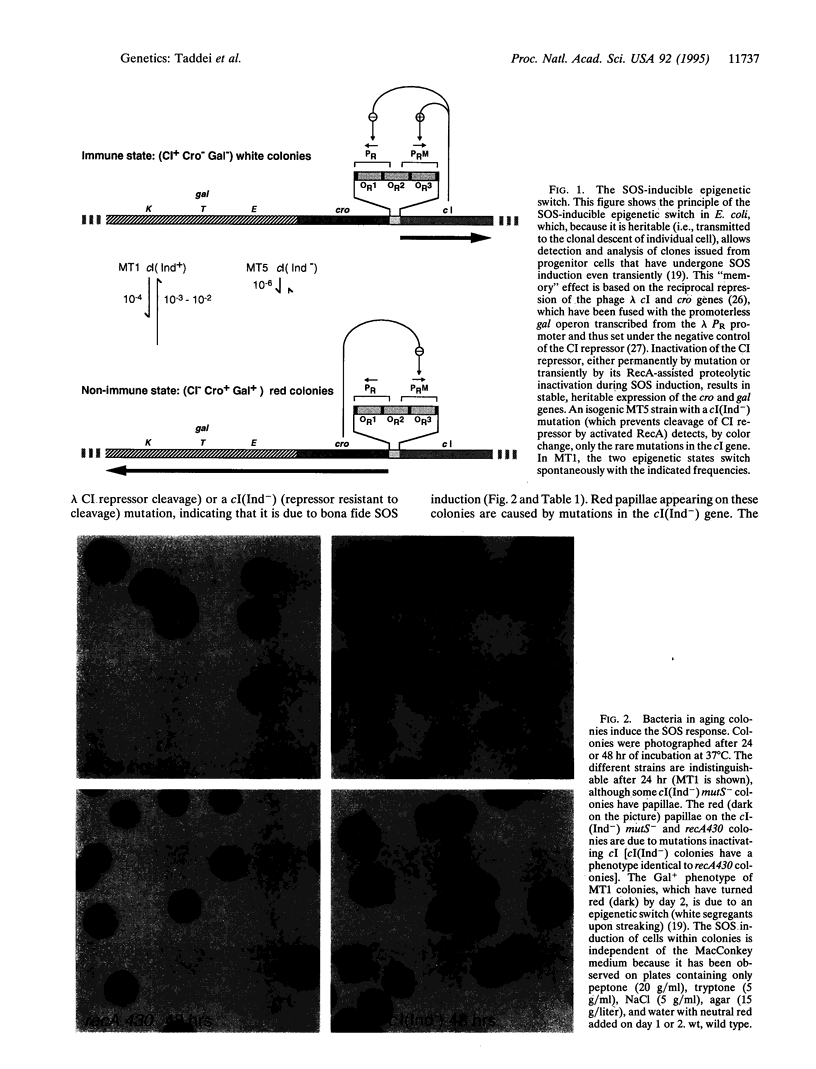
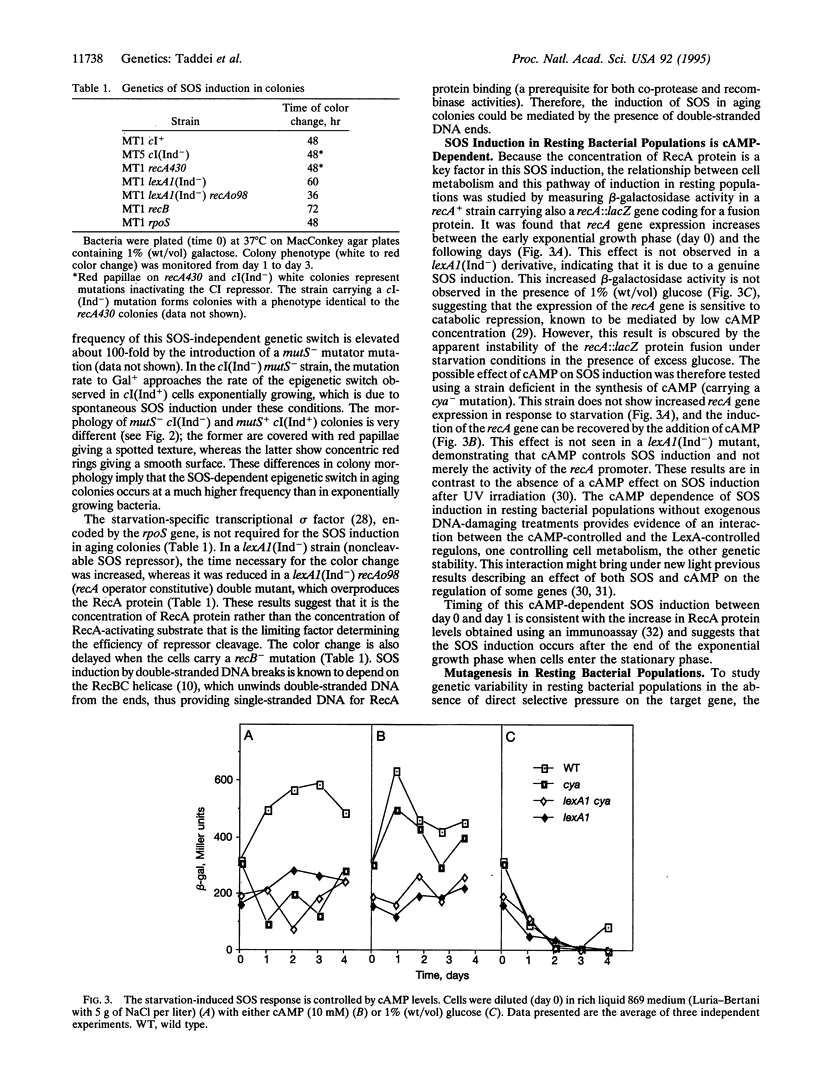
Abstract
The inducible SOS system increases the survival of bacteria exposed to DNA-damaging agents by increasing the capacity of error-free and error-prone DNA repair systems. The inducible mutator effect is expected to contribute to the adaptation of bacterial populations to these adverse life conditions by increasing their genetic variability. The evolutionary impact of the SOS system would be even greater if it was also induced under conditions common in nature, such as in resting bacterial populations. The results presented here show that SOS induction and mutagenesis do occur in bacteria in aging colonies on agar plates. The observed SOS induction and mutagenesis are controlled by the LexA repressor and are RecA- and cAMP-dependent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbé J., Gibert I., Guerrero R. Modulating action of cyclic AMP on the expression of two SOS genes in Escherichia coli K-12. Can J Microbiol. 1987 Aug;33(8):704–708. doi: 10.1139/m87-123. [DOI] [PubMed] [Google Scholar]
- Botsford J. L., Harman J. G. Cyclic AMP in prokaryotes. Microbiol Rev. 1992 Mar;56(1):100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Maenhaut-Michel G. Nature of the SOS mutator activity: genetic characterization of untargeted mutagenesis in Escherichia coli. Mol Gen Genet. 1988 Aug;213(2-3):491–498. doi: 10.1007/BF00339621. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Maenhaut-Michel G., Radman M. SOS mutator effect in E. coli mutants deficient in mismatch correction. EMBO J. 1984 Apr;3(4):707–712. doi: 10.1002/j.1460-2075.1984.tb01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J., Foster P. L. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics. 1991 Aug;128(4):695–701. doi: 10.1093/genetics/128.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Dambly-Chaudière C., Gottesman M., Debouck C., Adhya S. Regulation of the pR operon of bacteriophage lambda. J Mol Appl Genet. 1983;2(1):45–56. [PubMed] [Google Scholar]
- Dimpfl J., Echols H. Duplication mutation as an SOS response in Escherichia coli: enhanced duplication formation by a constitutively activated RecA. Genetics. 1989 Oct;123(2):255–260. doi: 10.1093/genetics/123.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Takahara Y., Kishi F., Nakazawa A., Brent R. LexA protein is a repressor of the colicin E1 gene. J Biol Chem. 1983 Nov 10;258(21):13258–13261. [PubMed] [Google Scholar]
- Echols H. SOS functions, cancer and inducible evolution. Cell. 1981 Jul;25(1):1–2. doi: 10.1016/0092-8674(81)90223-3. [DOI] [PubMed] [Google Scholar]
- Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in lambda. Proc Natl Acad Sci U S A. 1970 Jul;66(3):855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escarceller M., Hicks J., Gudmundsson G., Trump G., Touati D., Lovett S., Foster P. L., McEntee K., Goodman M. F. Involvement of Escherichia coli DNA polymerase II in response to oxidative damage and adaptive mutation. J Bacteriol. 1994 Oct;176(20):6221–6228. doi: 10.1128/jb.176.20.6221-6228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N. V. Barbara McClintock (June 16, 1902-September 2, 1992). Genetics. 1994 Jan;136(1):1–10. doi: 10.1093/genetics/136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L. Adaptive mutation: the uses of adversity. Annu Rev Microbiol. 1993;47:467–504. doi: 10.1146/annurev.mi.47.100193.002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Trimarchi J. M. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science. 1994 Jul 15;265(5170):407–409. doi: 10.1126/science.8023164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Adaptive evolution that requires multiple spontaneous mutations. I. Mutations involving an insertion sequence. Genetics. 1988 Dec;120(4):887–897. doi: 10.1093/genetics/120.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday J. A., Glickman B. W. Mechanisms of spontaneous mutation in DNA repair-proficient Escherichia coli. Mutat Res. 1991 Sep-Oct;250(1-2):55–71. doi: 10.1016/0027-5107(91)90162-h. [DOI] [PubMed] [Google Scholar]
- Harris R. S., Longerich S., Rosenberg S. M. Recombination in adaptive mutation. Science. 1994 Apr 8;264(5156):258–260. doi: 10.1126/science.8146657. [DOI] [PubMed] [Google Scholar]
- Hiom K., Thomas S. M., Sedgwick S. G. Different mechanisms for SOS induced alleviation of DNA restriction in Escherichia coli. Biochimie. 1991 Apr;73(4):399–405. doi: 10.1016/0300-9084(91)90106-b. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Karu A. E., Belk E. D. Induction of E. coli recA protein via recBC and alternate pathways: quantitation by enzyme-linked immunosorbent assay (ELISA). Mol Gen Genet. 1982;185(2):275–282. doi: 10.1007/BF00330798. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Torrey T. A., Connaughton M. J. Induction of UV-resistant DNA replication in Escherichia coli: induced stable DNA replication as an SOS function. Mol Gen Genet. 1979 Oct 2;176(1):1–9. doi: 10.1007/BF00334288. [DOI] [PubMed] [Google Scholar]
- Kolter R., Siegele D. A., Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- Kuan C. T., Tessman I. Further evidence that transposition of Tn5 in Escherichia coli is strongly enhanced by constitutively activated RecA proteins. J Bacteriol. 1992 Nov;174(21):6872–6877. doi: 10.1128/jb.174.21.6872-6877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Lenski R. E., Mittler J. E. The directed mutation controversy and neo-Darwinism. Science. 1993 Jan 8;259(5092):188–194. doi: 10.1126/science.7678468. [DOI] [PubMed] [Google Scholar]
