.e. minimum inhibitory concentration ( MIC) of the carbapenem for the infecting organism should be <4 mg/l, the drug should be given as prolonged infusion in maximum possible dose, and carbapenem should be used in combination with another active antibiotic.[20]
Another type of novel carbapenemase is NDM1, which was first reported in 2008. Evidence regarding appropriate antibiotic regimen for organisms possessing these enzymes is lacking in literature. A study by Kumarasamy showed that 89% NDM1 isolates from UK were sensitive to colistin, whereas 64% were sensitive to tigecycline.[21] These isolates were resistant to all other tested antibiotics including carbapenems.
Combination therapy has also been studied for CRE Acinetobacter baumanii infections, but due to lack of randomized control trials, definite conclusions cannot be drawn. Most of the information is derived from animal models, in vitro studies, or small case series. Petrosillo et al., in their pilot study of combination therapy with rifampicin and colistin for CRE Acinetobacter baumannii infection reported improved microbiological clearance in nine of 14 (64%) patients.[17] A recently conducted larger study including 210 critically ill subjects also showed significant increase in microbiological eradication rate with rifampicin and colistin combination when compared with colistin alone.[22] However, the study failed to show mortality benefit in combination therapy group. The authors concluded that at present rifampicin should not be routinely combined with colistin in clinical practice. Many questions like appropriate antibiotic combination, appropriate duration of combination therapy, and appropriate dosage of various combination antibiotics remain unanswered and require exhaustive research in this field.
Therapy for sepsis patients with multiorgan failure
Severely ill patients also form a subgroup of patient in which combination antibiotic therapy can improve survival. Kumar et al., in a meta analysis of 50 studies demonstrated that combination antibiotic therapy benefited only those patients who were at high risk of death (monotherapy risk of death >25%).[5] The benefit of combination therapy was lost in 15 to 25% risk range and was associated with worse survival when used in patients with <15% risk of death with monotherapy. Such findings may be related to the fact severely ill patients have more microbial burden and their accelerated clearance may help in reversal of organ failure.
Therapy for severe community acquired pneumonia with bacteremia
All patients of community acquired pneumonia are not alike and treatment depends on risk stratification. A scoring system named as CURB 65 (confusion, urea >20 mg/dl, respiratory rate >30 breaths/min, systolic blood pressure <90 mmHg or diastolic pressure <60 mmHg, age >65 years) can be used for this purpose. Patients with a score of three or more require intensive care unit (ICU) care.[24] Other factors affecting choice of treatment in CAP are presence of comorbid illnesses and previous antibiotic exposure.
Around 10% of the patients with community acquired pneumonia also develop bacteremia. Though bacteremic patients form a small group, but mortality is high in this subgroup of patients. Weiss et al., reviewed four retrospective studies on bacteremic pneumococcal pneumonia and concluded that combination therapy with beta-lactam and a macrolide is superior to monotherapy.[25] The advantage with macrolides in such patients is inhibition of pneumolysin production and immunomodulatory action on neutrophils.
Empirical therapy for sepsis
Though sepsis guidelines suggest the use of combination therapy in empirical regimens for patients with difficult to treat infections, evidence from well-designed randomized trials is lacking. Ideally empirical regimens for life-threatening infections should cover all likely pathogens. If this is not possible by giving one drug, combination therapy can be used but should be streamlined to specific monotherapy as soon as the microbiology reports are available.
Figure 1 outlines an algorithm for choosing antibiotic therapy for MDR pathogen.
Figure 1.