- Livneh Z., Cohen-Fix O., Skaliter R., Elizur T. Replication of damaged DNA and the molecular mechanism of ultraviolet light mutagenesis. Crit Rev Biochem Mol Biol. 1993;28(6):465–513. doi: 10.3109/10409239309085136. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Buckman C., Benson F. E. Genetic analysis of conjugational recombination in Escherichia coli K12 strains deficient in RecBCD enzyme. J Gen Microbiol. 1987 Sep;133(9):2531–2538. doi: 10.1099/00221287-133-9-2531. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Directed mutation: paradigm postponed. Mutat Res. 1993 Jan;285(1):109–116. doi: 10.1016/0027-5107(93)90058-n. [DOI] [PubMed] [Google Scholar]
- Matic I., Rayssiguier C., Radman M. Interspecies gene exchange in bacteria: the role of SOS and mismatch repair systems in evolution of species. Cell. 1995 Feb 10;80(3):507–515. doi: 10.1016/0092-8674(95)90501-4. [DOI] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Matin A., Matin M. K. Cellular levels, excretion, and synthesis rates of cyclic AMP in Escherichia coli grown in continuous culture. J Bacteriol. 1982 Mar;149(3):801–807. doi: 10.1128/jb.149.3.801-807.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Ganem D., Lu P., Schmitz A. Genetic studies of the lac repressor. I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J Mol Biol. 1977 Jan 15;109(2):275–298. doi: 10.1016/s0022-2836(77)80034-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. M. In pursuit of a molecular mechanism for adaptive mutation. Genome. 1994 Dec;37(6):893–899. doi: 10.1139/g94-127. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. M., Longerich S., Gee P., Harris R. S. Adaptive mutation by deletions in small mononucleotide repeats. Science. 1994 Jul 15;265(5170):405–407. doi: 10.1126/science.8023163. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Observations on the formation of clones containing araB-lacZ cistron fusions. Mol Gen Genet. 1984;194(1-2):79–90. doi: 10.1007/BF00383501. [DOI] [PubMed] [Google Scholar]
- Sonti R. V., Roth J. R. Role of gene duplications in the adaptation of Salmonella typhimurium to growth on limiting carbon sources. Genetics. 1989 Sep;123(1):19–28. doi: 10.1093/genetics/123.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I., Kennedy M. A. DNA polymerase II of Escherichia coli in the bypass of abasic sites in vivo. Genetics. 1994 Feb;136(2):439–448. doi: 10.1093/genetics/136.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toman Z., Dambly-Chaudière C., Tenenbaum L., Radman M. A system for detection of genetic and epigenetic alterations in Escherichia coli induced by DNA-damaging agents. J Mol Biol. 1985 Nov 5;186(1):97–105. doi: 10.1016/0022-2836(85)90260-8. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K. RecA protein-dependent proteolysis of bacteriophage lambda repressor Characterization of the reaction and stimulation by DNA-binding proteins. J Biol Chem. 1981 Nov 10;256(21):10883–10888. [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]