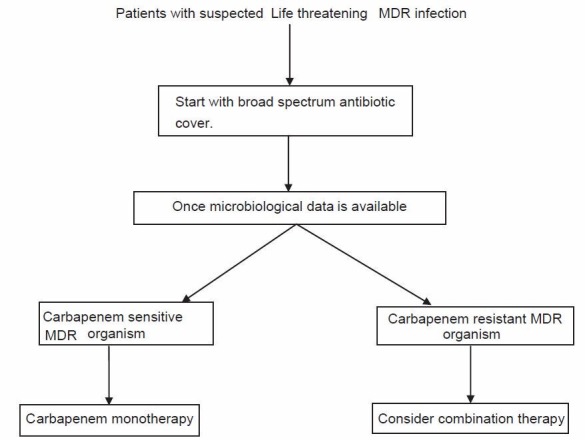
Proposed algorithm for choosing antibiotic therapy for life threatening infection with MDR pathogen. MDR: Multidrug resistant
Conclusions
With the emergence and rapid dissemination of MDR organisms, approach toward bacterial infections and antimicrobial therapy needs to be redefined. Limited evidence derived from various case series and case reports shows favorable results in only certain subgroups of patients when treated with certain antibiotic combinations. Large well-designed RCTs addressing this issue are lacking in the literature. Further research is needed in this field to guide rational use of combination antibiotics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin Infect Dis. 2011;53:60–7. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 3.Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: Systematic review and meta-analysis of randomised trials. BMJ. 2004;328:668. doi: 10.1136/bmj.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul M, Silbiger I, Grozinsky S, Soares-Weiser K, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2006:CD003344. doi: 10.1002/14651858.CD003344.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25:450–70. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Cooperative Antimicrobial Therapy of Septic Shock Database Research Group. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–48. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 7.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: Importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49:1306–11. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollef MH. Inadequate antimicrobial treatment: An important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31:S131–8. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]
- 9.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiellapneumoniae: Risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32:1162–71. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 10.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: A systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:913–20. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 11.Pournaras S, Vrioni G, Neou E, Dendrinos J, Dimitroulia E, Poulou A, et al. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int J Antimicrob Agents. 2011;37:244–7. doi: 10.1016/j.ijantimicag.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Patel G, Huprikar S, Calfee DP, Jenkins SG. Decreased susceptibility to polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. J Clin Microbiol. 2009;47:1611–2. doi: 10.1128/JCM.02466-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LF, Anderson DJ, Paterson DL. Overview of the epidemiology and the threat of Klebsiella pneumoniae carbapenemases (KPC) resistance. Infect Drug Resist. 2012;5:133–41. doi: 10.2147/IDR.S26613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold RS, Thom KA, Sharma S, Phillips M, Kristie Johnson J, Morgan DJ. Emergence of Klebsiella pneumoniae carbapenemase-producing bacteria. South Med J. 2011;104:40–5. doi: 10.1097/SMJ.0b013e3181fd7d5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): An emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010;65:1119–25. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 16.Lee GC, Burgess SD. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: A review of published case series and case reports. Ann Clin Microbiol Antimicrob. 2012;11:32. doi: 10.1186/1476-0711-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrosillo N, Chinello P, Proietti MF, Cecchini L, Masala M, Franchi C, et al. Combined colistin and rifampicin therapy for carbapenem-resistant Acinetobacter baumannii infections: Clinical outcome and adverse events. Clin Microbiol Infect. 2005;11:682–3. doi: 10.1111/j.1469-0691.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: Superiority of combination antimicrobialregimens. Antimicrob Agents Chemother. 2012;56:2108–13. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: Importance of combination therapy. Clin Infect Dis. 2012;55:943–50. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 20.Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiellapneumoniae: (When) might we still consider treating with carbapenems? Clin Microbiol Infect. 2011;17:1135–41. doi: 10.1111/j.1469-0691.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: A multicenter, randomized clinical trial. Clin Infect Dis. 2013;57:349–58. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 23.Petrosillo N, Giannella M, Lewis R, Viale P. Treatment of carbapenem-resistant Klebsiella pneumoniae: The state of the art. Expert Rev Anti Infect Ther. 2013;11:159–77. doi: 10.1586/eri.12.162. [DOI] [PubMed] [Google Scholar]
- 24.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003;58:377–82. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss K, Tillotson GS. The controversy of combination vs monotherapy in the treatment of hospitalized community-acquired pneumonia. Chest. 2005;128:940–6. doi: 10.1378/chest.128.2.940. [DOI] [PubMed] [Google Scholar]


